Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Using the gut to provide nutritional therapy by the enteral route plays a pivotal role in patient outcome in the critical care setting. Failure to use the gut for nutrition results in the gut becoming a proinflammatory organ, and results in increasing oxidative stress and higher risk of complications. Early enteral access and utilization of the gut in contrast, promote or support the mass of gut-associated lymphoid tissue (GALT), as well as mucosal-associated lymphoid tissue (MALT) at distant sites like the liver, lungs, and kidney. This process accounts for the “nonnutritional benefits” of nutritional therapy, contributing to an appropriate immune response, downregulation of inflammation, and a reduction in the rate of long-term complications. The sicker the patient, the greater the need to maintain gut integrity. Enteral nutrition support becomes a therapeutic tool or pharmacologic agent capable of changing outcome by reducing nosocomial infection, multiple organ failure, and hospital length of stay. Recent literature confirms that aggressive enteral tube feeding decreases the rate of complications when compared to “standard therapy,” which is defined as patients who are allowed to advance to either an oral diet on their own as tolerated or parenteral nutrition (PN).
However, obtaining enteral access early in the course of a critically ill patient may be difficult. Patients in this setting are at the height of the hypermetabolic response, often requiring high doses of narcotic analgesia and sedation; thus, they are prone to ileus, gastroparesis, and high gastric residual volumes. Transporting these patients to the radiology suite for placement of feeding tubes is difficult, as they are unstable. Such transport leads to delays in getting tubes placed and has been shown to increase the risk of complications (such as aspiration, hemodynamic instability, and new cardiac dysrhythmias). Bedside techniques to place feeding tubes are essentially blinded, which carries some additive risk. Although bedside techniques may be sufficient in a large number of patients in the critical care setting, the success rate for bedside placement decreases as disease severity increases, and there is greater need to place the tube lower in the gastrointestinal (GI) tract.
In long-term acute care and in the chronic management of patients recovering from stroke and neurologic injury, percutaneous endoscopic techniques provide a more reliable semi-permanent enteral access, affording a number of options in a variety of patients. Insertion of a feeding tube distal to the stomach and into the small bowel has been shown to reduce the incidence of regurgitation and aspiration. In a 2016 meta-analysis performed in preparation for societal guidelines, small bowel feeding was shown to significantly reduce the incidence of aspiration pneumonia when compared with gastric feeding. In patients with severe gastroparesis, percutaneous endoscopic techniques may provide a gastrostomy tube for decompression and a direct jejunostomy tube for continued enteral feeding. In patients with recurrent flares of chronic pancreatitis, placement of an endoscopic jejunostomy tube may provide therapeutic options which preserve nutritional status, decrease dependance on narcotic analgesia, and reduce the number of hospitalizations per year. In patients with dysphagia resulting from neurologic injury, these percutaneous endoscopic feeding tubes are easily removable should the patient recover function and resume adequate volitional oral intake.
The role of the endoscopist in these settings is critical. One must have the skills to place the tube at the appropriate level in the GI tract, and most techniques can be performed at the bedside in the intensive care unit (ICU) without transport to the radiology suite. The endoscopist has the expertise in gut physiology, thereby allowing for adequate monitoring of enteral nutrition therapy and distinguishing tolerance of feeds from intolerance. Endoscopists have the capabilities to provide simple techniques by which to manage complications. In the absence of such expertise for enteral access, the use of PN increases significantly, a change in management strategy that may negatively impact patient outcome.
Several important steps should be taken to set up an effective endoscopic enteral “tube service.” The nutrition literature is so strong regarding the beneficial effects of enteral feeding in the critical care setting that multidisciplinary nutrition teams are under significant pressure to get tubes in early and get feeds started quickly. The endoscopist should establish rapport with the multidisciplinary nutrition team and provide a timely response to consults for request for enteral access. This can be facilitated by flexibility with potential time slots in the endoscopy schedule to make room for add-on cases for enteral access. It is important to provide same-day service when a request for access is placed to deliver the best patient care. In general, it helps treat a request for enteral access for a patient in the ICU as one would respond to a request to evaluate a patient with GI bleeding.
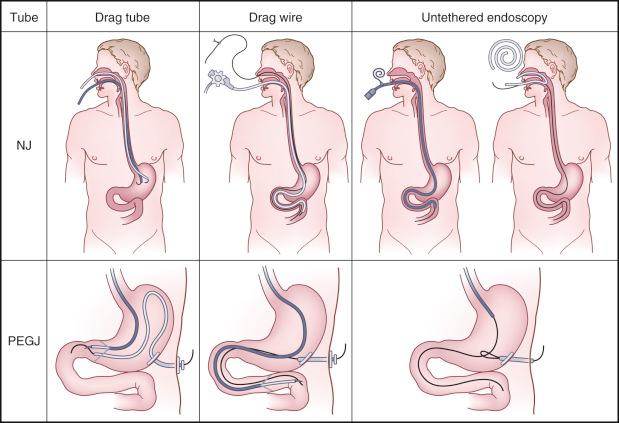
The two most important endoscopes needed to outfit an endoscopic enteral tube service are a pediatric colonoscope and a small-caliber gastroscope with an insertion tube outer diameter less than 6 mm. A pediatric colonoscope is usually the best choice for endoscopic nasoenteric tube (ENET), percutaneous endoscopic gastrojejunostomy (PEGJ), and direct percutaneous endoscopic jejunostomy (DPEJ) because of its length and relative stiffness. Although a push enteroscope is an adequate substitute for the pediatric colonoscope, the greater flexibility of the small bowel enteroscope promotes looping or curling in the stomach. The small-caliber gastroscope has the advantage that it can be passed via the transnasal route at the bedside with no sedation, making it an ideal choice in cases requiring ENET placement. The small-caliber endoscope can also be passed through a mature percutaneous endoscopic gastrostomy (PEG) tract ostomy for placement of a one-piece PEGJ. When a 28-Fr PEG is in place, this same endoscope may be passed through the PEG itself for purposes of conversion to a two-piece PEGJ. The endoscopist is encouraged to learn one technique well for each of the four procedures (ENET, PEG, PEGJ, and DPEJ), and then later experiment with many other techniques to find his or her personal favorite. When choosing an enteral access technique, priority should be placed on ones that allow “untethered” endoscopy to place a wire. Techniques which require dragging a wire down through the GI tract, or even worse, dragging the feeding tube itself, are less successful, more frustrating for the operator, and more likely to be displaced ( Fig. 42.1 ).
Fluoroscopy is valuable early in the learning curve, but all these techniques may be performed easily at the bedside or in the endoscopy suite without fluoroscopy. The ability to come to the ICU and place these tubes endoscopically without fluoroscopy avoids the need to transport the patient out of the ICU, increases flexibility in scheduling, and avoids the cost and exposure to radiation involved with fluoroscopy. Having appropriate ancillary devices such as wires, biopsy forceps, and snares that are long enough (such that they can be passed out through the colonoscope and still have a workable length beyond the tip of the scope) and having key accessories such as a hemoclip (to secure the distal tube tip to the intestinal mucosa) or bandage clips (to secure the proximal end of the tube to the skin), all improve the efficiency and success rate for these procedures. It is important to maximize lubrication when working with feeding tubes, both by activating the hydrophilic lubricant on the inner surface of the tube by water infusion, and by applying a vegetable spray or surgical lubricant to the outer surface.
Endoscopic nasoenteric tubes (ENET) placement is most commonly required in the critical care setting. Severity of critical illness, complications of sepsis and multiple organ failure, and therapeutic strategies such as placement on mechanical ventilation are all factors indicating the need for early enteral access. Establishing enteral access and initiating enteral feeds is considered part of the basic resuscitation of these patients. ENET placement deep into the jejunum is specifically reserved for those critically ill patients who demonstrate intolerance to initial nasogastric feeding (due to poor gastric emptying, ileus, regurgitation, or aspiration) and those who are at high risk for aspiration. Several techniques have been described for ENET placement.
The over-the-guidewire technique may be more difficult technically than other ENET procedures because of the oronasal transfer and wire exchanges (removing the endoscope from the wire and placing the feeding tube over the wire) ( Fig. 42.2 ). However, this technique is the one ENET procedure that most reliably places the feeding tube at or below the ligament of Treitz. Before performing an endoscopy, the oronasal transfer tube is placed through one nostril, brought out of the mouth, and then clamped to the side using a hemostat. The pediatric colonoscope is passed through the mouth, down the esophagus and stomach, and into the small bowel. As the endoscopist traverses the duodenum, it is important to pay attention to landmarks of the duodenal bulb, and the C-loop. The long, straight segment immediately after the duodenal C-loop is the distal duodenum leading up to the ligament of Treitz. The ligament of Treitz is the first turn after this long segment. Paying attention to these landmarks helps assure the endoscopist as to the location of the tip of the endoscope within the GI tract. Passing the endoscope one to two loops below the ligament of Treitz helps anchor the tip of the wire during subsequent wire exchanges (see Fig. 42.2A ). Once the endoscope has been passed as deep as possible, the wire is extended out from the end of the endoscope until it meets gentle resistance.
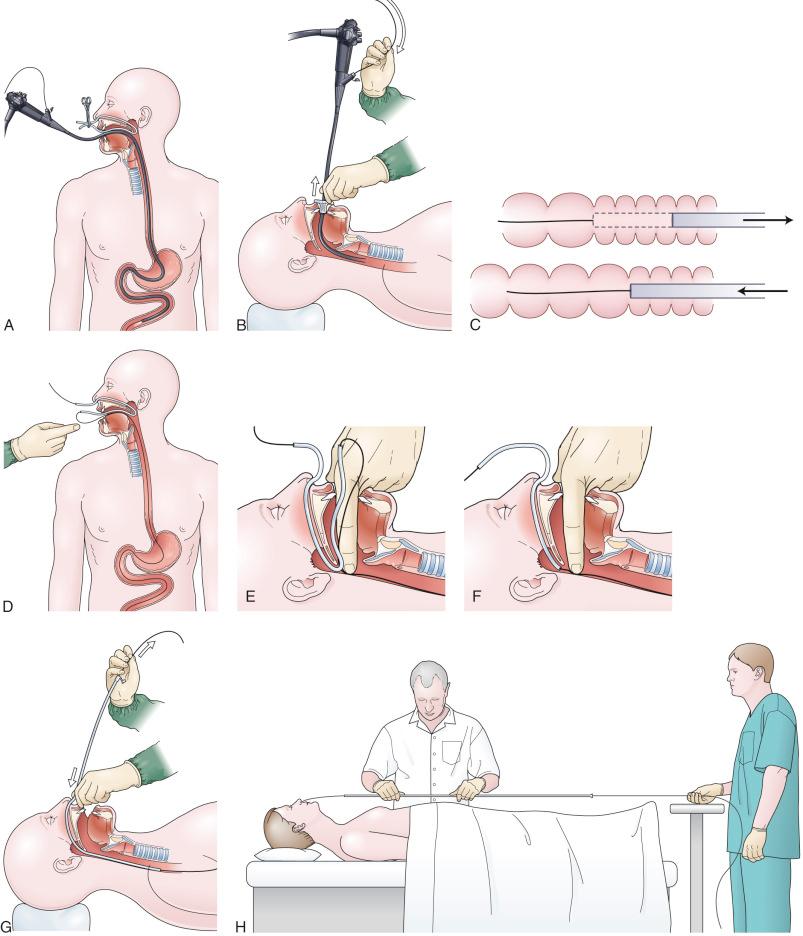
The first wire exchange involves removing the endoscope off the wire without displacing the tip. The key point to this aspect of the procedure is that the endoscopist places one hand on the endoscope as he or she removes it from the mouth and the other hand on the wire as it is passing into the operating channel of the endoscope at the other end (see Fig. 42.2B ). An assistant may support the weight of the scope, keeping it from bowing in the middle during the wire exchange. As the endoscopist pushes the wire out the end of the tube with one hand, and withdraws the scope with the other, a “keyhole technique” should be used. To keep the folds of the small bowel from passing off the end of the endoscope too quickly, a rolling motion should be used where the scope is withdrawn 5 cm and then pushed back in 2 to 3 cm (the same technique used to keyhole the pylorus in order to visualize the duodenal bulb as the scope is withdrawn from the small bowel on esophagogastroduodenoscopy). As a result, the bowel will come off the end of the scope in measured fashion 1–2 folds at a time (see Fig. 42.2C ). The point at which the colonoscope has been withdrawn off the wire, the tip of the wire is protruding from the patient's mouth. The oronasal transfer of the wire, if done incorrectly, will cause a loop to form in the mouth and/or displacement of the tip of the wire from the small bowel back into the stomach. The tip of the wire is placed through the oronasal transfer tube, passing the excess wire out of the end of the transfer tube protruding from the nose (see Fig. 42.2D ). Before the final loop protruding from the mouth is withdrawn or eliminated, the index finger is passed through the mouth, pinning the wire against the posterior wall of the oropharynx (see Fig. 42.2F ). While firmly holding the wire against the posterior pharyngeal wall, traction is placed on the end of the wire protruding from the nose, completely eliminating the loop protruding through the mouth (see Fig. 42.2E ). With the wire now protruding from the nose, the second and final wire exchange is made. This latter wire exchange may be accomplished using one of two different techniques. One technique involves carefully passing the feeding tube over the wire in a manner similar to the first wire exchange (see Fig. 42.2G ). Again, the endoscopist is careful to place one hand at the nose as he or she inserts the tube, with the other hand at the opposite end of the feeding tube where the wire is being withdrawn. The rate of the tube passing down into the nose should match exactly centimeter for centimeter the rate of the wire being withdrawn at the other end, to avoid deflecting the tip of the wire (see Fig. 42.2G ). An alternative technique for this second wire exchange involves pinning the end of the wire to a bed rail or bedside table establishing a “point in space” (see Fig. 42.2H ). Assistants help keep the wire straight and level while the endoscopist slides the feeding tube over the fixed wire into final position.
This technique is facilitated by placing one or two extra guidewires (for a total of 2 or 3) through the nasoenteric tube prior to placement. Two to three centimeters of the soft tip of one wire should protrude out through the distal end of the tube. This assembly is then passed down through the nose into the stomach, followed by passage of the endoscope alongside the tube, down through the mouth, and into the stomach. Once in the stomach, a long biopsy forceps is used to grab the soft tip of the wire protruding from the feeding tube. The endoscope holding the wire is then passed into the small bowel, hopefully down to or beyond the ligament of Treitz ( Fig. 42.3A ).
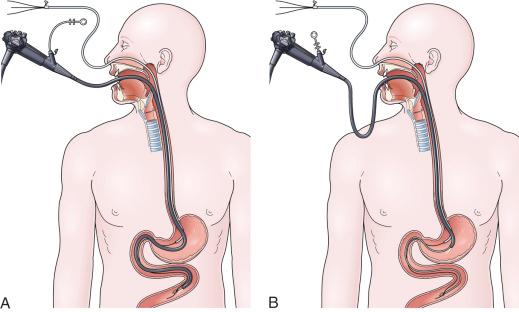
From the point of deepest insertion, the endoscope is then slowly withdrawn back toward the stomach as the biopsy forceps holding the wire is advanced, holding the tip of the wire in place in the small bowel. Once the endoscope is positioned back into the stomach, the feeding tube is advanced over the wire down to its tip, which is still being held by the biopsy forceps (see Fig. 42.3B ). Only at this point are the biopsy forceps opened and the wire is released. The biopsy forceps are then withdrawn back into the endoscope and the endoscope is slowly withdrawn back out through the esophagus and mouth. The keys to success for this procedure are a pair of biopsy forceps that are long enough (≥ 240 cm), and the stiffening of the feeding tube with extra guidewires (which facilitates removal of the endoscope without displacing the tube back into the stomach) (see Fig. 42.3B ).
Availability of a small-caliber gastroscope affords the endoscopist the opportunity for a simple technique for ENET placement. The key to success with this technique is the placement of a biopsy forceps or a Savary guidewire down through the operating channel, which serves to stiffen the instrument and increase the ease with which it may be passed through the bowel. Transnasal passage of the endoscope is tolerated well by the patient and sedation is not usually required. After intubating the esophagus and stomach, the endoscope is passed as far as possible, usually to the third or fourth portion of the duodenum. At this point, the stiffening device is withdrawn and a guidewire is placed down through the operating channel out as far as possible until meeting gentle resistance ( Fig. 42.4 ). Using the wire exchange system described in the over-the-guidewire technique, the small-caliber gastroscope is then withdrawn off the wire. Obviously, no oropharyngeal transfer of the wire is required, and the feeding tube may be immediately passed directly over the wire. Wire exchanges are somewhat more tenuous and difficult with this procedure because the wire is usually not passed as deep into the small bowel as in the over-the-guidewire technique described earlier, and the tip may be displaced more easily back into the stomach.
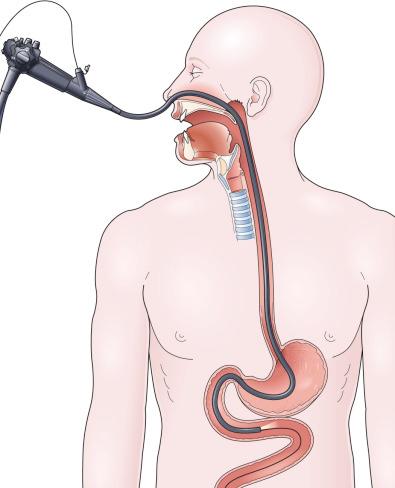
In a simpler version of the previously mentioned drag and pull technique, a knotted suture line is attached to the distal end of the feeding tube, and then the tube is passed through the nares down into the stomach. The endoscopist passes the endoscope alongside the tube through the mouth, down into the stomach, grabbing the knotted suture with biopsy forceps ( Fig. 42.5A ). It can be surprisingly difficult and frustrating to drag the tip of the feeding tube through the pylorus and down into the duodenum. The success of this sometimes awkward procedure is improved by using a knotted suture line instead of a loop or single strand on the tip of the tube (see Fig. 42.5B ), by adding a second guidewire to stiffen the tube to prevent displacement upon withdrawal of the endoscope, and by keeping the biopsy forceps 1 to 2 cm away from the tip of the endoscope to enhance visualization (see Fig. 42.5A ).
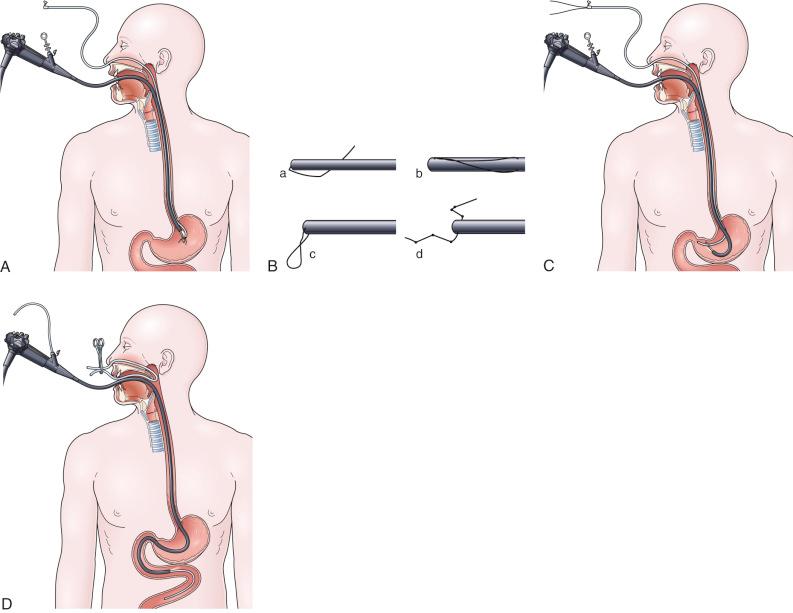
Another alternate technique utilizes adding one or two extra guidewires (for a total of two or three) to the feeding tube to increase stiffness, and then passing the tube through the nares down into the esophagus and stomach. The endoscope is passed through the mouth alongside the tube down into the stomach, and the tip of the stiffened tube is simply pushed or nudged using open biopsy forceps through the pylorus into the duodenal bulb (see Fig. 42.5C ). Continuing to watch endoscopically from the stomach, the endoscopist pushes the stiffened feeding tube from the outside proximal end in an effort to pass the distal tip around the C-loop and into the third and fourth portion of the duodenum (see Fig. 42.5C ).
The most reliable of the three alternate methods uses an 8-Fr nasoenteric tube, which is passed through the operating channel of the endoscope after it has been passed through the esophagus and stomach into the small bowel. This procedure's success is enhanced by using a large channel therapeutic endoscope and a small-bore (8-Fr) nasoenteric tube which has a removable proximal feeding cap. Because the endoscope is passed through the mouth, it does require placement of an oronasal transfer tube and the subsequent transfer of the tube from the mouth out through the nose (see Fig. 42.5D ), using the method described in the over-the-guidewire technique.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here