Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Aged related macular degeneration (AMD) and retinitis pigmentosa (RP) are two degenerative diseases that affect mainly the photoreceptors layer, rendering the retina unable to translate light into biological signals and causing blindness in patients. In the United States, 700,000 new AMD patients are diagnosed every year and 1 in 4000 live births are afflicted with RP. In these conditions, inner retinal neurons (bipolar cells and ganglion cells) are less affected by disease, allowing the retina to be electrically stimulated to restore a sense of vision.
Visual prostheses have been studied as a method to restore vision for decades, starting in 1929 when Foerster, a German neurosurgeon, exposed the occipital pole of one cerebral hemisphere and electrically stimulated the visual cortex in a sighted patient, reporting that his patient saw a small spot of light ( ). In , Krause and Schum were able to create a sensation of light by replicating Foerster’s experiment in a patient who had been blind for over 8 year. Similar experiments kept being performed over the decades ( ). In , Brindley and Lewin implanted the first visual prosthesis in a blind 52-year-old woman. The extracranial part of the implant consisted of an array of 80 radio receivers encapsulated in silicone rubber, that connected through a cable to the intracranial part of the implant, which consisted of an array of 80 platinum electrodes placed in a silicone rubber cap that was placed on her visual cortex. Patient reported seeing spots of white light in 39 different locations, based on the position of the electrode that was being stimulated. Six years after implantation a new publication reports that the device was still partially functional ( ).
In the years since Brindley’s experiments, various attempts were made to develop visual cortex prostheses. Although stimulating the visual cortex showed promise, its organization is very complex and the surgical access is difficult, which why different locations for a visual prosthesis started to be explored. As retinal surgery begun to develop in the 1970s, it followed that retinal prostheses were investigated as an alternative to visual cortex prostheses. Other implant areas along the visual pathway that have been studied include the optic nerve ( ) and cortical ( ), but this chapter will focus on retinal implants.
A retinal prosthesis consists of multiple components. In most cases, an external acquisition system is used to capture images from the outside; wearable computers convert this image into a stimulation pattern, a telemetry system delivers power and data to the implanted stimulator, the stimulator generates electrical pulses, and an electrode array delivers the stimulus pulses to the retina ( ). Placement and complexity of implanted components are the main differences that distinguish the various systems. In particular, the placement of the microelectrode array is a critical, defining feature of retinal prostheses. There are three different placements for the retinal prostheses electrode array. Epiretinal prostheses are attached to the inner surface of the retina, nearest the ganglion cells. Subretinal prostheses are placed between the retina and the choroid, in the space where the photoreceptors were prior to degeneration and suprachoroidal prostheses are implanted between the sclera and choroid, behind the retina ( ). Fig. 101.1 .
Epiretinal prostheses refer to implants where the microelectrode array is on the ganglion cell side. In practice, the electrode array is fixed to the retina with a tack ( ). This type of placement allows direct stimulation of ganglion cells (the final output of the retina), which may be advantageous in retinal degeneration diseases where the retinal network is altered. Another advantage of epiretinal implantation involves the use of the vitreous cavity for implantation and heat dissipation ( ). Surgical access is easier, with reduced risk of retinal detachment. However, fixation of the device to the retinal surface remains a challenge ( ). Since the array is tacked at only a single location, parts of the array away from the tack can separate from the retina. The three main groups that have reported clinical studies of epiretinal prostheses are:
Second Sight Medical Products Inc. (SSMP), Sylmar, California, United States of America
Intelligent Medical Implants (now Pixium Vision), Paris, France
EpiRet GmbH, GieBen, Germany
Two devices have been developed by SSMP: Argus I and Argus II. The external system is virtually the same for both, but the implant is different. The external system has a small camera placed on a pair of glasses that captures the image and feeds it to an external video processing unit (VPU) for image processing. The VPU software determines the average brightness of each region in a camera field of view and uses this information to program stimulation parameters for the implant. These parameters are encoded into a serial data stream and transmitted via a radio frequency (RF) signal to the implant. The RF signal also provides adequate energy to allow the implant to recovery power for operation ( ). Fig. 101.2 .
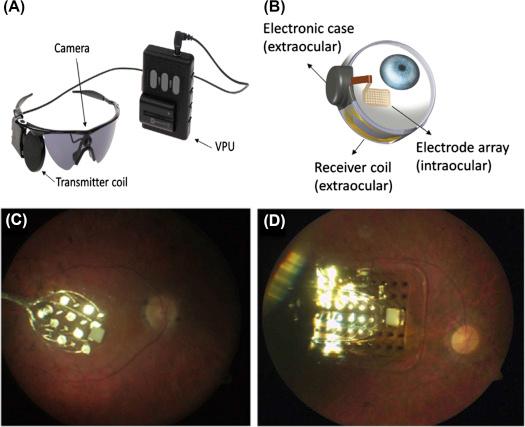
Argus I is a first generation epiretinal prosthesis approved for an investigational trial by the US Food and Drug Administration (FDA). The main goal of this trial was to demonstrate the safety of long-term retinal stimulation. The electronics were implanted behind the ear, since the electronics were based on cochlear implant technology, with a cable running along the temple to the orbit. A 4 × 4 electrode array, at the end of the cable, was implanted into the eye and attached to the retina with a tack. Platinum electrodes were 520 or 260 μm in diameter, the center-to-center electrode spacing was 800 μm ( ). The electrodes were supported on a silicone rubber substrate. Six subjects were implanted between 2002 and 2004. After some training, patients could use the Argus I to accomplish simple visual tasks, like localizing a white square on a black computer display, report the direction of motion of a moving bar, find a black door on a white wall, and follow a white line on the floor. In general, patients performed these tasks better with the system on than with the system off ( ). A recent report showed maintained functionality of the Argus I 10 years after implantation ( ). Overall, Argus I demonstrated adequate safety and some improvements in visual function, thus motivating the development of Argus II.
Argus II is a commercially available device that obtained the CE mark in 2011 and FDA approval as a humanitarian device in 2013. Over 100 devices have been implanted to date ( ). The electrode array consists of a 6 × 10 grid of roughened platinum disks. Each disk is 200 μm diameter and the center-to-center spacing is 575 μm. Similar to the Argus I, the electrode array is attached to the retina with a tack ( ). The Argus II device was developed with the objective of having a commercially available retinal prosthesis, so the safety of chronic implantation was central to early clinical studies. During phase I/II, clinical trial reports results on 30 patients who were evaluated for a minimum of 6 months and up to 2.7 years. Multiple tasks were given to the subjects and their performance was evaluated by comparing the results with the system ON versus the system OFF. Patients performed statistically better with the system ON than with the system OFF in object localization (96% of subjects), motion discrimination (57%), and a discrimination of oriented grating (23%). The best visual acuity recorded was 20/1260 ( ). In another study, 30 patients in 10 different centers in the United States and Europe were evaluated. Twenty-four out of thirty patients remained implanted with functioning Argus II systems. Patients were asked to perform three real-world functional tasks after 5 years of implantation: Sock sorting, sidewalk tracking, and walking direction discrimination tasks. All patients performed better with the system ON than with the system OFF. This result supports the long-term safety profile and benefit for blind patients ( ).
Overall, the Argus II has shown that retinal implants can partially restore vision and improve people’s quality of life by helping them be more independent. The main limitations found in Argus II are limited resolution due the relatively large electrodes, a head-fixed camera that requires patients to scan to change an image and may result in confusing input by ignoring eye movements, and the use of the retinal tack, which by necessity damages the retina and may cause the array to tilt ( ).
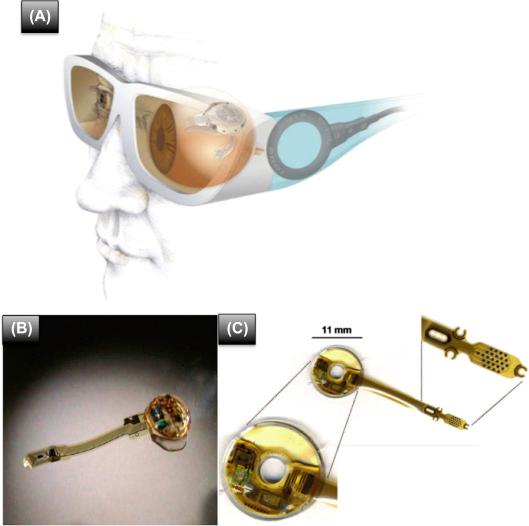
EpiRet3 is a retinal prosthesis designed by the EpiRet consortium in Germany. It is distinguished by the fact that the implant fits entirely inside the eye ( Fig. 101.3 ). The intraocular component consists of a receiver coil, a receiver chip, and stimulator chip, positioned in the location of the lens, and an electrode array with 25 3D-stimulating electrodes, 25 μm height and 100 μm diameter with a center-to-center spacing of 500 μm ( ). The electrodes are formed by electroplating of gold to achieve a height of 25 μm but are covered with a thin layer of iridium oxide, which is a better material for neural stimulation.
Six legally blind patients were implanted for a 4-week period in 2006. No external system was available for home use, but the implant could be activated in the clinic. During the 4-week period patients were evaluated at day 7, 14, and 27. All patients reported the occurrence of visual perceptions during stimulation, by pressing a button or verbally describing the perceptions like dots, line, or circles depending of the stimulation applied. Due to their 3D stimulating electrodes, close proximity to the retina was achieved and low thresholds (between 73.2 and 7.8 μC/cm 2 ) were measured during the experiments ( ).
The EpiRet device was removed after 4 weeks, but the retinal tack was left in place to avoid trauma from removal. The electrode array and tack were designed to allow explantation of the array without removal of the tack. 2 years after explantation, patients were reexamined for long-term side effects. No structural alterations were found, quality of life was consistent with their initial baseline, and moderate epiretinal gliosis was reported in the area were the tack was implanted ( ).
Their website ( http://www.eyenet-aachen.de ) claims that EpiRet project is currently in Phase III; a prototype device is currently fabricated and biocompatibility tests are being planned.
Intelligent Medical Implants (IMI) was founded in 2002 to commercialize retinal prosthesis technology initially developed at the Fraunhofer Institute. Their retinal prosthesis design consists of a retinal stimulator with an extraocular coil attached to the outer wall of the eyeball, and an intraocular multielectrode array attached to the retina with a tack, a visual interface (camera, data, and energy transmitter) mounted in a pair of eyeglasses and a pocket processor for image processing and power supply ( Fig. 101.3 ) ( ). Two wireless links are used: one RF transmission for power and one infrared link for data. The intraocular component has an infrared receiver that translates the optical signals into electrical impulses and sends them to a polyimide 49 contact electrode array with 360 μm diameter electrodes and an integrated microcable with conducting lines ( ). The pocket processor included a retinal encoder designed to replicate retinal signal processing.
Twenty subjects were tested between 2003 and 2004 with short-term electrode array implants (less than 3 h in a surgery room setting). Nineteen out of the twenty subjects reported light perception during stimulation. Even if only one electrode was stimulated, subjects described light perceptions as points, circles, triangles, rectangles, etc. Different colors as white, yellow, and blue were also reported ( ).
Based on these results, the implant was developed and four patients were implanted chronically to test the feasibility and safety of the surgery. Follow-ups were done during a 9-month period, reporting tolerance to the implant. During stimulation patients were able to distinguish between different points and simple patterns such as horizontal bars ( ).
IMI recently transitioned to a company named Pixium and their system IRIS is in clinical trials. Iris has 150 electrodes but otherwise the configuration is similar to the IMI prototype. Their design allows explantation of the device with minimal retinal damage. The first implantation was performed in January 2016, and they expect to enroll a total of 10 patients in their clinical trials ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here