Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Advances in neonatology, perinatology, and molecular genetics have defined new disease processes and continue to raise exciting questions in the field of nephrology. Prenatal maternal-fetal care and ultrasound imaging continue to improve the diagnosis of many urinary tract anomalies, which allows for improved prenatal and perinatal care. The use of invasive vascular catheters introduced new complications, including renal artery and aortic thrombosis. The administration of loop diuretics and steroids to infants with bronchopulmonary dysplasia (BPD) has significantly increased rates of neonatal hypertension and nephrocalcinosis (NC). Simultaneously, genetic similarities among children whose conditions were formerly considered phenotypically distinct are redefining congenital anomalies of the kidneys and urinary tract (CAKUT).
This chapter reviews the anatomic and physiologic development of the kidney, outlines the recommended approach to evaluation of the neonate with suspected kidney disease, and comments on the more common nephrologic and urologic problems seen in preterm and term neonates.
Mammalian kidney development begins at 5 weeks’ gestation, and the fetal kidney begins to produce urine by 10 weeks. This developmental process continues until approximately 36 weeks’ gestation and stops at birth, even in preterm babies born before 36 weeks. Formation of the metanephric kidney is complex and requires coordinated development from three separate embryological processes: branching morphogenesis, epithelial-mesenchymal transformation, and angiogenesis. Disruptions in normal kidney development can lead to CAKUT, which accounts for 40% to 50% of chronic kidney disease (CKD) worldwide and 36% of end-stage renal disease (ESRD) in children.
Formation of the renal collecting system begins as an outgrowth of the mesonephric duct called the ureteric bud. The ureteric bud elongates and interacts with the surrounding metanephric mesenchyme through a coordinated process that requires several well-characterized signaling interactions. The ureteric bud then undergoes several rounds of branching, giving rise to the renal pelvis, calyces, and collecting ducts. The length of the ureteric bud proximal to the first branch matures to become the ureter. Abnormal development of the renal collecting system can result in defects such as renal agenesis, dysplasia, and urinary obstruction ( Table 15.1 ).
| Developmental Process | Associated CAKUT Phenotype | Associated Genes |
|---|---|---|
| Ureteric bud initiation and elongation | Renal agenesis Renal dysplasia Vesicoureteral reflux |
RET BMP4 PAX2 GATA3 SIX1 SIX2 EYA1 SALL1 ROBO2 |
| Branching morphogenesis | Renal dysplasia | AGT AGTR |
| Epithelial-mesenchymal transformation | Renal agenesis Renal dysplasia Renal hypoplasia |
FGF20 WNT4 |
| Genetic patterning of nephron | Cystic kidney disease | UMOD |
| Unassigned but known to cause CAKUT in humans | Renal agenesis Renal dysplasia Renal hypoplasia Cystic dysplasia Vesicoureteral reflux UPJ obstruction Horseshoe kidney VACTERL association |
REN HNF1B CHD1L DSTYK KAL1 SIX5 SOX17 TNXB TRAP1 UPK3A MUC1 |
The nephrons of the definitive kidney arise from a separate process of epithelial-mesenchymal transformation. The developing ureteric bud invades and undergoes reciprocal signaling with the surrounding metanephric mesenchyme, inducing mesenchymal cells to condense into epithelial vesicles. These vesicles then use genetic signals to pattern themselves into an elongated S-shaped vesicle with proximal, middle, and distal portions that will mature into the definitive nephron segments. Finally, the renal vesicles fuse with the ureteric bud, becoming the junction of the distal tubule and collecting duct of the mature nephron ( Fig. 15.1 ). Failure of this complex process of nephrogenesis can result a wide variety of congenital anomalies, including renal agenesis, hypoplasia, dysplasia, or cyst development ( Table 15.1 ).
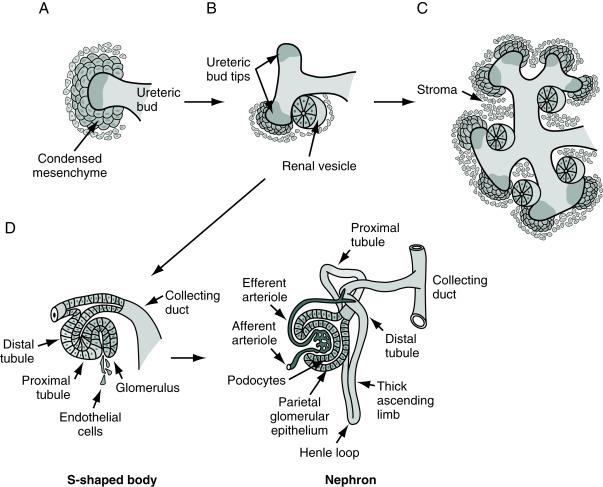
The glomerulus also arises from the metanephric mesenchymal cells and forms by the same mechanisms as other capillary beds. This process involves both vasculogenesis (creating new blood vessels) and angiogenesis (sprouting from existing vessels). The development of glomerular cells is intimately linked to the developing nephron segment that will become the podocytes of Bowman’s capsule and requires regulation by several molecular signals. Interestingly, glomeruli appear to retain some developmental potential after preterm birth, offering an exciting potential target for regenerative science and possible future therapies for preterm infants with prematurely arrested renal development.
During fetal development, the kidneys play a minor role in regulating salt and water balance, as this function is performed by the placenta. The fetal kidneys primarily function to produce large amounts of urine to provide adequate amniotic fluid. With the loss of placental function at birth, the kidneys quickly adapt to the extrauterine environment and assume the role of regulating water and mineral balance. Rapid maturation of renal blood flow, glomerular filtration, and tubular handling of solutes occurs in the first 2 to 3 postnatal weeks. This physiological maturation then continues slowly for the first 2 years of life, after which these functions reach adult levels.
The mature kidney receives a large percentage of cardiac output relative to its small size, which is necessary to continually regulate the extracellular fluid (ECF) composition. At birth, renal blood flow represents 4% to 6% of the cardiac output, and this value increases quickly during the first postnatal week, reaching the adult value of 20% to 25% by the end of the first year ( Table 15.2 ). Renal blood flow is initially low because of high renal vascular resistance caused by elevated levels of circulating vasoconstrictor hormones such as renin, angiotensin, aldosterone, endothelin, and catecholamines that counteract fetal prostaglandins. Renal blood flow increases as concentrations of these hormones decrease and systemic mean arterial pressure and cardiac output increase, more than doubling renal blood flow during the first postnatal week. The increase in renal blood flow is attenuated and delayed in preterm infants.
| Age | Glomerular Filtration Rate (mL/min/1.73 m 2 ) | Renal Blood Flow (mL/min/1.73 m 2 ) | Maximal Urine Osmolality (mOsm/kg) | Serum Creatinine (mg/dL) | Fractional Excretion of Sodium (%) |
|---|---|---|---|---|---|
| Newborn | |||||
| 32–34 wk gestation | 14 ± 3 | 40 ± 6 | 480 | 1.3 | 2–5 |
| Full Term | 21 ± 4 | 88 ± 4 | 800 | 1.1 | <1 |
| 1–2 wk | 50 ± 10 | 220 ± 40 | 900 | 0.4 | <1 |
| 6 mo–1 yr | 77 ± 14 | 352 ± 73 | 1200 | 0.2 | <1 |
| 1–3 yr | 96 ± 22 | 540 ± 118 | 1400 | 0.4 | <1 |
| Adult | 118 ± 18 | 620 ± 92 | 1400 | 0.8-1.5 | <1 |
In addition to the increase in overall renal blood flow, there is a marked change in distribution of blood flow within the neonatal kidney in the postnatal period. Because of a preferential decrease in vascular resistance in the outer cortex, there is a pronounced increase in blood flow to the outer cortex, which is the site of most glomerular filtration.
Glomerular filtration rate (GFR) in the fetal kidney is significantly lower than adult values and increases with gestational age. By 34 weeks’ gestation, a GFR of approximately 14 mL/min/1.73 m 2 is achieved, and the rate further increases to 20 to 30 mL/min/1.73 m 2 at term ( Table 15.2 ). In preterm infants, the GFR is even lower, especially in very low-birth-weight infants and infants with NC. The GFR continues to increase postnatally, achieving adult values of 120 mL/min/1.73 m 2 by 2 years of age.
Several factors account for the marked increase in GFR in the first postnatal weeks. The primary determinant of GFR is renal blood flow, so the GFR increases in parallel. Second, higher mean arterial pressure increases glomerular hydrostatic pressure, which increases GFR. The subsequent rise in GFR during the first 2 years results from continued increases in renal blood flow and glomerular pressure as well as maturation of superficial cortical nephrons, which expands the glomerular filtration surface area.
During the first week of postnatal life, an infant’s GFR passes through three distinct phases to maintain fluid and electrolyte homeostasis. The prediuretic phase, which occurs in the first 24 hours of life, is characterized by a transient increase in GFR followed by a return to low baseline GFR and minimal urine output regardless of salt and water intake. This phase may extend up to 36 hours of life in the preterm infant. The diuretic phase follows, in which the GFR increases rapidly and the infant experiences diuresis and natriuresis regardless of salt and water intake. The postdiuretic phase typically begins around day 4 to 5 of life, at which time the GFR begins to increase slowly with maturation, with salt and water excretion varying according to intake.
Importantly, the duration and timing of these phases differ among infants, so that individualization of fluid and electrolyte therapy is required. If insensible fluid losses are overestimated during the prediuretic phase, excess fluid intake may result in dilutional hyponatremia. On the other hand, a deficiency in fluid intake during the diuretic phase may lead to dehydration and hypernatremia.
The change in distribution of intracellular fluid (ICF) and extracellular fluid (ECF) in the fetus and newborn infant is summarized in Table 15.3 . In the healthy term infant, ECF volume decreases and ICF volume increases in the first few days of life, with a net loss of total body water that manifests clinically as weight loss after birth. This diuresis and ECF fluid loss is often delayed in neonates with respiratory distress syndrome (RDS). The change in ICF during the first week of life is variable and dependent on total energy intake. In preterm infants, the ICF volume increase can be attenuated so that more than a 10% loss in body weight during the first week of life may represent only ECF contraction without an increase in ICF.
| Percent Body Weight | |||
|---|---|---|---|
| Age | Extracellular Fluid | Intracellular Fluid | Total Body Fluid |
| Gestational | |||
| 14 wk | 65 | 27 | 92 |
| 28 wk | 55 | 25 | 80 |
| 40 wk | 45 | 30 | 75 |
| Postnatal | |||
| 14 wk | 25 | 40 | 65 |
Water movement between the vascular and interstitial fluid compartments is also higher in the neonatal period than it is later in life, which leads to a relatively large interstitial fluid compartment. This phenomenon may be attributed to a number of factors, including increased hydrostatic pressure, decreased intravascular osmotic pressure, and increased levels of atrial natriuretic peptide (ANP), vasopressin, and cortisol. The relatively large interstitial fluid compartment can partially replace lost vascular volume and enables the neonate to better tolerate hemorrhage or physiological diuresis, but it also reduces the ability to excrete a free water load.
Serum sodium concentration is maintained in a tight physiological range that does not differ between neonates and adults, and renal excretion is the only active mechanism of regulation. Although neonates retain approximately 1 mEq/kg/day of sodium because of somatic growth of new tissues, in general the renal excretion of sodium adjusts to account for a range in daily intake. Healthy term neonates have basal sodium handling with a fractional excretion of sodium (FE Na ) around 1%; this can be slightly higher in formula-fed babies than in those fed on human milk because of the difference in the sodium content of these solutions. Urinary sodium losses may be increased in certain conditions, including renal dysplasia, hypoxia, respiratory distress, hyperbilirubinemia, acute tubular necrosis (ATN), polycythemia, and the use of theophylline or diuretics. Pharmacologic agents, such as dopamine, labetalol, propranolol, captopril, and enalaprilat, that influence adrenergic neural pathways in the kidney and the renin-angiotensin axis may also increase urinary sodium losses in the neonate.
Renal sodium losses are inversely proportional to gestational age, and the FE Na may exceed 3% in infants born before 28 weeks’ gestation ( Fig. 15.2 ). As a result, preterm infants younger than 35 weeks’ gestation may display early negative sodium balance and hyponatremia because of high renal sodium losses and inefficient intestinal absorption. Sodium deficiency in early postnatal life leads to early and sustained growth retardation, and intake of 4 to 5 mEq/kg/day may be necessary in preterm infants to offset these renal losses during the first weeks of life.
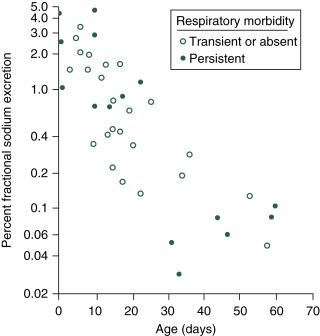
The mechanisms responsible for increased urinary sodium losses in the preterm infant are multifactorial. Compared with adults, neonates have decreased sensitivity to angiotensin and aldosterone, and their kidneys have increased sensitivity to ANP, all of which promote sodium excretion. Inappropriate sodium wasting occurs during glomerulotubular imbalance, the condition where the GFR exceeds the reabsorptive capacity of the renal tubules. This imbalance occurs in preterm neonates because of tubular immaturity, large extracellular volume, and reduced oxygen availability necessary for active transport.
Control of plasma osmolarity is necessary to maintain cellular volume and is especially critical for brain function and homeostasis. The regulation of serum osmolarity relies on the kidney to respond to antidiuretic hormone (ADH) and regulate free water excretion through the concentration or dilution of urine. As noted in Table 15.2 , the maximum renal concentrating ability is low at birth and progressively increases following delivery from about 600 milliosmoles (mOsm)/kg H 2 O in the first 2 weeks of life to adult values of 1200 mOsm/kg H 2 O by 2 years of age. This improvement in the ability to concentrate urine is primarily due to the maturation of the renal medulla, which contains the loop of Henle and collecting ducts. With age, these structures lengthen, which allows for increased medullary interstitial sodium and urea concentration. Also, the sensitivity of the collecting duct to ADH improves, allowing water movement along the medullary osmolarity gradient.
Similarly, the ability of the neonatal kidney to excrete excess free water by diluting the urine is limited compared with the adult kidney. Term and preterm newborns can dilute their urine to an osmolality of 70 mOsm/kg and 100 mOsm/kg, respectively, whereas adults can dilute their urine to 50 mOsm/kg. This inability to maximally dilute the urine is as a result of the reduced GFR and immaturity of transporters in the early distal tubule (diluting segment) and can predispose neonates to hyponatremia.
Regulation of the extracellular pH is tightly controlled by both the lungs and kidneys as well as extracellular buffers. The role of the kidney is primarily to regulate the excretion of buffers, especially bicarbonate and titratable acids such as ammonia. The range of neonatal serum bicarbonate levels is lower than that of adults, and healthy infants maintain a mild metabolic acidosis ( Fig. 15.3 ).
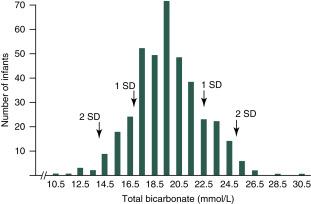
The limitation in acid–base homeostasis seen in neonates, particularly preterm infants, is related to immaturity of both proximal and distal tubular function. The proximal tubular bicarbonate transport threshold is much lower in neonates than in adults, leading to incomplete bicarbonate reabsorption. Proximal tubular bicarbonate wasting is more pronounced in preterm infants, resulting in an expected transient renal tubular acidosis. Limited distal tubular excretion of titratable acid and incomplete development of tubular ammonia production also contribute to the relative metabolic acidosis of the newborn. Treating neonates acutely with intravenous bicarbonate for metabolic acidosis provides little benefit because of renal bicarbonate wasting, adds risks of intraventricular hemorrhage and cardiac deterioration, and can worsen intracellular acidosis.
Historically, neonates with metabolic acidosis were routinely given sodium bicarbonate despite the lack of evidence to support the practice. As noted in a seminal commentary by Drs. Aschner and Poland, cleverly titled “Sodium Bicarbonate: Basically Useless Therapy,” the detrimental effects of the therapy often outweigh the benefits, and instead the focus should be on “understanding and treating the underlying cause of the acidosis” ( Pediatrics 2008;122:831).
In the first 24 hours of life, an early combined respiratory and metabolic acidosis may develop as a result of stress during birth and disturbances in cardiopulmonary adaptation. Late metabolic acidosis, on the other hand, develops after the first week of life because of net excess of acid intake compared with the capacity for acid excretion. Late acidosis is more pronounced in preterm neonates and those receiving parenteral nutrition but usually resolves by the end of the first month of life with renal maturity; if persistent, late acidosis can result in poor weight gain or skeletal growth.
An important consequence of chronic metabolic acidosis in the newborn is enhanced urinary calcium loss and negative calcium balance, which may contribute to the bone demineralization and osteopenia of prematurity. The mechanism for this process is multifactorial. Acidosis causes release of calcium from bones, increases parathyroid hormone secretion, inhibits intestinal calcium absorption, impairs activation of vitamin D, and increases urinary calcium excretion. Therefore chronic metabolic acidosis should usually be corrected with enteral supplementation with a goal of achieving a serum bicarbonate level of greater than 18 mEq/L.
Within 24 to 48 hours after birth, the serum calcium concentration decreases, a phenomenon that is most pronounced in preterm infants. Although the mechanism of neonatal hypocalcemia is not completely known, it appears to be as a result of suppressed parathyroid hormone secretion and elevated serum phosphate concentration. In most neonates, the ionized calcium level remains above a physiologically acceptable concentration, and the infant experiences no clinical symptoms. Symptomatic hypocalcemia may occur, however, in neonates stressed by illness or in the presence of aggressive fluid administration, diuresis, and sodium supplementation, all of which increase urinary calcium losses.
The normal serum phosphorus level in the newborn ranges from 4.5 to 9.5 mg/dL, which is significantly higher than that of adults and is necessary for positive phosphorus balance needed for bone and cellular growth. Factors leading to the higher neonatal serum phosphorus level include the generous phosphorus content of cow’s milk formulas, low GFR, and higher tubular reabsorption of phosphorus. Phosphorus reabsorption is lower, however, in preterm infants, which can lead to relative phosphate wasting and can contribute to inadequate bone mineralization and osteopenia. Therefore supplementation of phosphorus and calcium is often needed in preterm infants to promote adequate bone development.
The evaluation of a newborn with suspected kidney disease must be comprehensive and begins with a careful history and physical examination. Selected laboratory studies may be useful in determining the cause and severity of renal dysfunction. Focused radiologic evaluation may be useful in clarifying renal anatomy or function and detecting complications of vascular catheters.
When neonatal kidney disease is suspected, the medical history should focus on identifying prenatal risk factors that aid diagnosis as well as pre- and postnatal factors that influence therapy and prognosis. The cause of congenital kidney disease cannot always be determined, but genetically inherited conditions and nephrotoxic teratogens are becoming increasingly identified.
Review of the family medical history should include information on any prior fetal or neonatal deaths, or family history of kidney disease, congenital renal malformations, or recurrent urinary tract infection (UTI) that may signal urinary reflux or obstruction. When syndromes involving multiple organ systems are being considered, additional information about familial hearing loss, vision loss, cleft lip/palate, limb or vertebral skeletal malformations, congenital heart defects, or renal malignancy such as Wilms tumor should be elicited. It is increasingly recognized that most forms of CAKUT have a genetic etiology, even though affected family members may have significantly different pathology from the same genetic variant (e.g., mother with vesicoureteral reflux [VUR] has a child with unilateral renal agenesis). Thus, information about any structural, cystic, or congenital kidney disease is significant.
The maternal antenatal history should be reviewed, with particular attention given to medications, toxins, or unusual exposures during the pregnancy. Many prescribed medications have known teratogenic effects and are commonly prescribed to women of childbearing age. Treatment for conditions such as hypertension, acne, seizures, malignancy, autoimmune disease, and many psychiatric diseases commonly use such medications, and presence of these conditions should prompt a detailed history of current and past medication usage.
Results of prenatal ultrasonography should be reviewed with attention to kidney size, echogenicity, malformations, amniotic fluid volume, and bladder size and shape. The presence of small or enlarged kidneys, renal cysts, hydronephrosis, bladder enlargement, or oligohydramnios may suggest significant renal or urologic abnormalities. Also, developmental anomalies of other structures, such as skeletal or cardiac malformations, should raise suspicion for syndromes that may include renal malformations.
Evaluation of blood pressure and volume status is critical in the newborn with suspected kidney disease. Hypertension is often present in neonates with autosomal recessive polycystic kidney disease (PKD), acute kidney injury (AKI), or renal thromboembolism. Hypotension, on the other hand, may suggest volume depletion, hemorrhage, or sepsis, which may lead to renal ischemia and AKI. Edema or anasarca may be seen in oliguric AKI, hydrops fetalis, or congenital nephrotic syndrome. Isolated ascites may be seen with urinary tract obstruction with rupture of the bladder or renal pelvis, so-called “urinary ascites.”
The lower pole of both kidneys should be palpable on abdominal examination because of the reduced abdominal muscle tone of the neonate. The majority of neonatal abdominal masses are genitourinary in origin, so an abdominal mass in a newborn should be assumed to involve the urinary tract until proven otherwise. The most common renal cause of an abdominal mass is hydronephrosis followed by multicystic dysplastic kidney (MCDK). Less common causes include PKD, renal vein thrombosis, ectopic or fused kidneys, renal hematoma or abscess, and renal tumors. The newborn bladder is often palpable just above the pubic symphysis, but a lower urinary tract obstruction should be suspected when the bladder is enlarged. Absence or laxity of the abdominal muscles may suggest Eagle-Barrett (prune belly) syndrome.
A number of associated anomalies should raise suspicion for underlying renal defects. Because the genital and urinary systems are derived from a common developmental precursor, any anomaly of genital structures should elicit evaluation of the kidneys and bladder. Other features associated with disrupted development of the urinary system include small or malpositioned ears, aniridia or coloboma, microcephaly, spinal cord dysraphism and neural tube defects, pectus excavatum, hemihypertrophy, limb deformities, imperforate anus, and congenital heart disease. There appears to be an association between presence of a single umbilical artery and underlying renal anomalies. Historically, these newborns received a thorough evaluation with labs and imaging studies, but that approach has fallen out of favor with improvements in prenatal ultrasonography, and newer data showing only 2% of neonates with an isolated single umbilical artery have clinically significant renal anomalies.
A constellation of physical findings called Potter syndrome can be seen in neonates with fetal deformation by uterine wall compression. Characteristic facial features include wide-set eyes, depressed nasal bridge, beaked nose, receding chin, and posteriorly rotated, low-set ears. Although it was originally described in babies with bilateral renal agenesis, this syndrome can occur in any infant with a history of severe oligohydramnios, most commonly resulting from obstructive uropathy and cystic kidney diseases. This condition has a high mortality from associated pulmonary hypoplasia, and surviving infants often have significant morbidity because of the underlying renal disease and associated anomalies such as a compressed chest wall and arthrogryposis.
Some 98% of full-term infants void in the first 30 hours of life, with as many as 25% doing so in the delivery room. A delay in urination can be normal and should not cause immediate concern in the absence of an enlarged bladder, abdominal mass, or other indications of renal disease. Failure to urinate in the first 48 hours should prompt further investigation for structural or obstructive urinary tract anomalies and may necessitate placement of a urinary catheter.
Evaluation of the urine is a vital part of the examination of any neonate suspected of having a urinary tract abnormality. Collection of an adequate, uncontaminated specimen is difficult in the neonate. A specimen collected by cleaning the perineum and applying a sterile adhesive plastic bag enables analysis of urinary blood, protein, or electrolytes. For cultures, bladder catheterization produces a reliable specimen but may be technically difficult in preterm infants. Suprapubic bladder aspiration has been considered the collection method of choice in infants without intraabdominal abnormalities or bleeding disorders, although few clinicians choose this approach. A noninvasive clean-catch technique relying on external bladder stimulation to generate a urine stream is gaining popularity and appears to have contamination rates similar to urethral catheterization.
Point-of-care ultrasound (POC US) is increasingly available in the neonatal intensive care unit (NICU) for use by the neonatal team. Some studies, such as cardiac ultrasound, require significant training and experience to obtain proficiency. A relatively simple study is the use of POC US to evaluate the bladder before a catheter or suprapubic tap procedure to evaluate urine volume.
Analysis of the urine should include inspection of color and clarity, dipstick testing, and microscopic analysis. The urine of newborns is usually clear and nearly colorless. Cloudiness may be caused by either UTI or the presence of crystals. A dark yellow-orange color may be as a result of conjugated bilirubin but occasionally indicates simply concentrated urine in newborns. Urate crystals and certain drugs may stain the urine pink and be confused with bleeding. Brown urine suggests glomerular hematuria, hemoglobinuria from hemolysis, or myoglobinuria from muscle breakdown.
Urine dipstick evaluation can detect the presence of heme-containing compounds (red blood cells, myoglobin, and hemoglobin), protein, and glucose. The presence of leukocyte esterase and nitrites should raise suspicion of UTI, and a culture should be obtained for confirmation. Microscopic urinalysis should be performed if the urinary dipstick result is abnormal to assess for the presence of red blood cells, casts, white blood cells, bacteria, and crystals.
Clinical evaluation of neonatal renal function begins with measurement of serum creatinine. Normal values for serum creatinine vary with gestational and postnatal age because of differences in GFR discussed previously ( Table 15.2 ). The serum creatinine level is relatively high at birth and reflects maternal levels because of placental transfer. Creatinine decreases after birth as renal blood flow and GFR increase, reaching a nadir that reflects true neonatal GFR after 2 to 3 weeks, but this decline can be prolonged after preterm birth. In general, serum creatinine inversely correlates with GFR so that a doubling of the creatinine represents a 50% reduction in GFR. Although directly measuring the GFR is possible using radioisotopes or ionic contrast agents, these methods are generally difficult to accomplish and aid little in the clinical care of neonates. The “bedside” Schwartz formula uses serum creatinine and body length and often suffices for the care of preterm and term infants despite validation only in children older than 12 months. This equation estimates GFR standardized to body surface area:
Renal ultrasonography is the initial imaging modality of choice in infants with suspected renal disease. It offers a noninvasive anatomic evaluation of the urinary tract without the use of contrast agents or radiation exposure. Ultrasound provides useful information about the size and morphology of the kidneys and bladder and can detect the presence of hydronephrosis, cysts, stones, NC, masses, and abscesses. Doppler evaluation with ultrasound can illuminate renal parenchymal blood flow defects in cases of pyelonephritis and infarction. Doppler investigation of the renal vessels and aorta is useful in cases of neonatal hypertension where thromboembolism or vascular stenosis is suspected.
Voiding cystourethrography (VCUG) is the procedure of choice to evaluate the urethra and bladder and to detect VUR. The most common indications for this procedure are the evaluation of hydronephrosis or investigation following a UTI. VCUG is the gold-standard study for evaluation of suspected bladder outlet obstruction such as posterior urethral valves. This study involves urinary catheterization and fluoroscopy and thus confers a small infectious risk and radiation exposure. VCUG should be performed in all neonates with suspected urinary tract obstruction, those with severe hydronephrosis, and following UTI.
Other radiologic tests may occasionally be used for diagnostic purposes in the neonate. The radiolabeled isotope mercaptoacetylglycine (MAG-3) is cleared by the kidney, and imaging its rate of excretion after giving a diuretic is the preferred method to diagnose urinary tract obstruction. This test may be useful in an infant with clinically significant hydronephrosis with a normal VCUG. Dimercaptosuccinic acid is a radionuclide that is taken up by the kidney rather than excreted, making it useful to identify renal scarring or pyelonephritis. Computed tomography (CT) may be helpful in evaluating suspected renal abscess, mass, nephrolithiasis, or any condition where detailed anatomic information is needed.
Hematuria
At 60 days of age, a former 900-g 27-week infant with a history of BPD passes reddish-brown urine. Urinalysis shows 3+ blood with more than 250 red blood cells/mm 3 , no protein, positive leukocyte esterase, and nitrite. Urine culture grows greater than 50,000 Escherichia coli , and renal ultrasonography is normal with no NC or hydronephrosis. The infant is treated with IV antibiotics with resolution of macroscopic hematuria.
Hematuria can be classified into two categories: microscopic and macroscopic. Microscopic hematuria is defined as greater than or equal to five red blood cells on high-powered examination without visible urine discoloration. Macroscopic hematuria is defined as visibly discolored (red or brown) urine with greater than or equal to five red blood cells on high-powered examination. When a neonate is suspected of having macroscopic hematuria, it is important to rule out conditions mimicking hematuria. Urate crystals, bile pigments, and porphyrins may discolor the urine, but in these conditions the urinary dipstick test is negative for blood, and the microscopic examination reveals no red blood cells. Infants with myoglobinuria because of inherited metabolic myopathy, infectious myositis, or rhabdomyolysis may have red or brown urine and test dipstick positive for blood, but the microscopic examination of the urine reveals no red blood cells. Similarly, infants with hemoglobinuria because of hemolytic disease such as ABO incompatibility will have a positive urinary dipstick for blood but no red blood cells on microscopic examination. Vaginal bleeding or skin breakdown from diaper dermatitis can mimic gross hematuria.
Once it is determined that an infant has hematuria, there is a broad differential diagnosis. UTI should be suspected in infants with fever or other signs of instability. ATN or cortical necrosis should be considered in infants with a history of perinatal asphyxia. Renal vein thrombosis should be considered in infants with risk factors such as birth to a diabetic mother, cyanotic congenital heart disease, volume depletion, or an indwelling umbilical venous catheter. Coagulopathy related to hemorrhagic disease of the newborn should be considered in at-risk infants who have not received vitamin K prophylaxis. Urolithiasis or NC should be suspected in infants with a history of chronic lung disease and loop diuretic use. Other causes of hematuria include trauma from bladder catheterization or suprapubic aspiration, obstructive uropathy, and tumors. Glomerulonephritis, a common cause of hematuria in older children and adolescents, is an unlikely cause of hematuria in the neonate.
Evaluation of the neonate with hematuria includes formal urinalysis with microscopic examination, catheterized urine culture, complete blood count (CBC), serum electrolytes, creatinine, and kidney/bladder ultrasound. Further evaluation may include coagulation profile, urine calcium-to-creatinine ratio, abdominal CT scan, or urologic evaluation.
A 1900-g male infant is delivered at 34 weeks’ gestation to consanguineous parents. An enlarged placenta is noted at the time of delivery. At 5 days of age, the infant develops periorbital and peripheral edema as well as abdominal distension. Urinalysis shows 4+ protein and no blood, and serum albumin falls to 1.5 gm/dL. Serum creatinine is 0.4 mg/dL, and blood pressure is normal.
Proteinuria is defined as a urinary dipstick value of at least 1+ (30 mg/dL), with a specific gravity of 1.015 or less, or a urinary dipstick value of at least 2+ (100 mg/ dL), with a specific gravity of more than 1.015. Normal urinary protein-to-creatinine ratio is less than 0.5 mg/mg in infants and toddlers younger than 2 years of age. Nearly any form of renal injury can result in a transient increase in urinary protein excretion, including ATN, fever, dehydration, cardiac failure, high doses of penicillin, and recent administration of a contrast agent. Persistent heavy (4+) proteinuria, edema, and hypoalbuminemia in a neonate should prompt the consideration of congenital nephrotic syndrome, an autosomal recessive disorder characterized by massive proteinuria, failure to thrive, a large placenta, and CKD. Proteinuria associated with glucosuria, hypokalemia, hypophosphatemia, and metabolic acidosis raises concern for Fanconi syndrome, a rare condition characterized by generalized proximal tubular dysfunction; possible causes include cystinosis, Lowe syndrome, galactosemia, and mitochondrial disorders. False-positive urinary dipstick values for protein may be the result of highly concentrated urine, alkaline urine, infection, and detergents.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here