Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The human adrenal glands, each weighing only ~4 g, are located above the upper pole of each kidney in the retroperitoneal space. They produce four principal hormones: cortisol, aldosterone, epinephrine (adrenaline), and norepinephrine. Each adrenal gland is composed of an inner medulla and an outer cortex ( Fig. 50-1 ). Embryologically, the cortex is derived from mesoderm, whereas the medulla is derived from neural crest cells (see p. 261 ) that migrate into the developing cortex. The cortex produces two principal steroid hormones, cortisol and aldosterone, as well as several androgenic steroids. The medulla produces epinephrine and norepinephrine.
The adrenal cortex can be further divided into three cellular layers: the glomerulosa layer near the surface, the fasciculata layer in the midcortex, and the reticularis layer near the cortical-medullary junction. Aldosterone, the main mineralocorticoid in humans, is made in the glomerulosa cell layer. Cortisol, the principal glucocorticoid, is made in the fasciculata and to a small extent in the reticularis layer. The adrenal androgens —dehydroepiandrosterone (DHEA) and its sulfated form DHEAS—are made in the reticularis layer. Although both cortisol and aldosterone are made by enzymatic modification of cholesterol and are structurally quite similar, their actions on the body differ dramatically. Cortisol is considered a glucocorticoid because it was recognized early on to increase plasma glucose levels; deficiency of cortisol can result in hypoglycemia. Aldosterone is considered a mineralocorticoid because it promotes salt and water retention by the kidney. The activities of these two hormones overlap, particularly at high hormone levels, but this distinction is still very useful in identifying their most obvious functions. DHEA and DHEAS are weak androgens (compared to testosterone or dihydrotestosterone) and little is known about the regulation of their secretion. Plasma DHEA concentrations follow a diurnal pattern like that of cortisol. DHEAS circulates at much higher concentrations and shows no diurnal fluctuation.
In the adrenal medulla, chromaffin cells produce epinephrine (or adrenaline), a catecholamine that is synthesized from the amino acid tyrosine. Although the primary product of the medulla is epinephrine, it also produces variable amounts of the epinephrine precursor norepinephrine. These catecholamines are distinct from the steroid hormones both structurally and functionally.
Steroid hormones are divided into three major classes based on their actions: glucocorticoids, mineralocorticoids, and sex steroids. Cortisol is the prototypical naturally occurring glucocorticoid. The ability of cortisol to increase plasma [glucose] largely results from its ability to enhance mobilization of amino acids from proteins in many tissues and to enhance the ability of the liver to convert these amino acids into glucose and glycogen by activating gluconeogenesis.
The structures of cortisol and aldosterone ( Fig. 50-2 ) differ only slightly: aldosterone lacks the –OH group at position 17 and has an aldehyde (aldo) group at position 18. Despite the seemingly minor chemical difference, aldosterone at physiological concentrations has virtually no glucocorticoid activity.
Although classified as a glucocorticoid, cortisol affects more than the principal glucose-regulatory tissues, namely, the liver, fat, and muscle. Most body tissues, including bone, skin, other viscera, hematopoietic and lymphoid tissue, and the central nervous system (CNS), are target sites for glucocorticoid action. Although cortisol is the primary glucocorticoid in humans, in other species (e.g., the rat), corticosterone is the major glucocorticoid.
Glucocorticoids have numerous actions other than their ability to raise plasma glucose levels. These actions are described below and include potent immunosuppressive and anti-inflammatory activity, effects on protein and fat metabolism, behavioral effects due to actions on the CNS, and important effects on calcium and bone metabolism. Some of the diverse physiological effects of the glucocorticoids can be appreciated from clinical studies of excess glucocorticoid secretion (Cushing syndrome; Box 50-1 ) and glucocorticoid deficiency (Addison disease; see Box 50-1 ). The multiple actions of glucocorticoids, in particular, their “anti-inflammatory” action on leukocytes, has led to the development of numerous synthetic analogs that are more potent, have a longer half-life, and are more selective in their specific glucocorticoid actions than are either cortisol or corticosterone. Table 50-1 lists some of these compounds and indicates their relative potency as mineralocorticoids and glucocorticoids.
Glucocorticoid excess is most commonly seen clinically in individuals receiving glucocorticoids for treatment of a chronic inflammatory or neoplastic disorder. Less commonly, individuals overproduce cortisol either because of a primary cortisol-producing adrenal tumor or secondary to a pituitary tumor that produces ACTH, which in turn stimulates excess cortisol production by normal adrenal glands. In either case, the cortisol excess causes a constellation of symptoms and signs including adiposity of the trunk, neck, and facies; hypertension; loss of subcutaneous adipose and connective tissue in the extremities with associated easy bruising; loss of bone mineral; muscle weakness and wasting; and hyperglycemia. This constellation is referred to as Cushing syndrome after the famous American neurosurgeon who characterized this disorder. The specific therapy is based upon identifying whether the clinical picture arises from a tumor in the adrenal or in the pituitary gland, and then removing the culprit. When the pituitary gland is responsible, the disorder is referred to as Cushing disease. In the case of patients receiving glucocorticoid therapy, the signs and symptoms of Cushing syndrome are carefully monitored, and efforts are made to minimize these side effects. Unfortunately, all glucocorticoid drugs with anti-inflammatory actions also produce these other effects.
Glucocorticoid deficiency—which occurs in primary adrenal insufficiency, also called Addison disease, and affects both glucocorticoid and mineralocorticoid levels—can produce an array of symptoms and signs. Although tuberculosis was once a common cause of primary adrenal insufficiency, today autoimmune adrenal disease is the most common cause. Failure of adrenal cortical hormone secretion leads to increases in circulating concentrations of ACTH as well as other products of POMC (see p. 1023 ). Two of these products (α-MSH and γ-MSH) as well as ACTH (see p. 1023 ) cause skin hyperpigmentation. The lack of glucocorticoid predisposes to hypoglycemia. The combined absence of glucocorticoid and mineralocorticoid leads to hypotension and hyponatremia, ![]() N50-1 whereas aldosterone deficiency leads to hyperkalemia. Before the development of glucocorticoid and mineralocorticoid therapy, this disorder was uniformly fatal.
N50-1 whereas aldosterone deficiency leads to hyperkalemia. Before the development of glucocorticoid and mineralocorticoid therapy, this disorder was uniformly fatal.
| COMPOUND | GLUCOCORTICOID EFFECT | MINERALOCORTICOID EFFECT |
|---|---|---|
| Cortisol | 1 | 1.5 |
| Prednisone | 3–4 | 0.5 |
| Methylprednisone | 10 | 0.5 |
| Dexamethasone | 20 | 1 |
| Fludrocortisone | 12 | 125 |
* Relative potency is determined by a combined consideration of the compound's biological half-life and its affinity for the glucocorticoid or mineralocorticoid receptor.
Whereas aldosterone deficiency causes renal salt wasting, hypovolemia and hypotension do not directly cause hyponatremia (i.e., a low plasma [Na + ]). Rather, reduced effective circulating volume triggers the release of AVP (see pp. 846–847 ); AVP in turn provokes thirst and H 2 O retention in the collecting ducts, which dilutes plasma Na + and creates hyponatremia.
Most of the well-characterized actions of glucocorticoids result from their genomic actions to influence (either positively or negatively) the transcription of a variety of genes through glucocorticoid response elements (see p. 986 ). However, glucocorticoids also exert nongenomic actions (see p. 989 ) that occur promptly (0 to 3 hours) and are not inhibited by blockade of gene transcription.
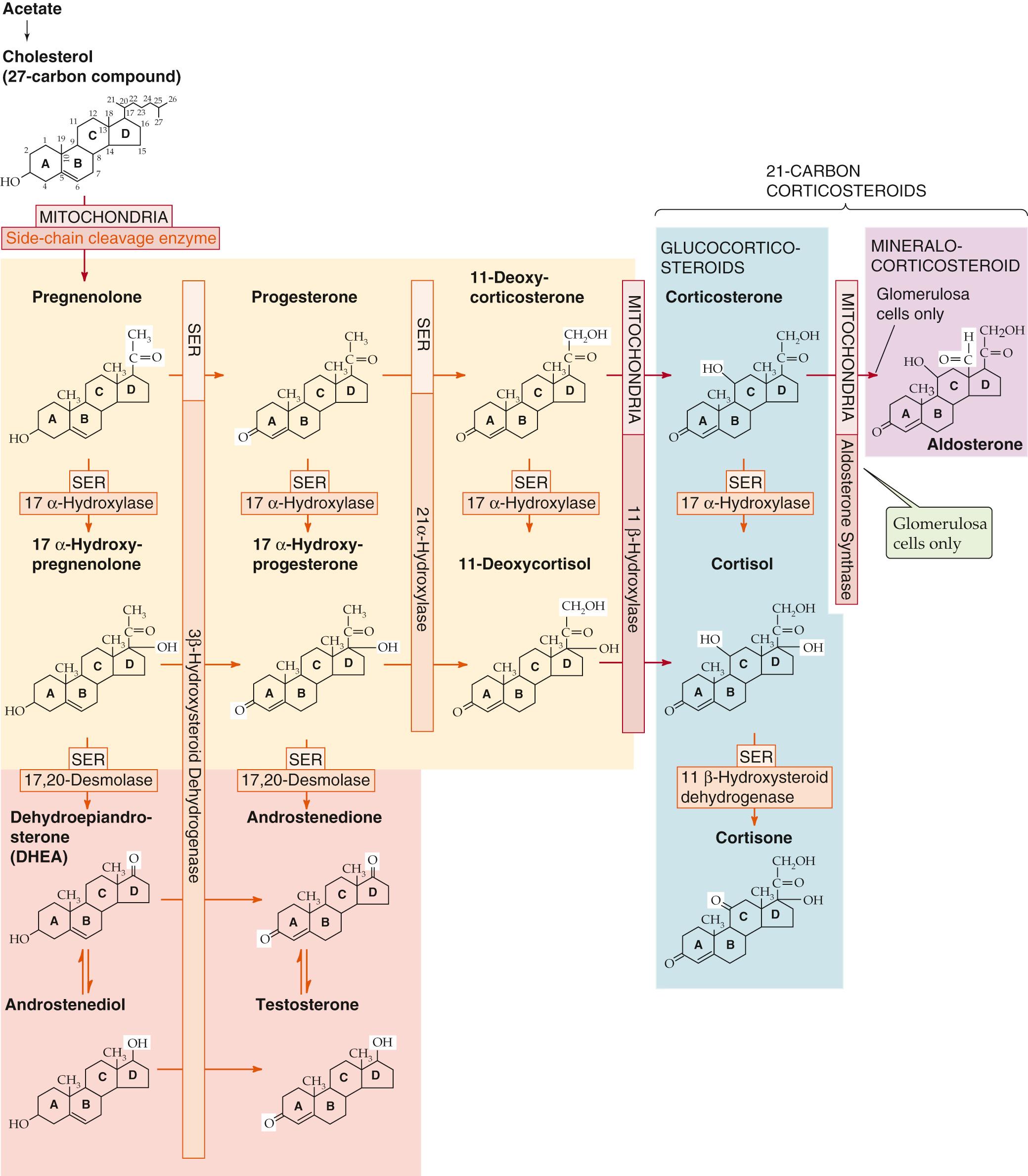
Synthesis of cortisol, as for all steroid hormones, starts with cholesterol (see Fig. 50-2 ). Like other cells producing steroid hormones, the adrenal gland has two sources of cholesterol (see p. 985 ): (1) it can import cholesterol from circulating cholesterol-containing low-density lipoprotein (LDL) cholesterol by means of LDL receptor–mediated endocytosis (see p. 42 ), or (2) it can synthesize cholesterol de novo from acetate (see Fig. 46-16 ). Although both pathways provide the steroid nucleus needed for cortisol and aldosterone synthesis, circulating LDL is quantitatively more important.
In the adrenal gland, cholesterol is metabolized through a series of five reactions to make either cortisol or aldosterone. All relevant enzymes are located in either the mitochondria or smooth endoplasmic reticulum (SER), and except for 3β-hydroxysteroid dehydrogenase (3β-HSD), belong to the family of cytochrome P-450 oxidases ( Table 50-2 ).
The pathway for cortisol and aldosterone synthesis begins in the mitochondria, where the cytochrome P-450 side-chain-cleavage (SCC) enzyme (also called 20,22-desmolase or P-450 SCC ) removes the long side chain (carbons 22 to 27) from the carbon at position 20 of the cholesterol molecule (27 carbon atoms). This enzyme, or the supply of substrate to it, appears to be the rate-limiting step for the overall process of steroid hormone synthesis.
The product of the SCC-catalyzed reaction is pregnenolone (21 carbon atoms), which exits the mitochondrion. The SER enzyme 3β-HSD ( not a P-450 enzyme) oxidizes the hydroxyl group at position 3 of the A ring to a ketone to form progesterone.
A P-450 enzyme in the SER, 17α-hydroxylase (P-450 c17 ), then adds a hydroxyl group at position 17 to form 17α-hydroxyprogesterone. However, as shown in Figure 50-2 , an alternative path to 17α-hydroxyprogesterone exists: the 17α-hydroxylase might first add a hydroxyl group at position 17 of pregnenolone and form 17α-hydroxypregnenolone, which the aforementioned 3β-HSD can then convert to 17α-hydroxyprogesterone.
In the SER, 21α-hydroxylase (P-450 c21 ) adds a hydroxyl at carbon 21 to produce 11-deoxycortisol.
In the mitochondria, 11β-hydroxylase (P-450 c11 ) adds yet another hydroxyl, this time at position 11, to produce cortisol.
| ENZYME | SYNONYM | GENE |
|---|---|---|
| Cholesterol side-chain cleavage | P-450 SCC | CYP11A1 |
| 11β-hydroxylase | P-450 c11 | CYP11B1 |
| 17α-hydroxylase | P-450 c17 | CYP17 |
| 17,20-desmolase | P-450 c17 | CYP17 |
| 21α-hydroxylase | P-450 c21 | CYP21A2 |
| Aldosterone synthase | P-450 aldo | CYP11B2 |
| Aromatase * | P-450 arom | CYP19 |
* P-450 arom catalyzes a reaction essential for the production of estrogens (see p. 1117 ).
The enzymes represented by the vertical bars in Figure 50-2 , as well as SCC, are present in all three cellular layers of the adrenal cortex. However, 17α-hydroxylase is not substantially present in the glomerulosa layer. Thus, the fasciculata and, to a much lesser extent, the reticularis layers synthesize cortisol.
The cells of the reticularis layers are principally responsible for androgen synthesis. These cells convert 17α-hydroxypregnenolone and 17α-hydroxyprogesterone into the adrenal androgens dehydroepiandrosterone and androstenedione. The enzyme that catalyzes this reaction is called 17,20-desmolase; however, it turns out to be the same SER enzyme as the 17α-hydroxylase that produced the 17α-hydroxypregnenolone and 17α-hydroxyprogesterone in the first place. The androgens formed by the adrenal are far less potent than either testosterone or dihydrotestosterone. However, other tissues (e.g., liver, kidney, adipose) can use 17β-hydroxysteroid dehydrogenase to convert androstenedione to testosterone (see p. 1097 ). In this manner, the adrenal can contribute significant amounts of circulating androgen, particularly in women. Increases in adrenal androgen production precede by 1 to 2 years the increases in gonadal steroid production that occur with puberty. This androgen production promotes growth of pubic and axillary hair and is referred to as adrenarche (see pp. 1088–1090 ).
The cortisol synthesized by the adrenal cortex diffuses out of the cells and into the blood plasma. There, ~90% of the cortisol is transported bound to corticosteroid-binding globulin (CBG), also known as transcortin, which is made in the liver. Transcortin is a 383–amino-acid glycoprotein whose affinity for cortisol is ~30-fold higher than that for aldosterone. An additional ~7% of the circulating cortisol is bound to albumin. Thus, only 3% to 4% of the circulating cortisol is free.
The clearance of cortisol from the body depends principally on the liver and kidney. An early step is the formation of an inactive metabolite, cortisone, by the action of either of two 11β - hydroxysteroid dehydrogenases (11β-HSDs). 11β-HSD1 is highly expressed in certain glucocorticoid target tissues, including liver and both subcutaneous and visceral adipose tissue. The enzymatic reaction is reversible. Indeed, when glucocorticoids were first developed as pharmaceutical agents, it was cortisone that was used to treat patients with a variety of inflammatory disorders (e.g., rheumatoid arthritis). For some time, investigators thought that cortisone was the active principle. Only later did it become apparent that the body must convert cortisone to cortisol, which is the biologically active agent. Because excess cortisol produces insulin resistance and many features of metabolic syndrome (e.g., glucose intolerance, hypertension, dyslipidemia; see Box 51-5 )—and 11β-HSD1 is expressed abundantly in adipose tissue—an interesting hypothesis is that increased 11β-HSD1 activity in adipose tissue locally produces cortisol and thus promotes the development of insulin resistance.
The second 11β-HSD isozyme (11β-HSD2) is expressed in the adrenal cortex (see Fig. 50-2 ; Box 50-2 ), although the adrenal gland does not normally make a significant contribution to the formation of cortisone. 11β-HSD2 is highly expressed in the renal distal tubule and collecting duct (see Fig. 35-13 C ), where it catalyzes an essentially irreversible conversion of cortisol to cortisone. This breakdown of cortisol allows aldosterone to regulate the relatively nonspecific mineralocorticoid receptor (MR) without interference from cortisol.
Mutations can affect one or more of the enzyme steps in steroid hormone synthesis and can produce unique clinical syndromes that are a direct result of failure to manufacture a particular hormone, accumulation of excessive amounts of precursor steroids, or both. The most common of these enzymatic disorders is 21α-hydroxylase deficiency. From Figure 50-2 we would predict that deficiency of this enzyme would lead to inadequate production of both glucocorticoid and mineralocorticoid hormones, which is indeed what occurs. Affected infants are ill with symptoms of “salt losing” (hypotension, dehydration) and glucocorticoid deficiency (hypoglycemia). The natural reaction of the body is to attempt to overcome this deficiency by increasing the secretion of ACTH, which stimulates the synthesis of cortisol and aldosterone. ACTH also causes growth of the adrenal gland. However, if the mutant enzyme is totally inactive, no cortisol or aldosterone synthesis will occur, although all other enzymes of the pathway involved in glucocorticoid and mineralocorticoid synthesis will be expressed in increased amounts. The result is greater than normal activity of SCC, 3β-HSD, 17α-hydroxylase, and 11β-hydroxylase, and the net effect is increased synthesis of both precursor molecules and adrenal androgens. The combination of inadequate production of glucocorticoids and mineralocorticoids, excessive production of androgens, and enhanced growth of the adrenal gland is the classical clinical syndrome of salt-losing, virilizing congenital adrenal hyperplasia. In female infants, the presence of excessive adrenal androgen in utero results in ambiguous genitalia at birth, a condition that should alert the pediatrician to the potential diagnosis. No such clue occurs in the male infant, in whom dehydration, hypotension, and hyperkalemia are the major manifestations.
The multiple hydroxylation reactions that convert cholesterol to cortisol result in a hydrophilic compound that, unlike cholesterol, is soluble in plasma, yet lipophilic enough to cross the plasma membrane of target tissue without requiring a membrane transporter. Cortisol, like all steroid hormones, binds to intracellular receptors within target cells (see pp. 71–72 ). Virtually all nucleated tissues in the body contain receptors for glucocorticoids. The glucocorticoid receptor (GR) is primarily located in the cytoplasm, where in its unbound form it is complexed to a chaperone protein (i.e., the heat shock protein hsp90, among others; see Fig. 4-15 A ). Binding of cortisol causes the chaperone to dissociate from the GR and this allows the cortisol-GR complex to translocate to the nucleus. There, the cortisol-receptor complex associates with glucocorticoid response elements (GREs) on the 5′ untranslated region of multiple genes to either enhance or diminish gene expression (see p. 90 ).
GRs are structurally similar to the receptors for mineralocorticoids, sex steroids, vitamin D, vitamin A, and thyroid hormone. These receptors, either homodimers or heterodimers, belong to the superfamily of nuclear receptors that contains domains A through F (see Fig. 3-14 ). Activity of the glucocorticoid-receptor complex requires dimerization of two identical receptor complexes (i.e., the GR functions as a homodimer ) at the near-palindromic nucleotide site of the GRE on the chromatin. Glucocorticoids mainly act by modulating gene transcription. One exception is the acute feedback effect of cortisol to block the release of preformed adrenocorticotropic hormone (ACTH) in the secretory granules of pituitary corticotrophs. This glucocorticoid effect is demonstrable within seconds to minutes and may relate to an as-yet undefined effect of glucocorticoid on membrane trafficking.
Although glucocorticoids are named for their ability to increase hepatic glucose and glycogen synthesis, they affect many somatic tissues. In liver, cortisol induces the synthesis of enzymes involved in gluconeogenesis and amino-acid metabolism in support of gluconeogenesis, thus enhancing hepatic glucose production. In muscle, cortisol stimulates the breakdown of muscle protein, which releases amino acids for uptake by the liver. Similarly, cortisol promotes lipolysis in adipose tissue. The fatty acids thus released provide an alternative fuel to glucose, whereas the accompanying glycerol provides another substrate for gluconeogenesis, thereby increasing the availability of glucose. For unknown reasons, although fat is mobilized from the extremities, some is also deposited centrally (see description of moon facies in Box 50-3 ).
The variety of glucocorticoid actions on body tissues is well illustrated by considering some of the clinically observed effects of hypercortisolism in patients receiving glucocorticoid drugs. Most strikingly, the entire body habitus changes. Body fat redistributes from the extremities to the face and trunk, producing (1) increased supraclavicular and dorsal interscapular fat (buffalo hump), (2) a rounded abdomen, and (3) a rounding of the face called moon facies, caused by increased subcutaneous fat in the cheeks and submandibular region. Conversely, the wasting of fat (and some supporting tissues) in the extremities produces thinning of the skin and fragility of cutaneous blood vessels. In bone, glucocorticoids reduce mineral density (osteopenia), which can lead to osteoporosis and bone fractures. The interference with normal immune function increases both the frequency and severity of infections. Rare malignancies can develop. Wasting of muscle tissue leads to a generalized weakness that is usually most prominent in the proximal muscles of the lower extremities. Finally, as would be expected from a glucocorticoid, patients become insulin resistant and even glucose intolerant (see p. 1038 ) or frankly diabetic (see Box 51-5 ). When cortisol is overproduced endogenously (from tumors producing either ACTH or cortisol), hypertension is common. The hypertension most likely results from the weak mineralocorticoid action of cortisol. Exogenous synthetic glucocorticoid therapy rarely produces hypertension because most of these drugs lack the mineralocorticoid activity of the endogenous hormone.
Cortisol has effects unrelated to its glucocorticoid actions that lead to its extensive clinical use in disorders like vasculitis, arthritis, malignancies, and in prevention of organ transplant rejection. Glucocorticoids also act on the cellular elements of trabecular bone (see pp. 1068–1069 ), decreasing the ability of osteoblasts to synthesize new bone. They also interfere with absorption of Ca 2+ from the gastrointestinal tract. As a result, long-term glucocorticoid use causes osteoporosis. In addition, glucocorticoids act on the CNS and can cause a variety of effects, including alterations in mood and cognition.
As summarized in Figure 50-3 , regulation of the synthesis and secretion of cortisol begins with the release of corticotropin-releasing hormone (CRH) from hypothalamic neurons as part of either a normal daily circadian rhythm or a centrally driven stress response. CRH stimulates the release of ACTH, also called corticotropin, from the anterior pituitary. ACTH directly stimulates the adrenal fasciculata layers to synthesize and secrete cortisol. Circulating cortisol exerts negative-feedback control on the release of both ACTH and CRH.
![Figure 50-3, Hypothalamic-pituitary-adrenocortical axis. Small-bodied neurons in the paraventricular nucleus of the hypothalamus secrete CRH, a 41–amino-acid peptide that reaches the corticotrophs in the anterior pituitary via the long portal veins. CRH binds to a GPCR on the corticotroph membrane, triggering the adenylyl cyclase (AC)–cAMP–PKA pathway. The activation of L-type Ca 2+ channels results in an increase in [Ca 2+ ] i that rapidly leads to the release of preformed ACTH. CRH also increases gene transcription and synthesis of the ACTH precursor, POMC. After its release by corticotrophs, ACTH binds to melanocortin-2 receptors on the cell membranes in all three layers of the adrenal cortex. This receptor triggers the AC–cAMP–PKA pathway, rapidly enhancing the conversion of cholesterol to pregnenolone and more slowly increasing the synthesis of several proteins that are needed for cortisol synthesis. The cerebral cortex can stimulate the hypothalamic neurons to increase their secretion of CRH. Cortisol exerts negative feedback at the level of both the pituitary and hypothalamus. In addition, ACTH produced by the corticotrophs negatively feeds back on the hypothalamic neurons in a “short loop.” Figure 50-3, Hypothalamic-pituitary-adrenocortical axis. Small-bodied neurons in the paraventricular nucleus of the hypothalamus secrete CRH, a 41–amino-acid peptide that reaches the corticotrophs in the anterior pituitary via the long portal veins. CRH binds to a GPCR on the corticotroph membrane, triggering the adenylyl cyclase (AC)–cAMP–PKA pathway. The activation of L-type Ca 2+ channels results in an increase in [Ca 2+ ] i that rapidly leads to the release of preformed ACTH. CRH also increases gene transcription and synthesis of the ACTH precursor, POMC. After its release by corticotrophs, ACTH binds to melanocortin-2 receptors on the cell membranes in all three layers of the adrenal cortex. This receptor triggers the AC–cAMP–PKA pathway, rapidly enhancing the conversion of cholesterol to pregnenolone and more slowly increasing the synthesis of several proteins that are needed for cortisol synthesis. The cerebral cortex can stimulate the hypothalamic neurons to increase their secretion of CRH. Cortisol exerts negative feedback at the level of both the pituitary and hypothalamus. In addition, ACTH produced by the corticotrophs negatively feeds back on the hypothalamic neurons in a “short loop.”](https://storage.googleapis.com/dl.dentistrykey.com/clinical/TheAdrenalGland/1_3s20B9781455743773000501.jpg)
Small-bodied neurons in the paraventricular nucleus of the hypothalamus (see Fig. 47-3 ) secrete CRH, a 41–amino-acid neuropeptide. The structure of CRH is highly conserved among species. In humans, CRH is also present in several tissues, including the pancreas and testes, as well as throughout the CNS, where it serves as a neurotransmitter. The hypothalamic neurons synthesize and release CRH via the classic secretory pathway (see pp. 34–35 ). Neurons store CRH in secretory vesicles located in synaptic terminals in the median eminence of the hypothalamus and can release CRH acutely in the absence of new synthesis. After release into the interstitial fluid of the median eminence, CRH enters the hypophyseal portal venous plexus (see p. 978 ) and travels to the anterior pituitary.
CRH arriving in the anterior pituitary binds to CRH-R1, a G protein–coupled receptor (GPCR) on the cell membrane of corticotroph cells. Hormone binding activates Gα s , which in turn stimulates adenylyl cyclase and raises [cAMP] i (see p. 53 ). Subsequent stimulation of protein kinase A (PKA) activates L-type Ca 2+ channels, leading to an increase in [Ca 2+ ] i , which stimulates the exocytosis of preformed ACTH. Over a much longer time, CRH receptor activation also leads to increased gene transcription and synthesis of the ACTH precursor (discussed later).
Although CRH is the major regulator of ACTH secretion, the paraventricular nuclei also make another hormone, arginine vasopressin (AVP; see Fig. 40-8 ). AVP is also a potent ACTH secretagogue and probably plays a physiological role in the regulation of ACTH secretion during stresses like dehydration or trauma.
A 39–amino-acid peptide hormone secreted by the corticotroph cells of the anterior pituitary, ACTH can also be produced by ectopic sources, particularly by small-cell carcinomas of the lung. Pituitary corticotrophs synthesize ACTH by complex post-translational processing of a large precursor protein (i.e., a preprohormone) called pro-opiomelanocortin (POMC). POMC is the precursor not only for ACTH but also for a variety of peptide hormones ( Fig. 50-4 ). In the anterior pituitary, POMC yields a long N-terminal peptide, a joining (J) peptide, ACTH, and β-lipotropin (β-LPH). During fetal life and pregnancy, the intermediate pituitary lobe—a small wedge of tissue between the more familiar anterior and posterior lobes—processes the same POMC in a very different manner to yield a different array of peptides: a short N-terminal peptide, γ-melanocyte–stimulating hormone (γ-MSH), J peptide, α-MSH, corticotropin-like intermediate-lobe peptide (CLIP), γ-LPH, and β endorphin. Other cells—such as the appetite-controlling POMC neurons in the hypothalamus (see p. 1002 )—can also synthesize POMC.
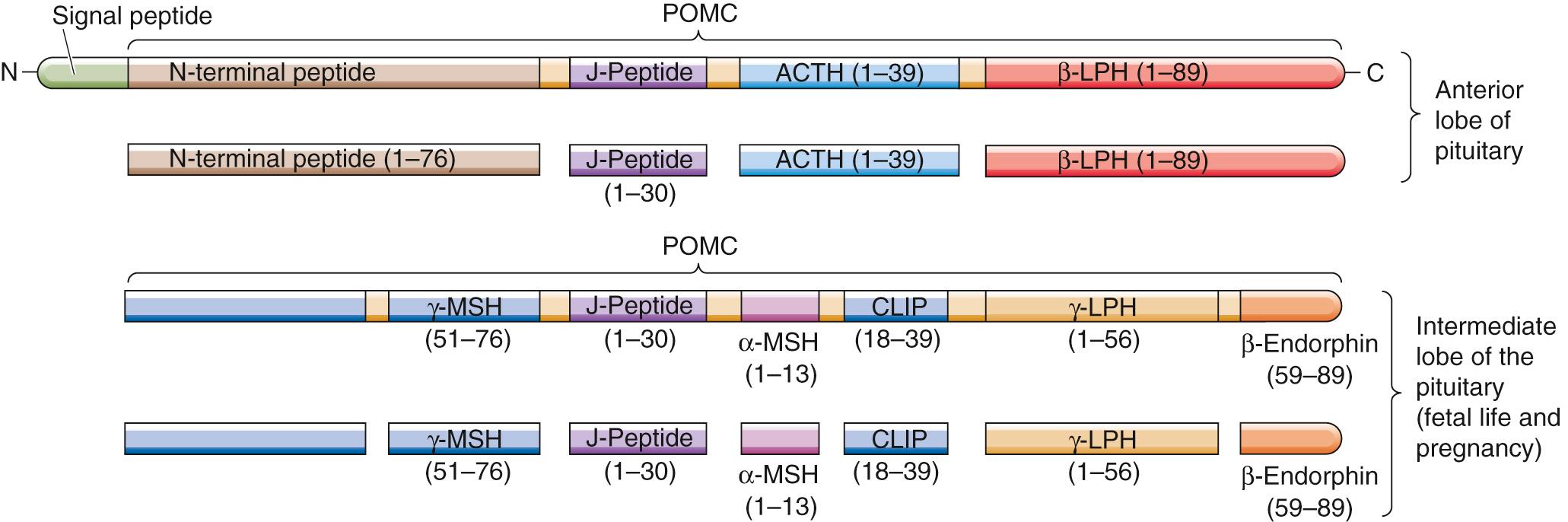
The melanocortins include ACTH as well as α-, β-, and γ-MSH and bind to a family of five GPCRs, the melanocortin receptors (MC1R to MC5R). α-MSH, γ-MSH, and ACTH act on MC1R receptors in melanocytes to increase the dispersion of pigment granules. In some patients who greatly overproduce ACTH, hyperpigmentation is a prominent clinical finding. Whether this hyperpigmentation is the result of increased production of MSH, increased production of β-LPH (which also has MSH activity), or the melanotropic action of ACTH per se remains uncertain. β-LPH and γ-LPH mobilize lipids from adipocytes in animals, although their physiological role in humans is unclear. β endorphin has potent opioid actions in the CNS (see p. 315 ), but its physiological actions (if any) in the systemic circulation are not known.
In the adrenal cortex, ACTH binds to MC2R on the plasma membranes of all three steroid-secreting cell types. However, because only the cells in the fasciculata and reticularis layers have the 17α-hydroxylase needed for synthesizing cortisol (see Fig. 50-2 ), these cells are the only ones that secrete cortisol in response to ACTH. ACTH appears to have few other actions at physiological concentrations. MC2R is coupled to a heterotrimeric G protein and stimulates adenylyl cyclase (see p. 53 ). The resulting increase in [cAMP] i activates PKA, which phosphorylates a variety of proteins. A rapid effect of ACTH is to stimulate the rate-limiting step in cortisol formation; that is, the conversion of cholesterol to pregnenolone via the SCC enzyme. In addition, ACTH—over a longer time frame—increases the synthesis of several proteins needed for cortisol synthesis: (1) each of the P-450 enzymes involved in cortisol synthesis (see Fig. 50-2 ), (2) the LDL receptor required for the uptake of cholesterol from blood (see p. 42 ), and (3) the 3-hydroxy-3-methylglutaryl–coenzyme A (HMG-CoA) reductase that is the rate-limiting enzyme for cholesterol synthesis by the adrenal (see p. 968 ).
Thus, ACTH promotes the acute synthesis of cortisol—and, as discussed later, aldosterone to a lesser extent—by the adrenal and increases the content of adrenal enzymes involved in steroidogenesis. In the absence of pituitary ACTH, the fasciculata and reticularis layers of the adrenal cortex atrophy. The glomerulosa layer does not atrophy under these conditions because in addition to ACTH, angiotensin II (ANG II) and high levels of K + are trophic factors that act on the glomerulosa layer. The atrophy of the fasciculata and reticularis layers occurs routinely in people treated with glucocorticoid drugs and leaves the person with an iatrogenic form of adrenal insufficiency when use of the drug is abruptly discontinued. Conversely, chronic stimulation of the adrenals by ACTH, such as can occur with pituitary tumors (Cushing disease) or with the simple physiological ACTH excess that can occur with chronic stress, can increase the weight of the adrenals several-fold.
Cortisol exerts negative-feedback control on the very axis that stimulates its secretion (see Fig. 50-3 ), and it does so at the level of both the anterior pituitary and hypothalamus.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here