Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neuromodulation requires a means of affecting the function of neurons and/or axons. This can be achieved by physical means such as changing temperature or mechanical pressure. Manipulation of the chemical environment by changing pH, ionic concentration, or the release of specific chemicals can also influence neuronal activity. However, the application of electrical fields, either directly or through electromagnetic induction, may be the simplest and most easily controlled neuromodulatory effector. Since this requires an electrode, it is important to understand the properties of the electrode in great detail which is the goal of this chapter.
Although electrodes can be made from many different materials, it is useful to consider the properties of a metal electrode. When a metal electrode is placed inside a physiological medium, such as extracellular fluid (ECF), an interface is formed between the two phases. In the metal electrode and in the attached electrical circuits, charge is carried by electrons. In the physiological medium, charge is carried by ions, including sodium, potassium, and chloride, in the ECF. The central process that occurs at the electrode–electrolyte interface is a transduction of electrons in the metal electrode to ions in the electrolyte.
In the study of electrode properties, two electrodes are often placed in an electrolyte, and electrical current passed between them. One of the two electrodes is termed a working electrode (WE), and the second is termed a counter electrode (CE). The WE is defined as the electrode that is under study and the CE is one with known properties which completes the electrical circuit. In neuromodulation, electrodes that are planned to modulate a neural system are termed the active electrodes and other electrodes are called reference electrodes.
There are two primary mechanisms of charge transfer at the electrode–electrolyte interface, illustrated in Fig. 7.1 . One is a nonfaradaic reaction, where no electrons are transferred between the electrode and electrolyte. Nonfaradaic reactions include redistribution of charged chemical species in the electrolyte. The second mechanism is a faradaic reaction, in which electrons are transferred between the electrode and electrolyte, resulting in reduction or oxidation of chemical species in the electrolyte.
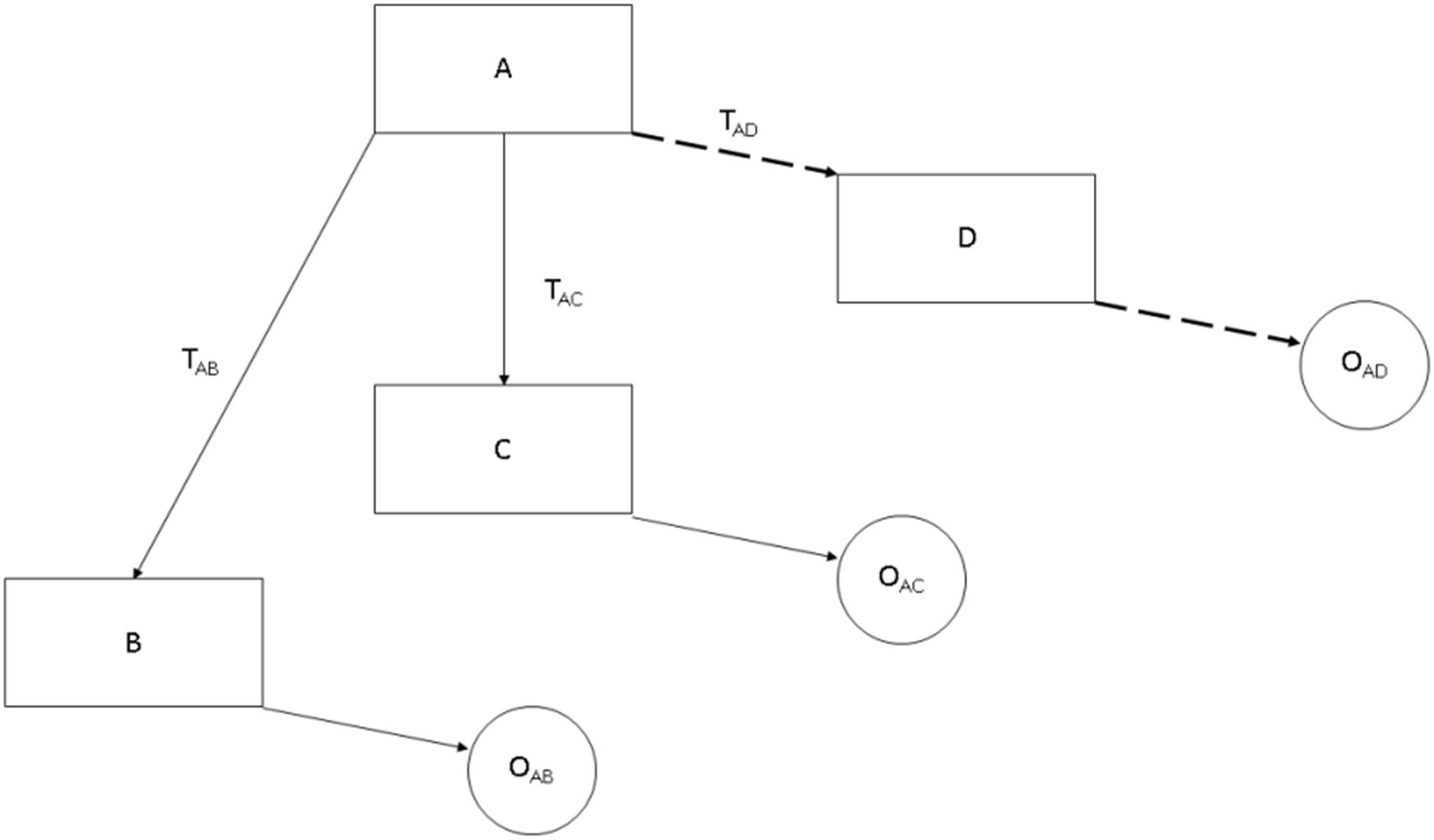
If only nonfaradaic redistribution of charge occurs, the electrode/electrolyte interface may be modeled as a simple electrical capacitor called the double layer capacitor C dl . This capacitor is formed due to several physical phenomena [ ]. When a metal electrode is placed in an electrolyte, ions in the solution are drawn to the electrode by electrostatic forces and chemical reactions occur at the electrode surface. In addition, the presence of the electrode locally orders even the uncharged molecules near the interface by disrupting the intermolecular forces responsible for creating the liquid state. These effects produce an interfacial layer between the electrode and the bulk solution ( Fig. 7.1 ). When the electrochemical composition of the interface is relatively unchanged with changes in the voltage at the electrode surface, injection of charge to the electrode produces no net flow of ions across the interface although charge can accumulate at either edge of the interface. In this regime, the electrode behaves as a capacitor. In this situation, the change in electrical potential when a certain amount of charge is injected is exactly the opposite of that when the same amount of charge is removed.
Charge may also be injected from the electrode to the electrolyte by faradaic processes of reduction and oxidation. Reduction, which requires the addition of an electron, occurs at the electrode that is driven negative, while oxidation, requiring the removal of an electron, occurs at the electrode that is driven positive. Unlike the capacitive mechanism, faradaic charge injection forms products in solution that cannot be recovered upon reversing the direction of current if the products diffuse away from the electrode. Fig. 7.1B illustrates a simple electrical circuit model of the electrode–electrolyte interface, consisting of two elements [ ]. C dl is the double layer capacitance, representing the ability of the electrode to cause charge flow in the electrolyte without electron transfer. Z faradaic is the faradaic impedance, representing the faradaic processes of reduction and oxidation where electron transfer occurs between the electrode and electrolyte.
The following are examples of faradaic electrode reactions. Cathodic processes, defined as those where reduction of species in the electrolyte occur as electrons, are transferred from the electrode to the electrolyte, include such reactions as:
Anodic processes, defined as those where oxidation of species in the electrolyte occur as electrons are transferred to the electrode, include:
Reaction 7.1 is the irreversible reduction of water forming hydrogen gas and hydroxyl ions. The formation of hydroxyl raises the solution pH. Reversible reactions, where species remain bound or close to the electrode surface, are demonstrated by reactions 7.2 through 7.4 . Reactions 7.2 and 7.3a , 7.3b , and 7.3c are the reversible formation and subsequent reduction of an oxide layer on platinum and iridium, respectively. Reaction 7.4 is reversible adsorption of hydrogen onto a platinum surface, responsible for the so-called pseudocapacity of platinum. In reaction 7.5 , water molecules are irreversibly oxidized, forming oxygen gas and hydrogen ions, thus lowering the pH. Reaction 7.6 is the corrosion of a platinum electrode in a chloride-containing medium.
The electrode interface model of Fig. 7.1B demonstrates the mechanisms of charge injection from an electrode; however, it neglects the equilibrium interfacial potential Δϕ that exists across the interface at equilibrium. This is modeled as shown in Fig. 7.2A , along with the solution resistance R S (also known as the access resistance R A or the ohmic resistance R Ω ) that exists between two electrodes in solution.
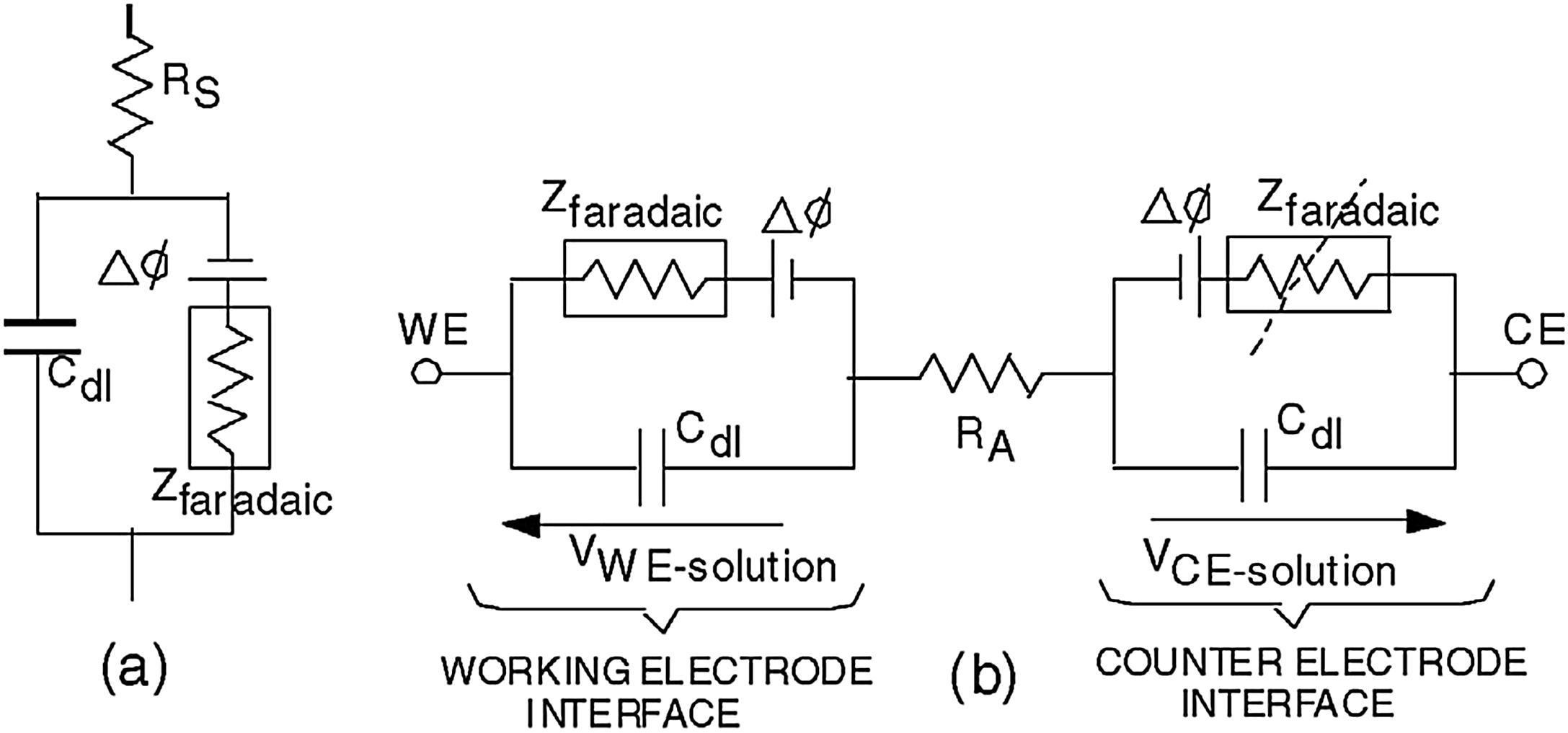
If one begins with a system that is in equilibrium and then forces the potential of an electrode away from its equilibrium value, for example, by connecting a voltage or current source between the working and CE, the electrode is said to become polarized. Polarization is measured by the overpotential η , which is the difference between an electrode's potential and its equilibrium potential (both measured with respect to some third reference electrode):
The net current density across an electrode/electrolyte interface is related to the overpotential, according to the equation [ ]:
where i net is the net faradaic current density across the electrode–electrolyte interface, i 0 is the exchange current density, [ O ](0, t ) and [ R ](0, t ) are concentrations of the oxidized and reduced species at the electrode surface ( x = 0) as a function of time, [ O ] ∞ and [ R ] ∞ are bulk concentrations, a c is the cathodic transfer coefficient and equals ≈ 0.5, n is the number of moles of electrons per mole of reactant oxidized, f ≡ F / R T , F is Faraday's constant ≈ 96,485 C/mol of electrons, R is the gas constant ≈ 8.314 J/mol- o K, and T is the absolute temperature.
In the case that the concentration of both species near the electrode is equal to the concentration in the bulk, (7.8) simplifies to:
For a sufficiently small overpotential (a small potential excursion away from equilibrium), there is little faradaic current, and current flows primarily through the capacitive branch of Fig. 7.1 . As the magnitude of the overpotential increases, the total current increases exponentially, making up increasing amounts of total electrode current. For substantial cathodic overpotentials, the left term of Eq. (7.8a) dominates; for substantial anodic overpotentials the right term dominates.
Faradaic reactions are divided into reversible and irreversible reactions [ ]. A reversible process is one where the reactants are reformed from the products upon reversing the direction of current. The degree of reversibility depends on three factors: the time scale of the charge injection, the rate of forward and reverse chemical reactions, and the speed of mass transport of chemicals out of the interfacial region. For simplicity, take T S as the time scale over which the stimulus is applied, T F and T R as the time scales (concentration dependent) over which reactions in the forward and reverse directions occur and T D be the time it takes a chemical entity of interest to diffuse away from the interfacial region. If T D >> T F , T R , and T S , the reactions are clearly reversible. But if either T F , T R , or T S are longer or of the same magnitude of T D , then, the reactions are irreversible. Irreversible products may include species that are soluble in the electrolyte, precipitate in the electrolyte, or evolve as a gas (e.g., reactions 7.1 and 7.5 ). Irreversible faradaic reactions result in a net change in the chemical environment, potentially creating chemical species that are damaging to tissue or the electrode. As a general principle, the biocompatibility of an electrode decreases as the amount of irreversible Faradaic reactions increase.
As illustrated in Fig. 7.1 , there are two primary mechanisms of charge injection from a metal electrode into an electrolyte. The first consists of charging and discharging the double layer capacitance, C dl . Total capacitance of an electrode is proportional to its area: C dl = C dl S × area, where C dl S Capacitance/area is an intrinsic property of the electrode in a given solution. For a metal electrode in aqueous solution C dl S has values on the order of 10–20 mF/cm 2 of real area (geometric area multiplied by the roughness factor). As charge is injected into the electrode, the potential of that electrode changes according to the basic formula for capacitance:
where q is the charge on the capacitor and v is its voltage. As the injected charge increases so does the potential and consequently the overpotential so that faradaic flow begins to increase.
In addition to the double layer capacitance, some metals have the property of pseudocapacity [ ], where a faradaic electron transfer occurs but, because the product remains bound to the electrode surface, the reactant may be recovered (the reaction may be reversed) if the direction of current is reversed. Although electron transfer occurs, in terms of the electrical model of Fig. 7.1 , the pseudocapacitance is better modeled as a capacitor, since it is a charge storage (not dissipative) process. Platinum is commonly used for stimulating electrodes as it has a pseudocapacity (by reaction 7.4 ) of 210 mC/cm 2 real area [ ], or equivalently 294 mC/cm 2 geometric area using a roughness factor of 1.4. 1
1 The relationship between capacitance and stored charge is given by Eq. (7.9) . A 1 V potential excursion applied to a double layer capacitance of 20 mF/cm 2 yields 20 mC/cm 2 stored charge, which is an order of magnitude lower than the total charge storage available from platinum pseudocapacitance.
The optimal design of electrical stimulation systems should avoid the onset of irreversible faradaic processes which may potentially create damaging chemical species, and keep the injected charge at a low enough level where it may be accommodated strictly by reversible charge injection processes. Unfortunately, this is not always possible because a larger injected charge may be required to cause the desired effect (e.g., initiating action potentials).
The net current passed by an electrode is the sum of currents through the two parallel branches shown in Fig. 7.1 , given by:
where i C is the current through the capacitance and i f is the current through the faradaic element.
The current through the Faradaic element is given by the current–overpotential Eq. (7.8) . The current through the capacitance is given by:
The capacitive current depends upon the rate of potential change, but not the absolute value of the potential. The faradaic current, however, is exponentially dependent upon the overpotential. As an electrode is driven away from its equilibrium potential, essentially all charge initially flows through the capacitive branch since the overpotential is small. As the overpotential increases, the faradaic branch begins to conduct a relatively larger fraction of the injected current. When the overpotential becomes great enough, the faradaic impedance becomes sufficiently small that the faradaic current equals the injected current.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here