Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Flow cytometry is a powerful, rapid, and cost-effective technique for the identification and monitoring of hematopoietic neoplasms.
Successful implementation of flow cytometry requires careful attention to details of instrument and reagent performance.
Normal hematopoietic cells are characterized by a reproducible gain and loss of antigen expression with maturation.
Hematopoietic neoplasms show deviation from the normal patterns of antigen expression, allowing for their diagnosis and classification.
The flow cytometric detection of residual disease following therapy allows for the monitoring of therapeutic response.
The diagnosis, classification, and post-therapeutic monitoring of hematopoietic neoplasms have greatly benefited from the widespread application of immunophenotypic studies over the past 2 decades. The subdivision of hematopoietic neoplasms by their correspondence to normal hematopoietic lineages and stages of differentiation is a basic tenet of current classification systems (e.g., the World Health Organization [WHO] classification) ( ). This information is largely provided by immunophenotyping and has resulted in the incorporation of immunophenotypic data into the definition of many hematopoietic neoplasms to the extent that certain diagnoses cannot be confidently made without immunophenotypic studies.
Flow cytometry is a rapid, convenient, and widely available technique for generating immunophenotypic data. The ability to perform multiparametric analysis on an individual cellular basis is a unique feature of the technique that offers distinct advantages over competing immunophenotypic methods, such as immunohistochemistry. Guidelines on the use of flow cytometry for the immunophenotyping of leukemic cells in the clinical laboratory have been published ( ), and National Institutes of Health–sponsored consensus conferences on the flow cytometric analysis of leukemia and lymphoma were convened in 1995 ( ) and 2006 (Davis et al., 2007; ) to attempt standardization of clinical practice. Despite these efforts, considerable heterogeneity exists in the clinical application of this technique to the evaluation of leukemia and lymphoma. This lack of standardization represents a significant challenge to the ability to consistently provide high-quality results. Efforts to improve standardization in flow cytometry in a variety of disease entities and clinical settings are ongoing and have resulted in publication of several consensus guidelines ( ; ). Such efforts attempt to improve the quality of flow cytometric data generation and interpretation.
Medical indications for performing flow cytometry in the setting of hematopoietic neoplasms can be divided into four primary categories:
The diagnosis and classification of hematopoietic neoplasms. The ability to accurately identify abnormal subpopulations of cells, assign lineage, and determine maturational stage adds improved reproducibility to the diagnosis of hematopoietic neoplasms, particularly for chronic lymphoproliferative disorders and acute leukemias. Flow cytometry is widely used for these purposes.
Detection of antigens used as therapeutic targets. A variety of immunologic reagents are now available for therapeutic use that specifically target antigens expressed by hematopoietic neoplasms (e.g., CD19, CD20, CD22, CD25, CD33, CD52). Confirmation of expression of the antigen of interest by the tumor is often a necessary prerequisite for use of these expensive therapies.
Detection of residual neoplastic cells following therapy. The ability of flow cytometry to routinely identify and quantitate the presence of abnormal hematopoietic cells with a sensitivity of 0.01% or better is increasingly being used as a surrogate measure of response to therapy and impending relapse in a variety of hematopoietic neoplasms ( ; ; ; ; ; ; ; ; ).
Assessment of specific antigenic phenotypes associated with prognosis. This is perhaps the most controversial of the listed indications and is theoretical in many disease entities. However, the expression of specific molecules on the neoplastic population has been associated with clinical outcome in some settings. As examples, CD56 in cases of acute promyelocytic leukemia ( ), CD20 in adult acute lymphoblastic leukemia (ALL) ( ; ), and CD49d in chronic lymphocytic leukemia (CLL) ( ) have all been reported to be adverse prognostic factors. As will be discussed later, in some settings the prognostic impact conferred by flow cytometry can be accounted for by genetic signature. Thus, care must be taken in interpreting results on individual patients when the entire genetic context is not known.
Several technical considerations critical for producing high-quality flow cytometric data are discussed in this section. Readers interested in a more in-depth discussion of validation of various pre- and postanalytical variables applicable in flow cytometry are referred to the “Practice Guidelines from the ICSH and ICCS” for the “validation of cell-based fluorescence assays” ( ; ; ; ).
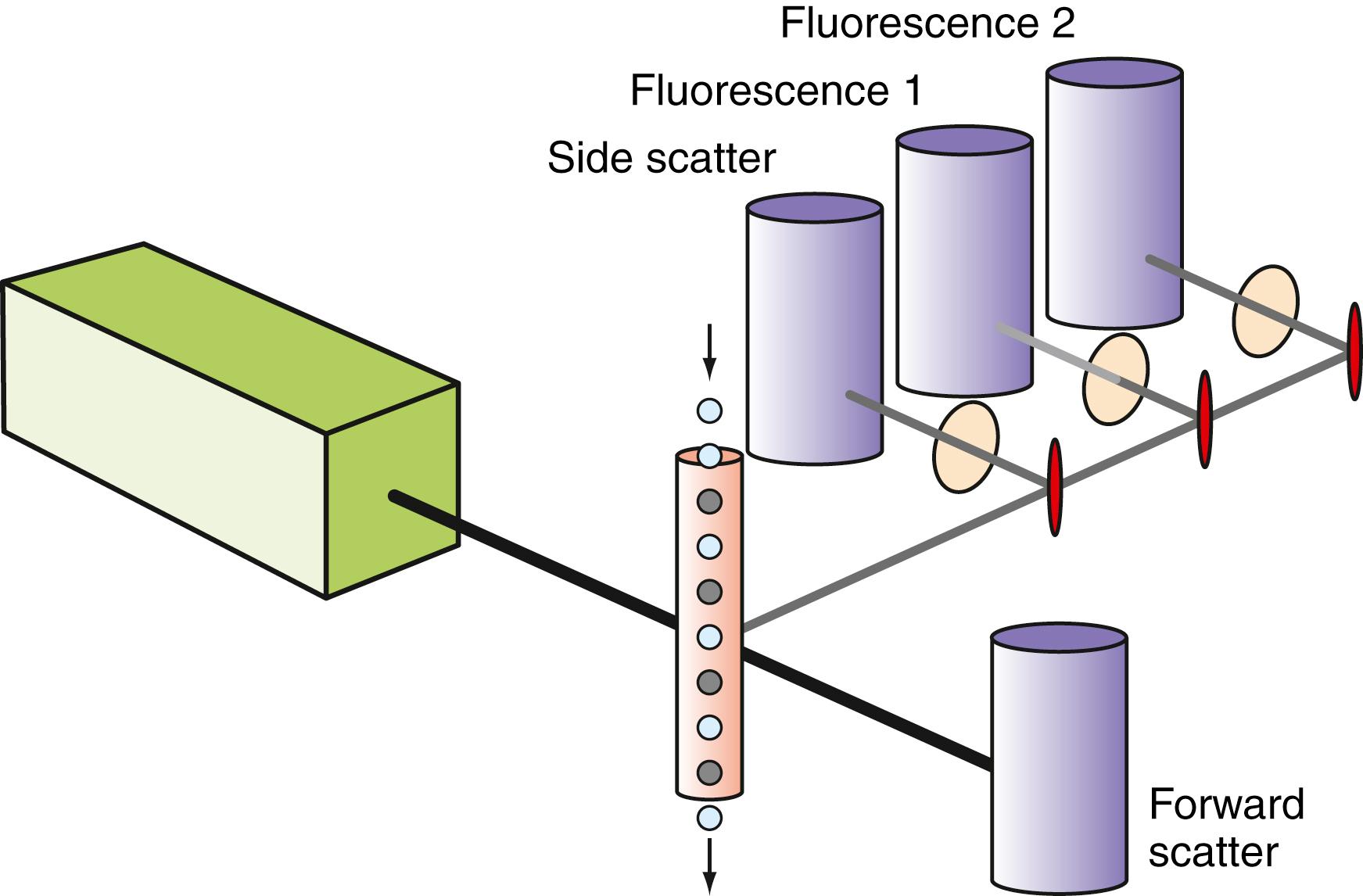
The basic principle of flow cytometry relies on the injection of a monodisperse suspension of particles (cells) into the center of a flowing stream of fluid (sheath) that then passes through a small quartz capillary tube at a constant velocity ( ). The sheath fluid serves to maintain the particles in the center of the flowing stream, where they may be illuminated by one or more focused light sources, typically lasers. The light scattered by the particles is collected by detectors positioned at a variety of angles around the capillary to obtain information about the cross-sectional area and, hence, size (low-angle or forward scatter) or complexity/granularity (high-angle or side scatter) of each individual particle as it transits the capillary. In addition, if fluorescent molecules or fluorochromes are attached to the particles, they may be excited by the incident light, giving rise to fluorescent emission that can be collected with the use of additional detectors and optical filters ( Fig. 35.1 ). The net result is a multiparametric analysis of each individual particle with the number of parameters evaluated dependent on the number of fluorescent molecules used and the complexity of instrument design. Modern clinical instrumentation is capable of assessing 10 and sometimes more simultaneous fluorochromes ( ).
Optimization and standardization of instrument performance are critical for the success of any flow cytometric study, but are of particular importance in the analysis of hematopoietic neoplasms because of the wide range of signal intensities that must be detected. The preferred approach is to define a set of instrument conditions that allow for optimal instrument performance and perform daily quality control of the instrument to consistently achieve that level of performance. Adherence to a consistent and fixed level of instrument performance greatly simplifies quality control and provides reproducible data for interpretation. Daily assessment of the fluorescence intensity and coefficient of variation, using particles that have stable, moderately bright fluorescence and assessment of instrument noise using nonfluorescent particles, is the cornerstone of instrument quality control.
The operator has control over relatively few instrument variables on a flow cytometer, principally detection threshold, detector gain, rate of sample flow and, for some multilaser instruments, laser delay time. The detection threshold or discriminator is associated with a single parameter and is the value that must be achieved for the system to recognize that a particle has passed through the capillary to initiate signal processing. Typically, forward scatter is used to identify particles that have a size greater than some desired value (e.g., cells equal to or larger than lymphocytes). Of particular importance is the gain or voltage supplied to each detector—commonly, a photomultiplier tube—as this has a direct impact on the ability to separate signals of interest from background instrument noise (signal-to-noise ratio). The voltage applied to each detector should be adjusted to optimize the signal-to-noise ratio and allow detection of the weakest required signals for each detector. This often results in positioning of negative cellular populations in such a way that they completely occupy the lowest decade of a logarithmic scale. Finally, for instruments having multiple lasers that intersect the capillary stream at different points (spatially separated lasers), a parameter must be supplied to allow synchronization of signal processing to account for the different times at which a single particle encounters each laser as it transits through the capillary. This laser delay time should be adjusted to maximize signal intensity for each laser. It should be noted that the laser delay time is highly dependent on the transit time of the particle through the capillary; thus, it requires a stable fluidic system to maintain consistency.
The rate at which the sample is introduced or aspirated through the system can be controlled by the operator but is limited by two factors: the rate at which the instrument electronics can process signals that are generated and sample concentration. If particles pass through the instrument more rapidly than they can be processed by the electronics, the data for a subset of the particles will be lost and another subset will show unusual and undesirable artifacts, typically variable loss of signal intensity over a wide range. It is critically important to select a sample aspiration rate and concentration appropriate to the instrument to be used. One additional phenomenon related to sample concentration is termed coincidence , the simultaneous presence of more than one particle in the laser, resulting in a single recorded event that has composite characteristics of the particles involved. Coincidence can represent a significant problem for rare event detection and can be minimized using methods for doublet discrimination during analysis—techniques beyond the scope of this chapter ( ).
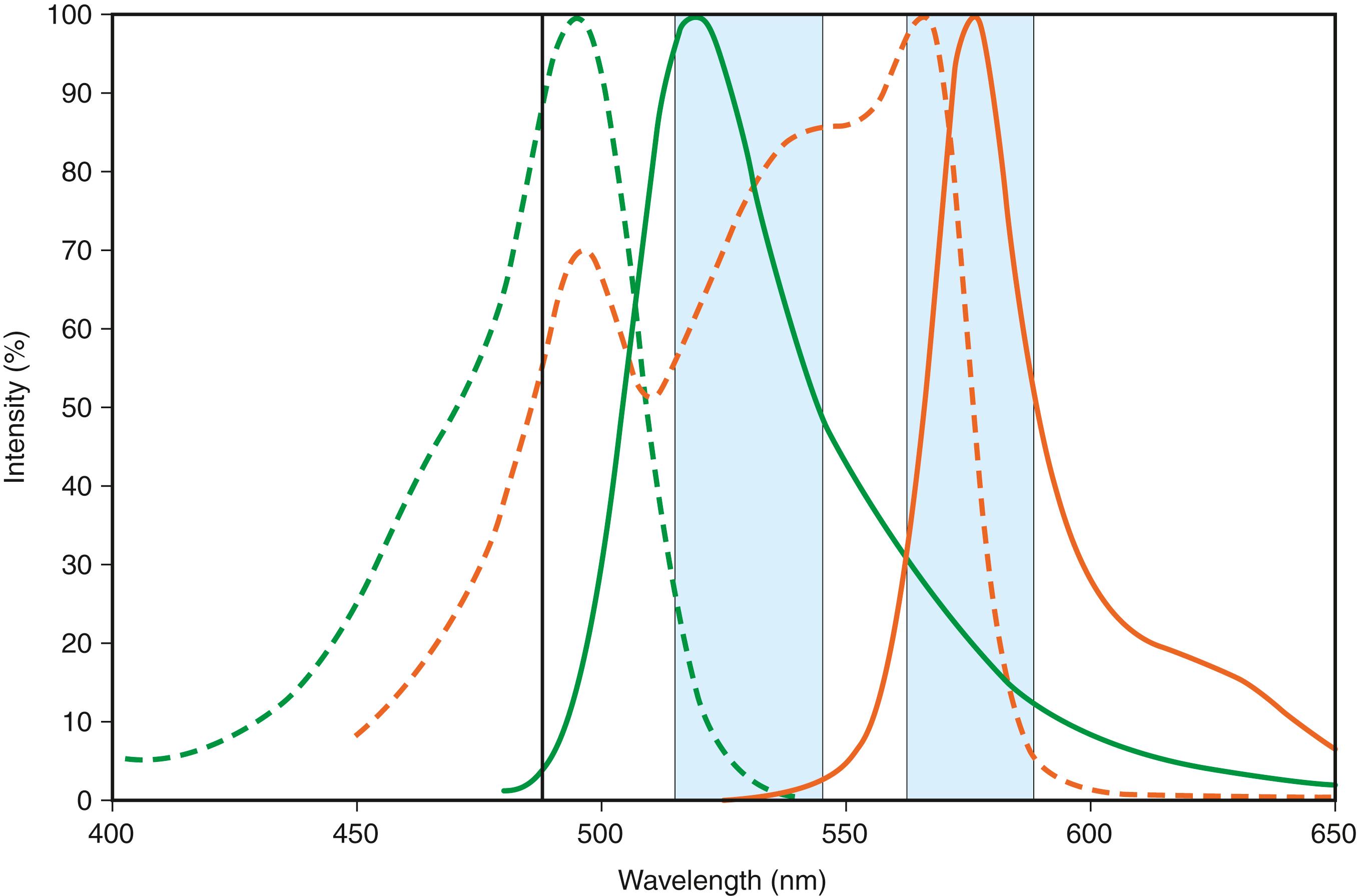
The identification of cellular characteristics beyond those provided by light scatter measurements commonly utilizes fluorochromes that directly interact with cellular structures such as DNA or that indirectly interact through conjugation to antibodies. Each fluorochrome has a unique excitation and emission spectrum that partly determines the performance characteristics of the reagent and dictates which fluorochromes may be used simultaneously ( Fig. 35.2 ). In an effort to increase the number of usable simultaneous fluorochromes possible from a single excitation source, tandem fluorochromes have been devised (e.g., PE-Cy5) that rely on excitation of a primary fluorochrome that transfers its energy to a secondary fluorochrome (Cy5), providing the predominant fluorochrome emission at a longer wavelength. Tandem fluorochromes are required for high-level multicolor flow cytometry, but they have more complex emission spectra as well as decreased fluorochrome stability under some circumstances. The other important fluorochrome characteristic, the intensity of emission, is related to the efficiency of excitation by the light sources utilized and the quantum efficiency of the fluorochrome itself ( Fig. 35.3 ). Emission intensity directly correlates with detection sensitivity and is a key consideration in the design of reagent panels, as discussed later.
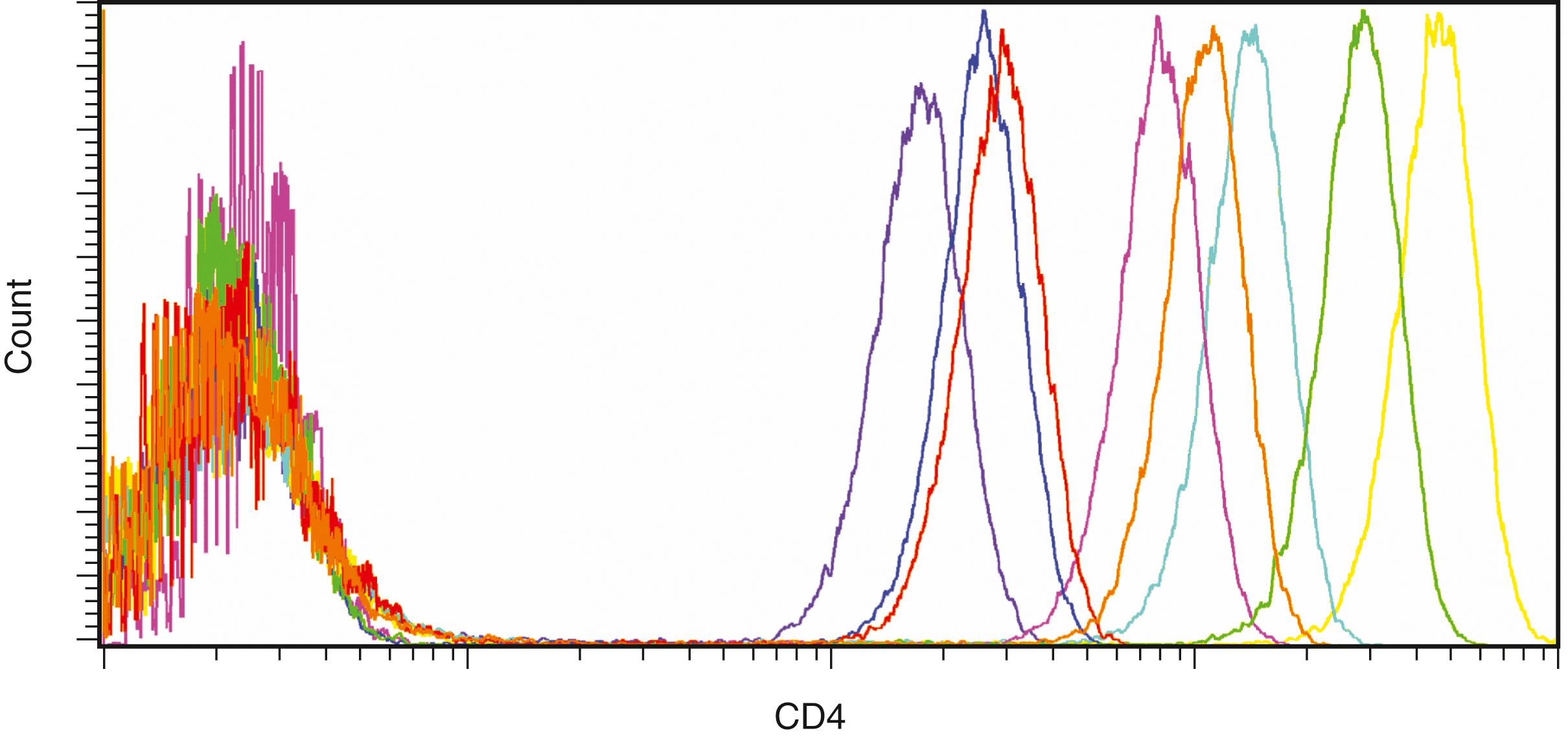
The use of multiple simultaneous fluorochromes (i.e., multicolor flow cytometry) is required for the analysis of leukemia and lymphoma. Although three was noted to be the minimum number recommended under early consensus guidelines ( ), the use of more simultaneous fluorochromes is typical in most modern clinical flow cytometry laboratories. The use of six or greater simultaneous fluorochromes is now common, with 10 or more being utilized in some clinical laboratory settings. The benefits of increased numbers of simultaneous fluorochromes is most pronounced in situations in which the abnormal population is small, the background is heterogeneous, and/or the abnormal population does not have uniform immunophenotypic characteristics. In addition, using more fluorochromes makes it possible to extract more information from limited samples. Because most fluorochromes have overlapping emission spectra, determination of the fluorescence due to a specific fluorochrome in a single detector requires subtraction of the portion of the signals contributed by each of the other fluorochromes in the sample, a process termed compensation . The appropriate amount of each signal to subtract (i.e., compensation coefficient) may be represented by an n -by- n matrix, where n is the number of fluorochromes. Consequently, as the number of fluorochromes increases, the number of compensation coefficients that must be determined increases geometrically, and software rapidly becomes required to perform this process correctly. To determine the compensation coefficients, a series of samples singly labeled with each fluorochrome are evaluated, with the positive population as bright as the brightest signal one plans to evaluate. Because the spectral characteristics of the tandem fluorochromes can vary significantly between lots and manufacturers, separate compensation controls are often required for each lot and conjugate of these reagents. Software, either on the instrument or on offline computers, is used to analyze the resulting data and to calculate compensation coefficients.
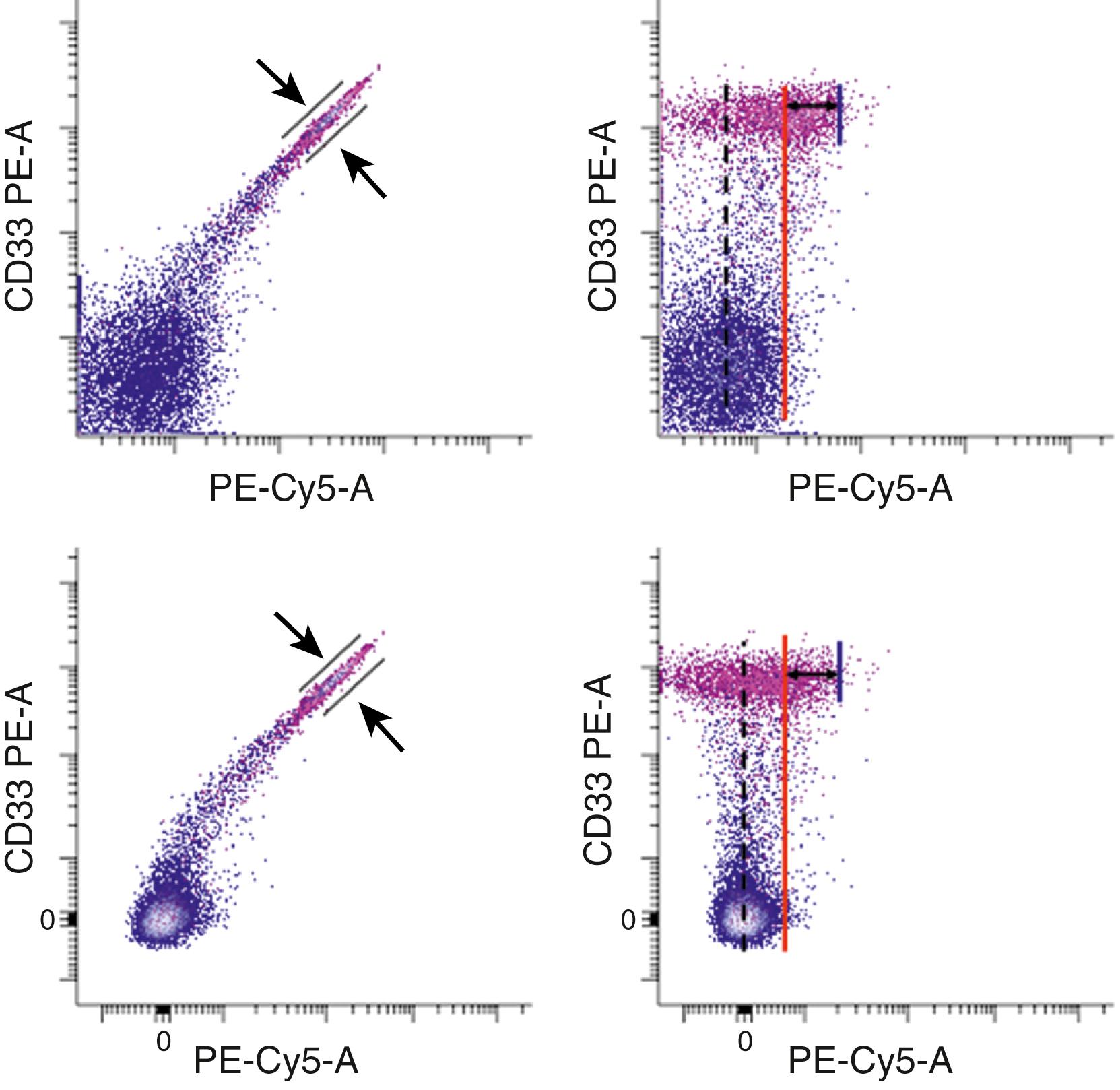
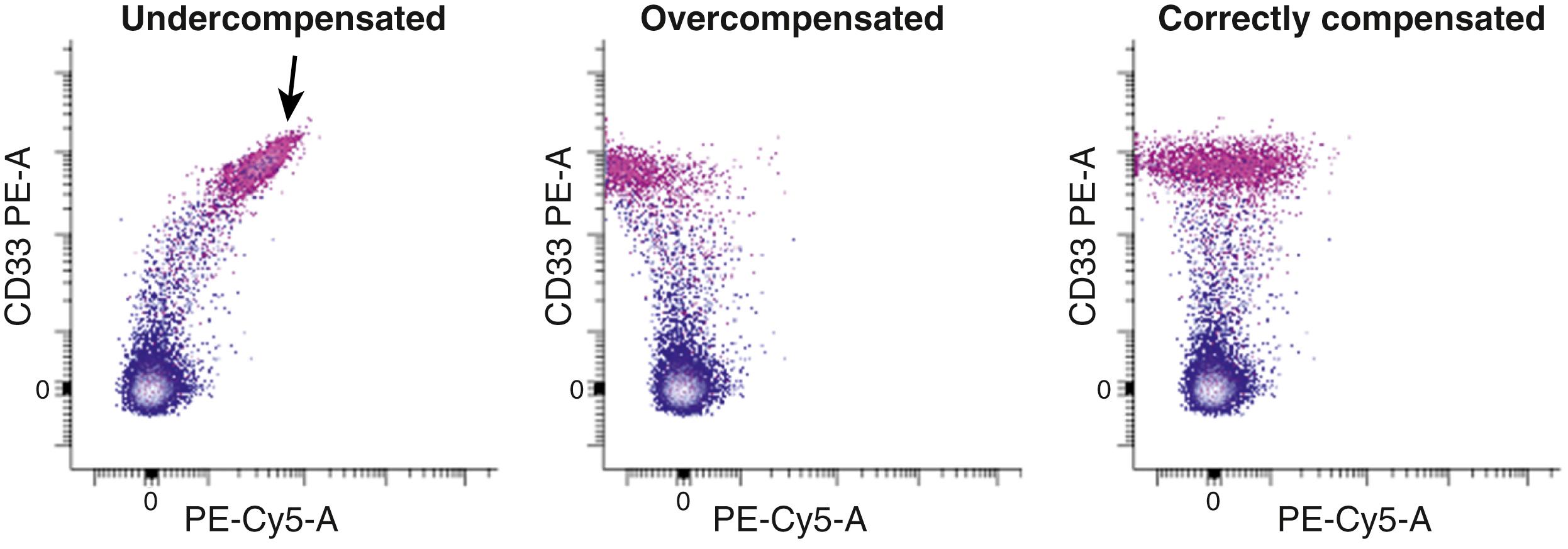
Although the process of determining and correctly applying compensation has been simplified using software, the use of multiple fluorochromes can result in significant display artifacts that are reflected in compromised low-level detection sensitivity ( ). This is a particular problem when one attempts to detect low-level signals in the presence of brightly overlapping fluorochrome emissions. Understanding the impact of compensation-related effects represents a major challenge in interpretation of multicolor flow cytometry. The implications of overlapping fluorochrome emission have direct consequences for panel design and data interpretation ( Fig. 35.4 ). When compensation settings are not appropriate for the reagents used, artifacts arise that can lead to both the misattribution of low-level positivity and an inability to recognize the presence of subpopulations ( Fig. 35.5 ). The use of software compensation provides a nondestructive method for data collection and allows the adjustment of compensation settings following acquisition, thereby supplying a way to correct for inadvertent compensation errors.
Antibodies are an integral part of the immunophenotypic evaluation of leukemia and lymphoma. It is important to determine the correct amount of each antibody to use by evaluating a series of samples labeled with different concentrations of antibodies (i.e., titering) ( ). The object of titering is to maximize the signal-to-noise ratio by providing as bright a signal as possible on positive populations while maintaining a low level of background nonspecific binding. For high-specificity antibodies used for cell surface labeling, one should be able to achieve saturation, a consistent intensity on positive populations at higher antibody concentrations, without significant increases in background on negative populations. The inability to do so indicates a poor-quality antibody. For intracellular labeling, saturation typically is not achieved because of relatively high levels of nonspecific antibody binding by intracellular constituents, generally a linear function of antibody concentration, and optimization of signal-to-noise by titering becomes critical for adequate reagent performance.
The pairing of reagents with appropriate fluorochromes is a key factor in ensuring appropriate signal intensity and detection sensitivity. The following four steps can aid in the construction of appropriate reagent panels:
Determine the purpose for each potential combination of reagents. Each reagent combination should have a clearly defined objective or should not be further pursued.
Select a group of cellular targets. The selected group of targets or antigens should be able to successfully address the purpose of the reagent combination and should be relatively independent of fluorochrome or availability of commercial conjugates. The reagent combination should be the minimum needed to answer the question being asked. Antibodies are commonly combined in a variety of ways for use in the identification of abnormal hematopoietic populations, but these generally fall into one of the categories outlined in Table 35.1 .
| Principle | Example Implementation |
|---|---|
| At least one reagent for positive and specific population identification | Lineage-associated antigens for specific cell lineage (e.g., CD19 for B cells or CD3 for T cells) |
| Multiple antigens of same lineage and maturational stage to identify aberrant expression levels | Use of CD2, CD3, CD4, CD5, CD7, or CD8 simultaneously to evaluate mature T cells |
| Multiple antigens of same lineage but different maturational stages to identify normal maturation and distinguish asynchronous antigen expression | Use of CD13 and CD16 simultaneously to demonstrate neutrophilic maturation |
| Separation of different cell lineages | Use of CD11b and CD15 simultaneously to separate monocytic and neutrophilic maturation |
| Demonstration of clonality | Use of κ and λ in combination with a B-cell lineage reagent (e.g., CD19) |
| Identification of frankly aberrant antigen expression | Use of T-cell or NK cell–associated antigens such as CD7 or CD56 in combination with CD34 to demonstrate aberrant nonlineage antigen expression on a myeloid blast |
Assign a fluorochrome to each target. The basic principle is that highly expressed antigens should be coupled with dim fluorochromes, and dimly expressed antigens should be coupled with bright fluorochromes. This principle provides reasonable signal intensities and avoids compensation problems due to excessively bright fluorescence. A second principle involves selecting fluorochrome combinations to avoid compensation problems. Three general classes of these problems include: (1) spillover from one fluorochrome into an adjacent fluorochrome detected at a higher wavelength; (2) cross-laser excitation, most commonly from direct excitation by the red laser of the cyanine dyes used in tandem conjugates (e.g., PE-Cy5 emitting as APC); and (3) tandem fluorochromes having variable emission of the parent fluorochrome (e.g., APC-Cy7 emitting as APC). For a more in-depth discussion of such considerations, the reader is referred to .
Test reagent combinations. The reagent combination must be empirically tested to ensure that signal intensities are as expected, that low-level sensitivity is appropriate where important, and that unexpected interactions between reagents do not occur. A sample prepared with all reagents of interest should be compared with the same sample prepared with a series of preparations, each lacking one of the component reagents (fluorescence-minus-one controls), as well as with a second series containing each single reagent independently (single-stained controls). Unexpected changes in intensity of one or more reagents indicate potential problems with the reagent combination.
Any specimen from which a single cell suspension can be generated is suitable for flow cytometric immunophenotyping, including peripheral blood, bone marrow, and lymph node, to name a few. Blood and bone marrow may be anticoagulated with ethylenediaminetetraacetic acid (EDTA), heparin, or acid citrate dextrose. Tissue specimens are best transported and stored in tissue culture media, such as RPMI 1640, and a single-cell suspension is generated by mechanical disaggregation using scalpel and forceps, needle and syringe, or wire screen mesh ( ), followed by filtration through fine-gauge wire mesh to remove aggregates. Specimens of all types are commonly stored at room temperature before preparation, although refrigeration will retard degradation when preparation must be delayed.
Analysis of white blood cells in peripheral blood or bone marrow requires erythrocyte removal for efficient evaluation. Density-gradient centrifugation (e.g., Ficoll-Hypaque) was historically used for this purpose, but it leads to selective loss of cellular subpopulations and gives relatively poor recovery on bone marrow specimens. Erythrocyte lysis using ammonium chloride or a variety of commercial preparations has become the standard technique for sample preparation in clinical laboratories ( ). Currently recommended methods involve the addition of antibodies to an aliquot of blood or marrow, followed by erythrocyte lysis and washing with a buffered salt solution such as phosphate-buffered saline to remove cell debris, the lysing reagent, and unbound antibody. Inclusion of a small amount of formaldehyde (0.25%–0.50%) with the lysing reagent or following washing is a convenient way to stabilize both the specimen and antibody binding. When analysis of light chain expression on B cells is required, plasma immunoglobulin (Ig) must be removed by repeated washing before antibody addition. Once prepared, samples should be acquired on the instrument as soon as reasonably possible to avoid sample and fluorochrome degradation, although fixation and refrigerated storage will delay degradation.
As the prepared sample is evaluated on the flow cytometer, it is important to collect enough events to allow detection of the population of interest. For populations that represent a large percentage of the total, relatively few events need to be acquired, but infrequent populations require the acquisition of larger numbers of events. Because in the evaluation of hematopoietic neoplasms one rarely knows what the likely population frequency will be for a given sample, it is most convenient to acquire a fixed number of total events that ensures the desired minimum level of sensitivity. For example, if a sensitivity of 0.1% is desired and 50 events are determined to be adequate for confident population identification, then 50,000 total white blood cell events will need to be acquired. The acquisition of fewer events will compromise the ability to detect low-frequency populations—an important consideration when evaluating for residual disease following therapy and in other situations in which the population of interest is present at low levels.
Instrument carryover between samples becomes a significant concern when evaluating for the presence of small abnormal populations. Most modern commercial instruments have a manufacturer’s specification for carryover of roughly 0.1%. Although low in the diagnostic setting, this level may be considered relatively high when one is looking for infrequent populations, as in the setting of low-level residual disease detection. To minimize carryover, a small amount of water or sheath fluid should be run between each aliquot of sample acquired until background returns to an acceptably low level. Failure to do so will result in the sporadic appearance of populations having unexpected immunophenotypes that can be mistaken for neoplastic disease.
The identification of hematopoietic neoplasia by immunophenotyping relies on the principle that neoplastic cells express patterns of antigen expression that are distinctly different from those of their normal counterparts. Antigen expression in normal cells is a tightly regulated process, resulting in a characteristic pattern of antigen acquisition and loss with maturation that is cell-lineage specific. Neoplastic cells commonly show nonrandom alterations in antigen expression that include the following:
Gain of antigens not normally expressed by cell type or lineage
Abnormally increased or decreased levels of expression of antigens normally expressed by a cell type or lineage, including the complete loss of normal antigens in some instances
Asynchronous antigen expression (i.e., expression of antigens normally expressed by cell type or lineage but at an inappropriate time during maturation)
Abnormally homogeneous expression of one or more antigens by a population that normally exhibits heterogeneous expression of that antigen
The consequence is that the immunophenotypic identification of hematopoietic neoplasia rests on a thorough knowledge of the normal patterns of antigens expressed by hematopoietic cells at different stages of maturation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here