Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The stomach is organized by five concentric tissue layers: (1) the mucosal epithelium, lining the lumen of the stomach; (2) a thin layer of smooth muscle called the muscularis mucosae; (3) the submucosa consisting of connective tissue and blood vessels; (4) the tunica muscularis, composed of two layers (inner circular and outer longitudinal) of smooth muscle; and (5) the serosa, facing the peritoneal cavity ( Fig. 38.1 ). The digestive juice is secreted from mucosa in which several types of secretory epithelial cells make up organized tubular gastric glands. The luminal surface of the gastric mucosa is covered by a layer of columnar epithelial cells, the surface mucous cells, which secrete mucus and bicarbonate. It has been proposed that they play a role in protecting the surface from direct acid exposure. The luminal surface is studded with numerous invaginations, or pits, that serve as conduits for secretions from the subadjacent gastric glands.
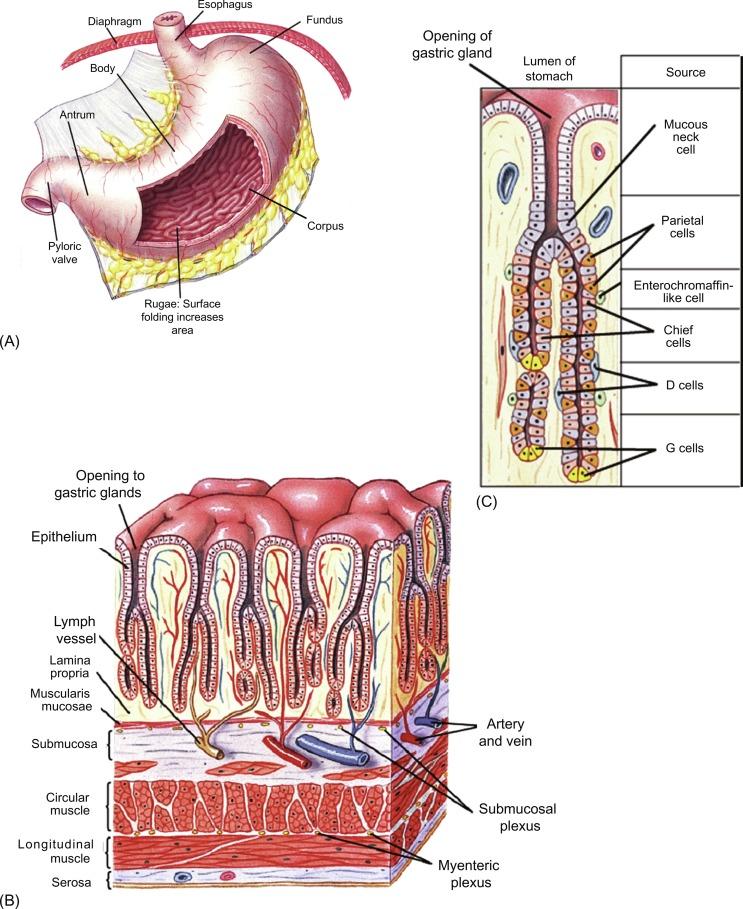
In mammals, gastric glands are composed of three major types of secretory epithelial cells: mucous neck cells, chief cells, and parietal cells. Mucous neck cells are small cells located in the region of transition at the base of the pits extending throughout the neck of the glands. Mucous neck cells secrete a mucous glycoprotein distinct from that of surface epithelial cells. They also serve as a differentiating precursor to the chief cell as they migrate toward the base of the glands. Chief cells are the principal source of pepsinogen, the inactive precursor form of the protease pepsin, and are abundantly distributed at the base of the glands. The large acid-secreting parietal cells are found throughout the length of the gland, but tend to be more abundant in the neck region of the gland. Because of their large size and relative abundance, parietal cells represent about 50%–60% of the mass of the secretory mucosa. Subadjacent to the glandular epithelial cells, there are a number of endocrine and paracrine cells that play important regulatory roles in gastric secretory function, such as gastrin-secreting G-cells and somatostatin-secreting D-cells. The cellular separation and specialization of acid secretion from pepsinogen release in mammals is an evolutionary landmark as the gastric glands of lower vertebrates (birds, reptiles, amphibians, and fish) use a single epithelial cell, named the oxyntopeptic cell, to secrete both acid and pepsin.
Gastric epithelial cells undergo dynamic renewal to orchestrate tissue homeostasis and digestive physiology. All gastric epithelial cells originate from a small population of pluripotent stem cells located in the upper neck region of the glands that represent the progenitor cells for differentiating into all functionally distinct epithelial cell types in gastric glands. These stem cells are the precursors of the constantly renewing gastric epithelium, which must be sustained by continued regeneration, differentiation, and migration both up the pit to differentiate into surface epithelial cells, and down the length of the gland to replace secretory cells. Each of the gastric epithelial cells has distinct turnover kinetics, or life span. Studies of 3 H-thymidine incorporation into cells of the mouse stomach provide an estimate of average cell turnover times. Because of their direct luminal exposure, surface epithelial cells have a relatively short life of about 3 days. Mucous neck cells spend about 40 days in the neck region of the gland, and as they migrate to the base, they transform into chief cells that have an average life span of about 250 days. Parietal cells have an average turnover time of 71 days. Newly differentiated parietal cells progressively migrate from the neck toward the base of the gland. Morphologic and functional criteria suggest that secretory activity may be downregulated as parietal cells age/migrate. Judging by their apical membrane dynamics and corresponding secretory activity, the younger cells that are in proximity to the luminal surface appear to be more active in HCl secretion than the cells located more basally in the gland.
The existence of multipotent stem cells in gastric glands has been inferred by elegant clonal marking studies, and recent characterization of differentiation markers for the fundic and antral regions of the human and mouse gastric mucosa, as well as the development of ex vivo organoid cultures will facilitate the characterization of the mechanisms underlying gastric epithelial stem cell dynamics and lineage. The leucine-rich repeat-containing, G-protein-coupled receptor 5 (Lgr5) has been established as a gastric epithelial stem cell marker in the gastric antrum. Lgr5 was expressed at the base of prospective corpus and antral glands, whereas expression in the adult was predominantly restricted to the base of mature antral glands. Lineage tracing revealed these Lgr5-positive cells to be self-renewing, multipotent stem cells responsible for the long-term renewal of the antral epithelium. Single Lgr5-positive cells efficiently generated long-lived organoids resembling mature antral glands in ex vivo culture. In addition, Lgr5 together with another stem cell marker, CD44, are expressed at high levels in proliferating mouse gastric organoids that are enriched for a stem cell-like niche for fundic stem cells. When these proliferating organoids are cocultured with immortalized stomach mesenchymal cells, they develop into organoids that contain all of the major cell types of the in vivo gastric gland: surface epithelial, mucous (neck), stem, parietal, chief, ECL, and other endocrine cells. Such organoids can be then used as an in vitro system to study gastric physiological function, such as acid secretion, gastric disease, epithelial cell biology, and tissue repair and regeneration.
In an exciting development for the field of human gastric epithelial cell physiology, human pluripotent stem cells have been differentiated from early-stage three-dimensional gastric posterior foregut spheroids into either fundic or antral mucosal gastric organoids in a Wnt/beta-catenin-dependent fashion. The differentiation of these spheroids into fundic organoids was dependent upon the activation of the Wnt/beta-catenin pathway, and disruption of this signaling pathway resulted in the differentiation of antral organoids. On the basis of the expression of markers of cellular differentiation, both types of organoids contained surface mucous cells, mucous neck cells, and endocrine cells for ghrelin, somatostatin, and serotonin. Gastrin-secreting endocrine cells, as well as the transcription factor Pdx1, were expressed exclusively in antral organoids, while histamine-secreting endocrine cells, parietal cells, and chief cells were expressed exclusively in fundic organoids. The transcription factors Irx2, Irx3, and Irx5 were highly expressed in the differentiating fundic organoids compared to antral organoids. In addition, only the fundic organoids displayed glandular budding morphogenesis. Thus, this system represents a potentially powerful in vitro model for the characterization of the development and physiology of the human fundus, as well as to be able to interrogate human diseases that are not easily recapitulated in animal models, such as H. pylori infection. Finally, human organoids are becoming increasingly useful platforms for drug discovery, development, and screening.
The parietal cell has several distinctive morphologic features that are fundamentally significant to function. The cell is generally pyramidal in shape with tight junctions surrounding the apex of the pyramid. In the resting, or non-secreting, cell, there is a limited area of apical membrane in direct apposition to the gland lumen, but more extensive luminal contact is provided by distinctive invaginations of apical plasma membrane deep into the cytoplasm, forming elaborate secretory canaliculi characteristic of parietal cells ( Fig. 38.2 A). The entire apical surface, including the canaliculi, is lined by short, stubby microvilli in the non-secreting state. A cross section shows that the parietal cell cytoplasm abounds with tubular and vesicular membrane profiles, especially prominent in the apical pole, the system of so-called tubulovesicles (TVs). Three-dimensional reconstruction indicates that many of the TVs are, in fact, flattened and more cisternal in shape. The gastric proton pump responsible for gastric acid secretion is the H,K-ATPase, clearly the most abundant protein in this enormous pool of tubulovesicular membranes. Numerous large mitochondria occupying 30%–40% of the cellular mass are also characteristic of the parietal cell, consistent with the energy requirements and high oxidative capacity of these cells. The most remarkable aspect of parietal cell morphology is its ability to undergo massive morphologic rearrangement in response to the stimulation. Although limited by the optics of light microscopy, careful histologic examination allowed Golgi to illustrate the enlargement of canaliculi of secreting parietal cells in the late nineteenth century. Modern electron microscopic analyses have shown that within a few minutes of adding stimulants there are obvious changes in the apical plasma membrane lining the canaliculi. Parietal cell microvilli grow longer and more elaborate with a concomitant disappearance of TVs from the cytoplasm. The process of apical membrane expansion continues until the steady state of secretory activity is achieved ( Fig. 38.2 B). The maximally stimulated parietal cell has an apical membrane surface area that is 5–10 times that of the resting cell. Morphometric studies show that membrane surface area is conserved; the large apical plasma membrane expansion is quantitatively matched by the disappearance and decrease in the surface area of cytoplasmic TVs. Parietal cells retain this “secreting” morphology and sustain HCl secretion as long as the stimulus is applied and metabolic competence is maintained. Fig. 38.3 depicts the general morphologic change in going from rest to maximal secretion, and returning to the resting state. Removal of the secretagogue leads to a dramatic remodeling of the canalicular spaces, followed by a complex process of reincorporation of the apical surface, and therefore H,K-ATPase, back into tubulovesicular forms of the resting cell. The structural reorganization of the recovered cell has been documented; however, little is known about the forces and precise mechanisms that operate this massive recovery. The recent development of super-resolution fluorescence microscopic analyses, such as photoactivation localization microscopy and structured illumination microscopy, or even improvements with image-processing capabilities in confocal microscopy, combined with cell-type-specific genetic manipulation will allow illustration of the molecular dynamics underlying this massive membrane recovery process.
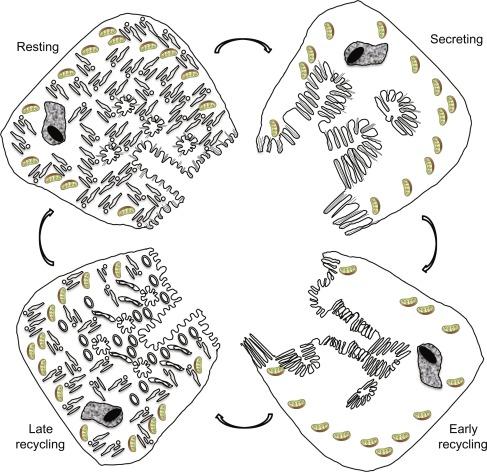
It has been proposed that the membrane-recycling hypothesis accounts for the mechanistic basis for the observed morphologic transformations to the functional output of hydrochloric acid in gastric parietal cells. In non-secreting cells, the H,K-ATPase is retained in a quiescent state in cytoplasmic TVs. This has been shown in biochemical analysis of isolated membranes and in intact cells by immunodetection of H,K-ATPase. Stimulation elicits a series of intracellular signaling events that orchestrate the translocation of TVs through a protein machinery involving TV docking, priming, and fusion, ultimately expanding the apical canalicular surface with H,K-ATPase-rich membrane and resulting in the secretion of voluminous, isotonic HCl. This chapter examines the experimental evidence that led to the proposition of this membrane-recycling model and recent advancements that illustrate the molecular details of the membrane-recycling model.
The biochemical nature of the gastric proton pump was first identified as unique K + -stimulated ATPase and K + -stimulated phosphatase activities in microsomal vesicles that were purified from homogenates of gastric mucosa. The microsomal vesicles are, in fact, parietal cell TVs purified on the basis of size and density. In microsomal vesicles from a non-secreting stomach, K + -stimulated ATPase activity also requires K + ionophores for maximal stimulation, and it was concluded that the isolated TVs have limited K + permeability and an internal K + activating site. Thus, the K + -stimulated ATPase was linked to HCl secretion on the basis of its location in the acid-secreting region of all vertebrate stomachs and its coincident expression with the functional onset of HCl secretion in early development.
The use of pH electrode and pH-sensitive fluorescent dye techniques linked the K + -stimulated ATPase activity of gastric microsomal vesicles to vectorial H + and K + transport with H + pumped into the vesicles in exchange for K + transport out. The pump was shown to be an electroneutral H + for K + exchange and became known as the H,K-ATPase. The pump-leak model as depicted in Fig. 38.4 A indicates that ATPase-dependent accumulation of H + by the vesicle critically depends on the relative permeabilities of the three principal ions: K + , H + , and Cl − . The interrelations between the pumps and leaks of ions could be observed experimentally and predicted mathematically from Nernst-Planck ion flux predictions and Michaelis-Menten enzyme kinetics. Thus, the H,K-ATPase is the source of gastric H + , operating as a cation exchange ATPase, using the hydrolysis of ATP to transport H + in exchange for K + , with a stoichiometry of 1H + :1 K + :1 ATP under physiological conditions.
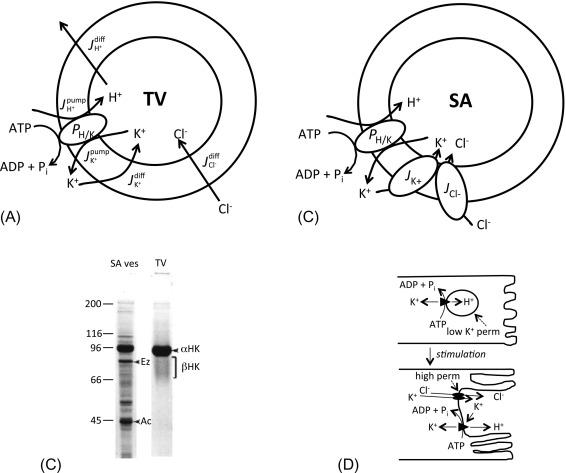
The pump-leak model carefully describes the interrelations among ATP utilization, proton pumping, and ionic dependencies in the isolated TVs, but there was an important factor missing for operating the pump in a functional parietal cell. The TV experiments required an exogenous K + ionophore, for example, valinomycin. What was the equivalent mechanism in vivo? The answer came from experiments contrasting ATPase and pump activities in vesicles derived from resting and actively secreting stomachs. The first surprise was that H,K-ATPase activity disappeared from the small TV fraction and transferred almost quantitatively to larger, denser membrane vesicles, which are called stimulation-associated (SA) vesicles. More importantly, the SA vesicles pump protons with almost all of the same characteristics as resting TVs, except that they operate independent of a K + ionophore. In fact, the SA vesicles possess relatively selective ion channels: both K + channels and Cl − channels ( Fig. 38.4 B). Comparison of the protein patterns of SA vesicles with TVs demonstrates some differences in composition (see Fig. 38.4 C). Both fractions are enriched in H,K-ATPase, but several additional protein bands are clearly obvious in SA vesicles, including prominent bands for actin and ezrin (apical cytoskeletal proteins discussed below), and several other proteins. These data clearly indicate that SA vesicles are derived from the apical plasma membrane of stimulated parietal cells: they are much larger and more structurally complex than TVs, they contain actin microfilaments and other apical cytoskeletal elements, and they possess K + and Cl − channels consistent with apical membranes. These observations thus provide the basis for pump operation in vivo, as well as the means to link the enzymatic and transport data with the ultrastructural changes associated with secretory activation. A current model of parietal cell activation and HCl secretion is shown in Fig. 38.4 D, placing H,K-ATPase in the context of the membrane-recycling hypothesis. In the resting cell, the cytoplasmic compartment is full of TVs that are rich in H,K-ATPase, but are functionally inactive because of low K + (and Cl − ) permeability. Secretagogue stimulation of the cell leads to recruitment of TVs to the apical membrane providing the source for membrane expansion and placing the pump in a functionally relevant position. K + and Cl − channels in parallel with the proton pump provide the necessary pathways for ionic transfer, with K + recycling through channel and pump as net H + and Cl − are secreted together with complementary osmotic flow of water. Removal of the secretagogue leads to re-sequestration of the membrane and pumps back into the cytoplasmic compartment and with return to the resting state. Exactly how the apical channels are activated and recruited to the apical membrane with stimulation is the subject of much current research, and more detailed discussion about the regulation of these channels is provided later in this chapter.
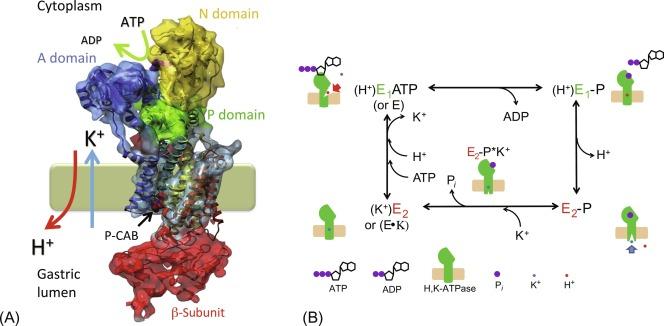
The gastric H,K-ATPase belongs to the P-type family of transport ATPases, which also includes the ubiquitous plasma membrane Na,K-ATPase and the Ca-ATPase. There are also H,K-ATPase isoforms other than gastric, including those performing H + and K + homeostasis in mammalian renal tubules and colon, and amphibian urinary bladder. In addition to a great deal of structural homology, the family of P-type cation transport ATPases shares the characteristic that their transport cycle depends on phosphorylation, dephosphorylation, and defined phosphoenzyme intermediates, thus designated P-type. P-type ATPases also exhibit two phenomenologically and structurally discrete conformations, designated E 1 and E 2 , which have distinct kinetic variables, such as affinities for substrates. Thus, P-type ATPases are also called E 1 –E 2 ATPases. The overall catalytic and transport cycle for the H,K-ATPase is made up of a series of partial reactions, as shown schematically in Fig. 38.5 . The initial kinase activity is an autophosphorylation step in which the γ-phosphate of ATP is transferred to the aspartate residue D387 (in the P domain discussed below), forming an acyl-phosphate intermediate, E-P. The phosphoenzyme has two conformer states, and their transition involves the release of a proton. One conformation (denoted by E 1 -P in Fig. 38.5 B) has high reactivity with ADP (and low reactivity with K + ), and thus can easily reverse itself; it comprises what is known as the ATP-ADP exchange reaction. Transition from E 1 -P to the second conformation, E 2 -P, is essentially an irreversible step involving the release of a proton to the luminal surface, which is important for the prevention of the reverse reaction of the transport cycle and proton back flow into cytosol. Subsequently, binding of luminal K + to the phosphozyme participates in formation of E 2 -P*K + and promotes another important enzymatic function, the K + -catalyzed dephosphorylation, or phosphatase activity. By this reaction, inorganic phosphate is liberated from the enzyme, which can then resume the phosphorylation cycle once K + is released to the cytoplasmic surface, K + in . The scheme in Fig. 38.5 B indicates that ATP can bind with either the free enzyme (E) or the K + -bound enzyme (E · K). The association of K + is not a simple, rapidly reversible, ionic bond, but rather is described as an occluded form, similar to that described for Na,K-ATPase. It turns out that the affinity of ATP for free E 1 is much higher than for E 2 · K. Thus, one of the rate-limiting steps in H,K-ATPase turnover is the rate of K + release from E 2 · K which, interestingly, is promoted by a high ATP concentration. Because the enzyme is embedded in a membrane with distinct sidedness for specific binding sites, the H,K-ATPase cycle provides vectorial transport of ions. As the concentration of K + in the cell is relatively high, ATP concentration in the millimolar range is necessary for the rapid conversion of E 2 · K to E 1 · ATP and high pump turnover. This is the plausible reason for the high sensitivity of H + secretion to a compromised metabolism or gastric blood flow.
The H,K-ATPase is made up of two transmembrane subunit peptides designated α- and β-subunits. The available data suggest that the functionally assembled H,K-ATPase is an oligomer of two closely associated (αβ) 2 heterodimers. The 110 kDa α-subunit contains the catalytically active sites such as the E-P phosphorylation site, and 10 membrane-spanning segments, in which amino acids involved in ion binding are located in the M4, M5, M6, and M8 transmembrane segments. The α-subunit has an estimated 73% of its mass residing on the cytoplasmic side of the membrane. The 32 kDa β-subunit has only a single transmembrane segment with more than 80% of its peptide mass, which also includes six or seven sites of N-linked glycosylation, residing on the extracellular side of the membrane. Although the β-subunit does not directly participate in ATP utilization, it is important for stability and “protection” of the holoenzyme. For example, precise folding of the enzyme during synthesis and targeting to the appropriate membrane compartment have been attributed to the β-subunit. Moreover, it has been proposed that the β-subunit may help to provide an answer to the age-old question of why the stomach does not digest itself. A combination of extensive, unique, β-subunit glycosylation and bonding forces within the extracellular domain of the enzyme are proposed to offer protection from the acidic and proteolytic conditions on the luminal aspect of the cell. The short cytoplasmic tail of the β-subunit with ~ 32 amino acid residues contains a tyrosine-based signaling sequence (FRHY) that appears to be important for membrane retrieval and recycling under secretagogue control. The cytoplasmic tail of the β-subunit also interacts with the α-subunit, and seems to prevent the E 2 P conformation as mentioned in Section 38.2.1.6 .
In the highly purified TV fraction, H,K-ATPase is the overwhelmingly predominant protein. The α-subunit is seen as an intensely stained and focused band at about 95 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), whereas the heavily glycosylated β-subunit runs as a poorly stained broad band extending from 60 to 80 kDa ( Fig. 38.4 C). When oligosaccharides are removed, the deglycosylated β-subunit is nicely focused at its core peptide size of 32 kDa.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here