Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the past 25 years, the field of clinical neonatal nephrology has expanded significantly in concert with major advances and changes in the care and survival of neonates, particularly premature neonates. The widespread use of invasive vascular catheters, for example, has resulted in a new set of complications, including acute kidney injury (AKI) and renovascular hypertension related to thromboembolic disease. The improved survival of infants with extremely low birth weight and bronchopulmonary dysplasia has led to the relatively new complication of neonatal nephrocalcinosis. The increased use of prenatal ultrasonography has created new paradigms for the prenatal management of urinary tract anomalies (see Chapter 11 ).
The goals of this chapter are to review the anatomic and functional development of the kidney, outline the recommended approach to the evaluation of the neonate with suspected renal disease, and provide an overview of the common nephrologic and urologic problems seen in preterm and term neonates.
Kidney and urinary tract development is a complex process involving interactions between genes involved in the formation and maturation of the glomeruli, tubules, renal blood vessels, extracellular matrix, and uroepithelium. This carefully coordinated process involves the regulated activation and inactivation of hundreds of genes that encode transcription factors, growth factors and receptors, structural proteins, adhesion molecules, and other regulatory proteins. Since the mid-1990s, the rapidly expanding field of molecular genetics has provided important new insights into the mechanisms involved in renal and urinary tract development. A detailed review of the genes and signaling pathways involved in renal development is beyond the scope of this chapter; therefore, the reader is referred to two detailed reviews of the subject. This section highlights some of the important pathways involved in these processes and provides examples of human kidney diseases resulting from abnormalities in normal development.
Kidney embryogenesis involves successive formation of three different kidneys: the pronephros, mesonephros, and metanephros. The formation of these structures during intrauterine development is illustrated in Fig. 93.1 . The pronephros, a vestigial structure of 7-10 solid or tubular cell groups called nephrotomes, develops in the cervical region and disappears by the end of the fourth week of gestation. As the pronephros regresses, the mesonephros appears and is characterized by excretory tubules that form an S-shaped loop, with a glomerulus and Bowman capsule at the proximal end. At the distal portion, the tubule enters the collecting duct (also referred to as the mesonephric or Wolffian duct), which does not drain into the coelomic cavity. During the second month of gestation, the urogenital ridge, which is the forerunner of the gonads, develops. By the end of the second month of gestation, most portions of the mesonephros disappear. However, a few caudal tubules remain in close proximity to the testis and ovaries, developing into the vas deferens in males and remaining as remnant tissue in females.
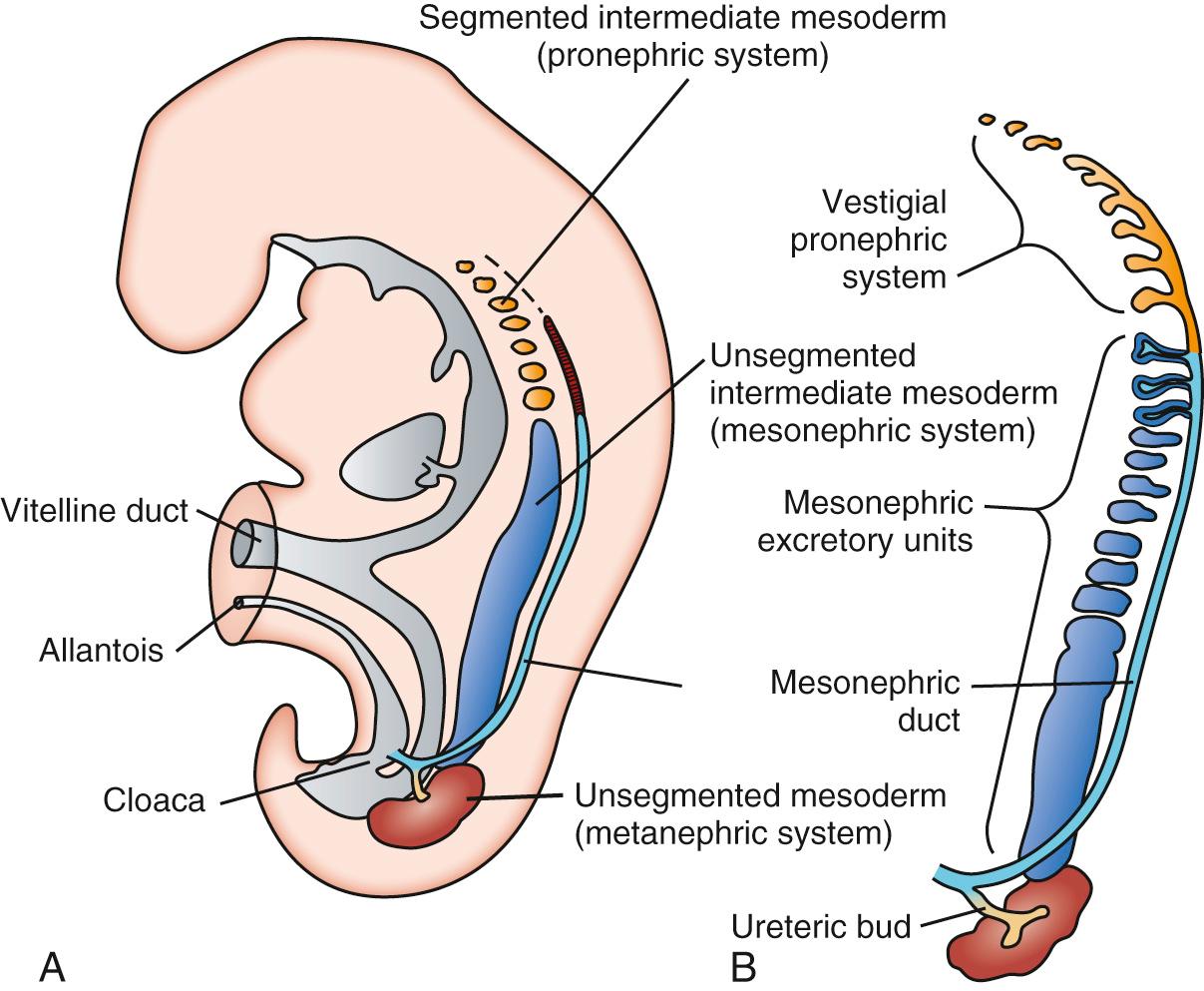
The metanephric, or definitive, kidney is derived solely from intermediate mesoderm and begins forming at 5 weeks of gestation, when a portion of the Wolffian duct swells to form the ureteric bud (UB). The UB, composed of epithelium, then invades the nearby metanephric mesenchyme. The outgrowth and subsequent branching of the ureteric bud is dependent on interaction between glial cell–derived neurotrophic factor (GDNF) expressed by the metanephric mesenchyme and Ret receptors present on the UB. The GDNF-Ret interaction leads to patterned, progressive divisions of the UB (called “branching morphogenesis”) that form the collecting ducts of the kidney as well as the major and minor caliceal system of the renal pelvis. At the tip of the branches, the mesenchymal cells of the metanephric blastema are induced by the advancing ureteric bud to differentiate into the epithelial cells that eventually become the glomeruli and renal tubules. The branching UB cells are dependent on the support of extracellular matrix components such as laminins and integrins. Foci of the metanephric blastema become condensed adjacent to the branching ureteric bud to form comma-shaped bodies that then elongate to form S-shaped bodies. The lower portion of the S-shaped body becomes associated with a tuft of capillaries to form the glomerulus, because the upper portion forms the tubular elements of the nephron.
The metanephric kidney ascends from the pelvic to the thoracolumbar region. This process is thought to occur as the result of a decrease in body curvature and body growth of the lumbar and sacral regions. In the pelvis, the metanephric kidney receives its blood supply from a pelvic branch of the aorta. During ascent, the metanephric kidney receives its blood supply from arterial branches at higher levels of the aorta. Although the pelvic vessels usually degenerate, persistence of these early embryonic vessels can lead to supernumerary renal arteries. The metanephric kidney becomes functional during the second half of pregnancy. Nephrogenesis, which is the formation of new nephron units, is complete at 34 weeks of gestation, when each kidney contains its definitive complement of approximately 800,000 to 1.2 million nephrons.
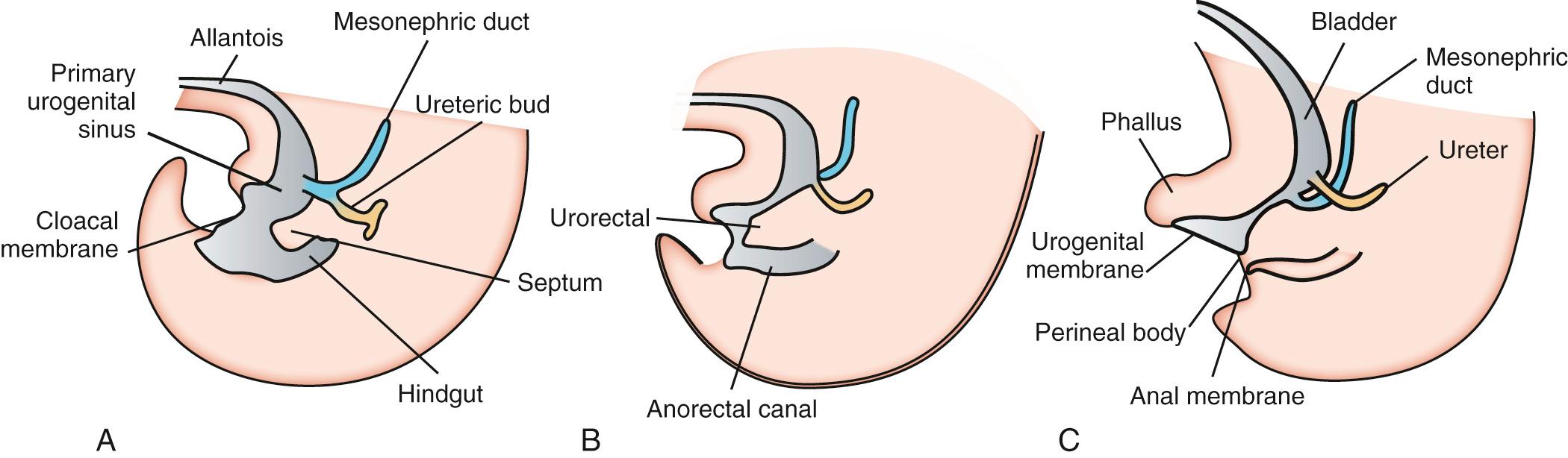
The bladder and urethra are formed during the second and third months of gestation, which is illustrated in Fig. 93.2 . During the fourth to seventh week of development, the cloaca, which is located at the proximal end of the allantois and is the precursor to the urinary bladder and urethra, is divided by the urorectal septum into the primitive urogenital sinus (anterior portion) and the anorectal canal (posterior portion). The primitive urogenital sinus develops into the bladder (upper portion), prostatic and membranous urethra (pelvic portion) in males, and the penile urethra (males) or urethra and vestibule (females). As the cloaca develops, the caudal portion of the mesonephric ducts is absorbed into the bladder wall. Similarly, the caudal portions of the ureters, which originate from the mesonephric ducts, enter the bladder. During these processes, the ureteral orifices move cranially and the mesonephric ducts move closer together to enter the prostatic urethra, forming the trigone of the bladder. At the end of the third month of gestation, the epithelial proliferation of the prostatic urethra forms outbuddings that constitute the prostate gland in males. In females, the cranial portion of the urethra forms buds that develop into the urethral and paraurethral glands.
Studies in animal models have highlighted the important role of multiple proteins and signaling mechanisms in nephrogenesis, including growth factors such as fibroblast growth factor (FGF), adhesion molecules such as integrin alpha 8, the transcription factor WT-1, the canonical Wnt/beta catenin signaling pathway, and the GDNF/Ret tyrosine kinase signaling pathway. Alterations in the actions of one or more of these proteins/pathways can result in renal or urogenital anomalies such as renal agenesis, renal dysplasia, cystic kidneys, and glomerulosclerosis.
A number of genes have been associated with congenital anomalies of the kidney and urinary tract (CAKUT) and often present with similar renal phenotypes ( Table 93.1 ). Genetic mutations associated with CAKUT may be inherited in an autosomal dominant or recessive pattern, but most often occur as sporadic, de novo mutations. In addition to monogenic causes, copy number variation (CNV) has been found in 10%-16% of patients with CAKUT, specifically in patients with posterior urethral valves or multicystic dysplastic kidneys. However, identified genetic mutations do not account for all patients with CAKUT, and the majority of patients with anomalies do not have a known mutation identified.
| Type of Malformation | Renal Phenotype | Gene | Cause |
|---|---|---|---|
| Renal agenesis | Absence of the ureter and kidney | RET, GDNF, FGF20, FRAS1, FREM2 | Lack of interaction between the ureteric bud and MM |
| Renal hypoplasia | Reduced number of ureteric bud branches and nephrons, small kidney size, often associated with dysplasia | Pax2, Sall1, Six2, BMP4, HNF1B, UMOD | Aberrant interaction between ureteric bud and MM |
| Renal dysplasia | Reduced number of ureteric bud branches and nephrons, undifferentiated stromal and mesenchymal cells, cysts, or cartilage | PAX2, HNF1B, UMOD, Nphp1 BMP4, Six2, XPNPEP3 | Aberrant interaction between ureteric bud and MM |
| MCDK | Absent glomeruli and tubules | HNF1B, UPIIIA, PEX26, ELN, HNF1B, ALG12, FRG1, FRG2, CYP4A11 | Aberrant interaction between ureteric bud and MM |
| Duplex ureters | Duplex ureters and kidneys or duplex ureters and collecting systems | Robo2, FoxC1, FoxC2, BMP4 | Supernumerary ureteric bud budding from the MD |
| Horseshoe kidney | Kidneys are fused at inferior lobes and located lower than usual | HNF1B | Defects in renal capsule |
| VUR | Urine refluxes to various degrees from bladder up into the collecting system | PAX2, ROBO2, SIX1, SIX5, SOX17, TNXB, CHD1L, TRAP1 | Aberrant insertion of ureter into bladder wall |
| Renal tubular dysgenesis | Absence of or incomplete differentiation of proximal tubule | ACE, AGT, AGTR1, REN | Impaired tubular growth and differentiation |
Certain prenatal factors have been associated with increased risk of CAKUT. Maternal diabetes mellitus, older maternal age, gestational hypertension, and BMI greater than 25 were associated with higher likelihood of having renal dysplasia or obstructive uropathy in the infant. Teratogenic effects of several drugs have been documented, most notably angiotensin converting enzyme inhibitors (ACE-I) and angiotensin II receptor blockers (ARB). ACE-I fetopathy presents with renal tubular dysgenesis, neonatal anuria, pulmonary hypoplasia, and occasional central nervous system abnormalities, most often after exposure to ACE-I or ARB during the second trimester of pregnancy. Animal studies have demonstrated nephrotoxic effects of nonsteroidal, anti-inflammatory drugs; immunosuppressive medications, such as corticosteroids, cyclosporine, and tacrolimus; and antibacterial medications, such as aminoglycosides and beta-lactams. These drugs are mostly associated with reduced nephron mass, but their effects in humans are still uncertain. Finally, heavy metal exposure can potentially damage fetal kidney development, and there may be other unidentified environmental toxins that may cause developmental abnormalities of kidneys.
During intrauterine life, the kidneys play only a minor role in regulating fetal salt and water balance, because this function is maintained primarily by the placenta. The most important functions of the prenatal kidneys are the formation and excretion of urine to maintain an adequate amount of amniotic fluid. After birth, there is a progressive maturation in renal function, which appears to parallel the needs of the neonate for growth and development ( Table 93.2 ).
| Age | GFR (mL/min/1.73 m 2 ) | RBF (mL/min/1.73 m 2 ) | Maximum Urine Osm (mOsm/kg) | Serum Creatinine (mg/dL) | Fe Na (%) |
|---|---|---|---|---|---|
| Newborn | |||||
| Premature (30-34 weeks) | 14 ± 3 | 40 ± 6 | 480 | 0.6-1.3 * | 2-6 |
| Term | 21 ± 4 | 88 ± 4 | 800 | 0.6-1 * | <1 |
| 1-2 weeks | 50 ± 10 | 220 ± 40 | 900 | 0.27-0.5 * | <1 |
| 6 months-1 year | 77 ± 14 | 352 ± 73 | 1200 | 0.18-0.29 * | <1 |
| 1-3 years | 96 ± 22 | 540 ± 118 | 1400 | 0.24-0.43 † | <1 |
| Adult | 118 ± 18 | 620 ± 92 | 1400 | 0.6-1.3 † | <1 |
Absolute renal blood flow (RBF) and the percentage of cardiac output directed to the kidneys increase steadily with advancing gestational age. The kidneys of a human fetus weighing more than 150 g receive approximately 4% of the cardiac output, compared with approximately 6% in the term infant. The relatively low RBF of the fetus is related to high renovascular resistance caused by the increased activity of the renin-angiotensin-aldosterone and sympathetic nervous systems. The RBF dramatically increases postnatally, reaching 8%-10% of the cardiac output at 1 week of life, and achieves adult values of 20%-25% of the cardiac output by 2 years of age. This rise in RBF is caused primarily by decreasing renovascular resistance and increasing cardiac output and perfusion pressure.
The glomerular filtration rate (GFR) in the fetal kidney increases steadily with advancing gestational age. By 32-34 weeks’ gestation, a GFR of 14 mL/min per 1.73 m 2 is achieved, which further increases to 21 mL/min per 1.73 m 2 at term. The GFR continues to increase postnatally, achieving adult values of approximately 120 mL/min per 1.73 m 2 by the age of 2 years. The achievement of adult GFR may be delayed in preterm infants, especially those with very low birth weights. The progressive increase in GFR during the initial weeks of postnatal life primarily results from an increase in glomerular perfusion pressure. Subsequent increases in GFR during the first 2 years of life are caused primarily by increases in RBF and the maturation of superficial cortical nephrons, which lead to an increase in the glomerular capillary surface area.
Newborn infants have a limited capacity to concentrate their urine, with maximal urinary osmolality of 800 mOsm/kg in a term infant compared to that of a 2 year old, which approximates adult values of 1400 mOsm/kg. In contrast, the term newborn infant has full ability to maximally dilute its urine in response to a water load, achieving adult values of 50 mOsm/kg. Preterm infants are unable to fully dilute their urine but can achieve urine osmolality of 70 mOsm/kg. However, the response of premature infants to an acute water load may be limited because of their low GFR and the decreased activity of sodium transporters in the diluting segment of the nephron. The excessive administration of water may place the newborn infant at a high risk for dilutional hyponatremia and hypervolemia. Urinary diluting and concentrating capacity in term and preterm infants is discussed in more detail in Chapter 92 .
The antenatal history should be reviewed thoroughly, with particular attention devoted to medications, toxins, and unusual environmental exposures during the pregnancy. Exposure to angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), particularly in the second and third trimesters, can result in oligohydramnios, renal failure caused by renal tubular dysgenesis, limb deformities, prolonged hypotension, pulmonary hypoplasia, and hypocalvaria. Structural and functional alterations of the newborn kidney have also been described in infants with antenatal exposure to nonsteroidal anti-inflammatory drugs; selective COX-2 inhibitors; mycophenolate mofetil; certain antiepileptic medications; and chemotherapeutic agents, such as doxorubicin and cyclophosphamide. A review of the family medical history is important, including any prior fetal or neonatal deaths. Certain congenital abnormalities of the kidney and urinary tract (CAKUT) (renal hypoplasia/dysplasia, multicystic dysplastic kidney, and vesicoureteral reflux) may have familial clustering. In other disorders, including polycystic kidney disease and congenital nephrotic syndrome, a clear genetic basis has been established. The results of prenatal ultrasonography should be carefully reviewed, with particular attention devoted to kidney size, echogenicity, structural malformations, amniotic fluid volume, and bladder size and shape ( Box 93.1 ). Although the bladder may be identified and its volume discerned at 15 weeks of gestation, the kidneys are not visualized until after the 16th-17th week of gestation in most fetuses. The presence of small or enlarged kidneys, renal cysts, hydronephrosis, bladder enlargement, or oligohydramnios suggests significant renal or urologic pathology.
Dilated renal pelvis/hydronephrosis: physiologic, vesicoureteral reflux, obstruction
Cystic kidney: MCDK, cystic dysplasia, ADPKD, severe obstruction
Echogenic kidney: renal dysplasia, ARPKD
Structural abnormalities: renal duplication, fusion abnormalities (e.g., horseshoe, crossed fused ectopia), ectopic kidneys (pelvic, thoracic)
Lack of visualization: renal agenesis, hypoplasia, ectopic
Enlarged kidneys: ARPKD, obstruction, overgrowth syndromes (Simpson-Golabi-Behmel, Perlman, Beckwith-Wiedemann)
Renal mass
Hydroureter
Dilation
Ureterocele
With thickened wall: posterior urethral valves
Without thickened wall: megacystis-megaureter syndrome, neurogenic bladder, EBS
Bladder exstrophy, cloacal exstrophy
Lack of visualization: minimal or absent fetal urine production; obstructive uropathy
Urinary ascites (typically associated with thickened bladder wall and/or abnormal kidneys)
Most often due to nonrenal causes; occasionally caused by bilateral renal cystic disease, urinary tract obstruction, congenital nephrotic syndrome
Bilateral renal dysplasia, urinary tract obstruction, ARPKD
Rupture of membranes, postmaturity, subacute fetal distress
Renal tubular disorder with urinary concentrating defect (NDI, Bartter syndrome)
Multiple gestation, upper gastrointestinal tract obstruction, neurologic disorders, maternal diabetes, fetal hydrops
Congenital nephrotic syndrome
ADPKD, Autosomal dominant polycystic kidney disease; ARPKD, autosomal recessive polycystic kidney disease; EBS, Eagle Barrett syndrome; MCDK, multicystic dysplastic kidney; NDI, nephrogenic diabetes insipidus.
The evaluation of blood pressure and volume status is critical in the newborn with suspected renal disease. Hypertension may be present in infants with polycystic kidney disease, acute kidney injury (AKI), renovascular or aortic thrombosis, or obstructive uropathy. Hypotension may be present in infants with volume depletion, hemorrhage, or sepsis, any of which may lead to AKI. Edema may occur in infants with AKI, hydrops fetalis, or congenital nephrotic syndrome. Ascites may be present in infants with urinary tract obstruction, congenital nephrotic syndrome, or volume overload. Special attention should be paid to the abdominal examination. In the neonate, the lower pole of each kidney is easily palpable because of the neonate's reduced abdominal muscle tone. The presence of an abdominal mass in a newborn should be assumed to involve the urinary tract until proved otherwise, because two-thirds of neonatal abdominal masses are genitourinary in origin. The most common renal cause of an abdominal mass is hydronephrosis, followed by a multicystic dysplastic kidney. Less common renal causes of an abdominal mass include polycystic kidney disease, renal vein thrombosis, ectopic or fused kidneys, and renal tumors. The abdomen should be examined for the absence of or laxity in the abdominal muscles, which may suggest Eagle-Barrett (“prune-belly”) syndrome. Distention of the newborn bladder may suggest lower urinary tract obstruction or an occult spinal cord lesion.
A number of anomalies should alert the physician to the possibility of underlying renal defects, including abnormal external ears, aniridia, microcephaly, meningomyelocele, hemihypertrophy, persistent urachus, bladder or cloacal exstrophy, an abnormality of the external genitalia, cryptorchidism, imperforate anus, and limb deformities. Although screening renal ultrasonography of infants with a single umbilical artery had previously been recommended, in the era of routine prenatal ultrasonography, this practice is no longer recommended.
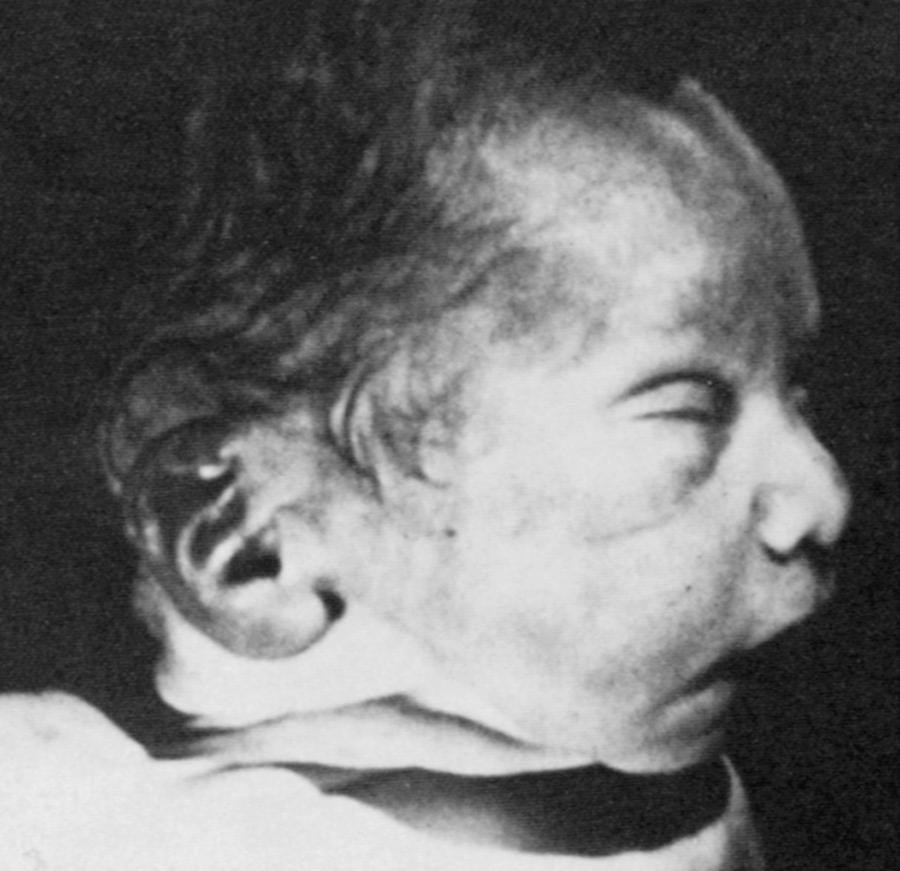
A constellation of physical findings designated the oligohydramnios (Potter) sequence may be seen in infants with severe neonatal kidney disease, including bilateral renal dysplasia, bilateral urinary tract obstruction, or autosomal recessive polycystic kidney disease. Marked reduction of fetal kidney function results in oligohydramnios or anhydramnios, which causes fetal deformation by compression of the uterine wall. The characteristic facial features include wide-set eyes; depressed nasal bridge; beaked nose; receding chin; and posteriorly rotated, low-set ears ( Fig. 93.3 ). Other associated anomalies include a small, compressed chest wall; arthrogryposis; hip dislocation; and clubfoot. Such patients often have respiratory failure caused by pulmonary hypoplasia; complications include spontaneous pneumothorax and/or pneumomediastinum resulting from their requirement for high ventilator pressures.
The examination of a freshly voided urine specimen provides immediate, valuable information about the condition of the kidneys. A urine specimen collected by cleaning the perineum and applying a sterile adhesive plastic bag is useful for obtaining urine for urinalysis but is not recommended for urine culture, as it may be associated with a false-positive result caused by fecal contamination. Bladder catheterization is a more reliable method for collecting a sample for culture but may be technically difficult in preterm infants. Suprapubic bladder aspiration is an alternative urinary collection method in preterm infants without intra-abdominal pathology or bleeding disorders.
Analysis of the urine should include inspection, urinary dipstick assessment, and microscopic analysis. The newborn urine is usually clear and nearly colorless. Cloudiness may represent a urinary tract infection or the presence of crystals. A yellow-brown to deep olive-green color may represent large amounts of conjugated bilirubin. Porphyrins, certain drugs such as phenytoin, bacteria, and urate crystals may stain the diaper pink and be confused with bleeding. Brown urine may suggest AKI, hemoglobinuria, or myoglobinuria.
The specific gravity of neonatal urine is usually very low (<1.004) but may be factitiously elevated by high-molecular-weight solutes such as contrast agents, glucose or other reducing substances, or large amounts of protein. Urinary osmolality may be a more reliable measurement of the concentrating and diluting capacity of the kidney. Urinary dipstick evaluation can detect the presence of heme-containing compounds (i.e., red blood cells, myoglobin, and hemoglobin), protein, and glucose. White blood cell products such as leukocyte esterase and nitrite may also be detected on urinary dipstick evaluation and should raise the suspicion of urinary tract infection, mandating collection of a urine culture. The microscopic examination of urinary sediment may detect the presence of red blood cells, white blood cells, bacteria, casts, or crystals.
Although 98% of term infants void during the first 30 hours of life, a delay in urination for up to 48 hours should not be a cause for immediate concern in the absence of a palpable bladder, abdominal mass, or other signs or symptoms of renal disease. A failure to void for longer than 48 hours may suggest impairment of renal function and should prompt further investigation. The serum creatinine level is the simplest and most commonly used indicator of neonatal kidney function. Immediately after birth, the serum creatinine concentration reflects the maternal creatinine concentration. In term infants, the serum creatinine level gradually decreases from a range of 0.6-1 mg/dL (depending on the mother's serum creatinine) at birth to a mean value of 0.4 mg/dL within the first 2 weeks of life ( Table 93.2 ). In preterm infants, the decline in serum creatinine level is slower and may not reach nadir for 1-2 months. In very preterm infants, the creatinine may actually rise transiently because of low GFR and tubular reabsorption of creatinine before falling to reach a nadir value at 2 months of age ( Table 93.3 ). Failure of the serum creatinine level to fall or a persistent increase in serum creatinine suggests impairment of renal function. Estimation of GFR is difficult in neonates, because formulas used in children have not been validated in infants less than 1 year of age. Measurement of serum cystatin C, a low molecular weight protein produced in all cells and filtered through the glomerulus, may offer more accurate estimation of neonatal kidney function but is not yet widely available for use.
| Age | 50th Percentile Value mg/dL (micromol/L) |
95th Percentile Value mg/dL (micromol/L) |
|---|---|---|
| 7 days | ||
| 25-27 weeks' gestation | 0.87 (76.9) | 1.23 (108.7) |
| 28-29 weeks' gestation | 0.84 (74.3) | 1.18 (103.9) |
| 30-33 weeks' gestation | 0.66 (58.3) | 0.95 (83.9) |
| 10-14 days | ||
| 25-27 weeks' gestation | 0.75 (66.3) | 1.10 (97.3) |
| 28-29 weeks' gestation | 0.69 (61) | 1.02 (90.3) |
| 30-33 weeks' gestation | 0.57 (50.4) | 0.84 (84.1) |
| 1 month | ||
| 25-27 weeks' gestation | 0.48 (42.4) | 0.72 (63.6) |
| 28-29 weeks' gestation | 0.41 (36.2) | 0.64 (57) |
| 30-33 weeks' gestation | 0.35 (30.9) | 0.57 (50.2) |
| 2 months | ||
| 25-27 weeks' gestation | 0.31 (27.4) | 0.51 (44.9) |
| 28-29 weeks' gestation | 0.33 (28.8) | 0.58 (51.6) |
| 30-33 weeks' gestation | 0.25 (22.2) | Data not available |
Ultrasonography is the most common method of imaging the neonatal urinary tract, as it is a readily available, noninvasive test that does not involve exposure to contrast agents or radiation. Ultrasonography is indicated in infants with a history of an abnormal antenatal ultrasound, as well as in infants with an abdominal mass, AKI, hypertension, hematuria, or congenital malformations or genetic syndromes with increased risk of urinary tract anomalies. Ultrasonography can identify hydronephrosis, cystic kidney disease, and abnormalities of kidney size and position. It also may be used as a screening tool in preterm infants at increased risk of developing nephrocalcinosis due to chronic lung disease and long-term loop diuretic therapy. A Doppler flow study of the renal arteries and aorta may be helpful in the evaluation of infants with suspected renovascular hypertension, renal arterial or venous thrombosis, or AKI.
Importantly, nonurgent postnatal ultrasounds, such as those performed to follow up mild to moderate antenatal hydronephrosis, should be delayed until at least 48 hours after birth. Ultrasounds performed in the first 24-48 hours of life may not show hydronephrosis because of the low urine output of the neonate in the first few days of life.
Voiding cystourethrography (VCUG) should be considered an important part of the radiologic examination in infants with significant hydronephrosis, hydroureter, or documented urinary tract infection. Voiding cystourethrography involves the instillation of a radiopaque contrast agent into the bladder by urinary catheterization. This study is the procedure of choice to evaluate the urethra and bladder and ascertain the presence or absence of vesicoureteral reflux and posterior urethral valves. Other radiologic tests may occasionally be used for diagnostic purposes in the neonate (see Chapter 38 ). Radioisotopic renal scanning may be of value in locating anomalous kidneys and identifying obstruction or renal scarring. Radioisotopic scans also provide information about the contribution of each kidney to overall renal function but may be difficult to interpret in the first few weeks of life because of the relatively low GFR in newborns. Abdominal computed tomography is useful in the diagnosis of renal tumors, renal abscesses, and nephrolithiasis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here