Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
With the discovery of nitrogen mustard as an anticancer agent in the 1940s, medicinal and hence systemic cancer therapy became a reality. The identification and application of cytotoxic agents dominated the next 60 years of cancer treatment. The success of imatinib for BCR-ABL rearranged chronic myelogenous leukemia (CML), the dramatic responses seen in patients with BRAF -mutated melanoma and ALK rearranged non–small cell lung cancer to targeted inhibitors, and the unparalleled ability to systematically characterize cancer genomes have catapulted the community into a new era of targeted cancer therapy. Nonetheless, there is much to learn from the 60-year experience in empiric-based therapy that should be directly applicable to the development of molecularly targeted therapy. Cytotoxic chemotherapy, although often regarded as largely unsuccessful, has led to substantial cure rates in a number of well-defined malignancies, particularly pediatric. In this chapter we will discuss the fundamental concepts, advantages, and disadvantages of cytotoxic cancer therapy and their relevance to targeted drug therapy for malignancy. We will define targeted therapy, its advantages and disadvantages, discuss the credentialing of targets, and provide a system for categorizing classes of targets and classes of therapies. We will then focus on particular lessons learned in the early development and testing of new targeted therapies for adult malignancies, with examples from each of the target classes. Our intent is to highlight illustrative examples rather than to provide an exhaustive list of all targeted therapies in development. We will conclude with a discussion of the challenges facing us in the development of targeted therapies for children and highlight emerging pediatric cancer-based targets.
Although conventional chemotherapy agents are often seen as nonspecific cytotoxic drugs, over the years more information has come to light about their mechanisms of action in killing tumor cells. In general, these agents were designed to interfere with molecular pathways required for nucleotide synthesis, DNA replication, and the mechanics of cell division. Thus these agents have significant but often predictable, or at least stereotypical, morbidity related to target-mediated suppression of hematopoiesis or of the cell-division process required for homeostasis of other organ systems. These agents are discussed in greater detail in Chapter 45 .
The use of cytotoxic chemotherapy agents is an imperfect science at best, but researchers have learned many lessons over the years about the medicinal treatment of cancer, lessons to strongly consider in the development of targeted therapy ( Box 44-1 ).
Combination therapy is required for cure.
Appropriate dose intensity is required for improved outcome.
Toxicity is acceptable when therapy is curative.
Establish curative regimens, and then attempt to reduce toxicity.
The goals of combination therapy are numerous. First, because it is difficult to predict which single agent will be most effective against an individual patient's tumor, the use of multiple drugs increases the potential for an initial complete response. Second, combination therapy is likely critical for preventing the development of therapeutic resistance. Third, drug combinations may have additive or synergistic interactions, increasing the total activity of the drugs and potentially enabling lower dosing and reduced toxicity. One example is the successful dose reduction of cytotoxic chemotherapy with the addition of all- trans -retinoic acid (ATRA) for the treatment of patients with acute promyelocytic leukemia (APL). With few exceptions, curative systemic therapy has required the combination of highly active agents rather than combinations of active and inactive agents.
The need for multiagent therapy was first appreciated in the treatment of acute lymphoblastic leukemia (ALL). At best, 60% of patients are estimated to achieve complete remission with single-agent chemotherapy. Despite continued use, almost all patients will relapse within 6 to 9 months with single-agent therapy. However, complete remission rates exceed 95% in patients treated with combination chemotherapy, and cure rates today for children with ALL exceed 80% with multiagent treatment regimens. Similarly, patients with APL had very poor remission and cure rates until the discovery of ATRA differentiation therapy for this disease. ATRA achieved a high complete remission rate in use as a single agent, but virtually all patients ultimately relapsed. It was not until the combination of cytotoxic chemotherapy with ATRA and the recent combination of ATRA with arsenic trioxide that these patients obtained the best long-term survival rates of any acute myeloblastic leukemia (AML) subtype.
A guiding principle in the delivery of cytotoxic chemotherapeutic agents is the concept of the maximum tolerated dose (MTD): the simple idea that more is better. Drugs are delivered at the maximum dose possible, a dose greater than which unacceptable toxicity is encountered. Here, without a clear cellular biomarker of cytotoxic activity, it is not possible to relate the maximum molecular effect to maximum achievable efficacy. Thus cytotoxic drugs are first evaluated in phase I studies with the primary study goal of establishing the MTD. Subsequent phase II and III trials are then based on this dosing schedule. MTD assumes that some toxicity is acceptable and, in fact, expected in the pursuit of cure. The most extreme example of this concept has been the use of autologous stem cell transplantation to deliver what otherwise would be lethal doses of chemotherapy. In this case the MTD is surpassed by replacing the dose-limiting factor, the hematopoietic system, with stem cells harvested from the patient before delivery of the conditioning chemotherapy. While clearly a brute force approach, the MTD paradigm also established an important corollary: significant side effects can be tolerated when therapy is administered with curative intent (discussed later).
Many retrospective studies of chemotherapy dose intensity and outcome support this premise, particularly in the pediatric setting. In high-stage neuroblastoma the addition of dose intensification with stem cell transplantation has improved the outcome for patients with metastatic disease. In pediatric ALL several studies suggest that dose intensification is important for long-term outcome, including one randomized study comparing standard versus dose-reduced methotrexate with mercaptopurine. Moreover, in Ewing sarcoma chemotherapy intensification through interval compression in a randomized trial was also associated with an improved outcome in patients with localized disease. However, fewer prospective studies than retrospective studies have been performed to address the issue of dose intensity and outcome for pediatric tumors. Significantly, while these studies begin to emerge, some previous assumptions about MTD have come into question. For example, retrospective studies support the notion that doxorubicin or methotrexate dose intensity is important for outcome in patients with localized high-grade osteosarcoma. However, prospective studies involving large patient numbers have called into question the role of further dose intensification for this disease. Furthermore, randomized trials for malignancies such as breast cancer have demonstrated that massively increasing dose with stem cell transplantion does not always improve long-term survival. Well-controlled prospective trials are critical to address the issue of dose intensification, even for cytotoxic agents.
The oncology community has accepted toxicity as inherent in the mission of cure. With the implementation of dose-intensive regimens, however, comes additional toxicity. Because of the relatively nonspecific nature of traditional chemotherapy, morbidity from these treatment regimens is significant. Acute toxicity takes the form of infection, transfusion dependence, renal injury, and hepatic injury. There are also potential long-term consequences such as cardiac injury, infertility, chronic renal insufficiency, hearing loss, skeletal injury, and secondary malignancies. Ongoing efforts are focused on decreasing these short- and long-term side effects. For example, to minimize acute toxicity, chemotherapy dosing is usually individualized on the basis of body surface area (mg/m 2 ). For some cytotoxic agents, such as methotrexate and busulfan, drug levels can be monitored to minimize toxicity. Dose intensity can also be achieved with regional tumor delivery, growth factor support, or rescue with stem cells. Furthermore, better supportive care, such as infection prophylaxis, has enabled tolerance of prolonged immunosuppression. To decrease long-term morbidity, “safe” maximum cumulative doses are being established, such as maximum cumulative doxorubicin to minimize late cardiac effects. Although toxicity can be tolerated, it is essential that the level of toxicity be commensurate with the level of benefit. Marginally effective therapeutic regimens that result in substantial toxicity should not become accepted standards.
Treatment strategies for most of the pediatric malignancies, including ALL and the solid tumors, have used disease stratification to decrease dose in treatment regimens. A dose-intensive regimen is initially used to establish efficacy. Then, patients with a good prognosis are treated with less intensive regimens, with the goal of comparable disease-free outcomes but decreased treatment-related morbidity. This is particularly important for pediatric disease, in which the long-term toxicity of treatment is experienced over a lifetime. Historically, these stratifications were based on factors such as age, stage, and histology, but now they include molecular genetic markers, such as MYCN amplification in neuroblastoma, and MLL rearrangement in pediatric ALL. For example, the National Wilms'Tumor Study evaluated a half-dose chemotherapy regimen for infants with low-risk disease in an attempt to decrease the incidence of toxic deaths without compromising long-term survival. These infants had acceptable toxicity with no toxic deaths, and long-term survival was not compromised. A similar approach has been taken with infants and young children with low-stage neuroblastoma, and therapy for children with ALL has been dose-reduced based on rapid clearance of minimal residual disease.
While our understanding of the molecular basis of malignancy improves, so will our ability to develop fine-grained prognostic categories and patient-tailored treatment regimens, perhaps even for traditional chemotherapy agents. While we implement targeted therapy, it is expected that disease stratification and patient selection will be based on molecular genetic findings related to the credentialed target. In the clinical development of targeted therapies, we must continue to consider these key concepts: combination therapy, appropriate dose intensification, tolerance of toxicity for substantial efficacy and cure, and treatment adjustment within established favorable prognostic groups.
Although clinicians have made great progress with the cytotoxic armamentarium, further strides in the treatment of cancer are limited with this approach alone. Several shortcomings of this approach are intimately related to the lack of specific target knowledge ( Box 44-2 ).
Toxicity is significant.
Optimization of anticancer effect versus side effects is difficult.
Mechanistic understanding of nonresponding and resistant disease is lacking.
Predictors of patient response to a specific drug are poor.
To date cytotoxics have explored a narrow range of cellular and molecular mechanisms.
Toxicity is significant.
Optimization of anticancer effect versus side effects is difficult.
Mechanistic understanding of nonresponding and resistant disease is lacking.
Predictors of patient response to a specific drug are poor.
Cytotoxics have explored a narrow range of cellular and molecular mechanisms.
One major shortcoming to cytotoxic therapy is morbidity. Because these agents are relatively nonspecific, there is significant toxicity to normal host cells. Patients can expect to experience short- and long-term morbidity from these drugs and even incur the risk of therapy-related fatality. Furthermore, because the most relevant targets of these drugs are poorly understood, it is difficult to optimize the anticancer effect versus the toxicity, and it is difficult to separate on-target from off-target toxicities. Often, there is no relationship between the administered dose (pharmacokinetics) or the MTD and inhibition of the putative target (pharmacodynamics). Developing a strong correlation between the pharmacokinetics of a drug and its pharmacodynamic effect is obviously impossible when the target of a drug is unknown. Similarly, in the absence of target knowledge, our understanding of resistant or refractory disease is poor at best, and it is very difficult to predict responders a priori. This has necessitated long clinical development programs with large trials to identify responsive subpopulations of patients and then follow-up trials to more thoroughly investigate response in this patient subset. Targeted therapy has the potential to address these shortcomings, to enable more rational tumor-specific therapy with less morbidity risk, and to explore previously unexploited cancer-related molecular mechanisms. With the sequencing of the human genome, the development of high-throughput genomic technologies, and an ever-increasing understanding of the molecular pathogenesis of cancer, this promise is likely to be realized.
Although we have made great strides with the cytotoxic armamentarium, further strides in the treatment of cancer are limited with this approach alone. We still fail to cure many patients with this approach, and several shortcomings of this approach are closely related to the lack of specific target knowledge (see Box 44-2 ). Targeted therapy, defined as a therapeutic procedure whose preclinical development focused on modulation of a single molecular target (typically inhibition but occasionally activation of the molecular entity), has the potential to address these shortcomings. Targeted therapy relies on the concept of cancer cell dependence—that is, the malignant cell or tumor as a whole depends for its survival or proliferation on the particular gene product or pathway. The discovery of targeted therapy differs significantly from that of cytotoxics. Historically, cytotoxic therapy discovery relied on simple, phenotypic cell death assays in which small-molecule libraries were screened in vitro against a panel of cancer cell lines. Because cytotoxic compounds are believed to cause nonspecific cell death, effective compounds should, in principle, have efficacy for all cancer types. In practice, however, this has not proved to be the case. Thus although phenotypic screens may be of significant value in identifying molecules with complex mechanisms of action, the direct prediction of clinical efficacy from limited in vitro testing remains problematic. This is in sharp contrast to the concept of targeted therapy.
There are three critical elements to the implementation of targeted therapy. First, on the basis of available data, a hypothesis is generated suggesting a causal or pathogenic link between a specific protein or gene (the potential drug target) and cancer. Experiments are then performed and data generated to test the validity of this hypothesis. Is this candidate target relevant to oncogenesis or the maintenance of the malignant phenotype? Second, there must be a valid therapeutic approach to interfering with the target activity through small molecules, antibodies, or protein- or cell-based biologics. Third, the clinician must estimate the likely impact of target manipulation in a relevant clinical application. For example, a targeted therapeutic may have limited clinical application if the disease state is already readily cured. The process of evaluating potential targets that emerge is target “validation.”
Targeted therapy offers several potential advantages over nonspecific cytotoxic treatments ( Box 44-3 ).
Improved patient selection is enabled.
Drug efficacy and target inhibition are linked, enabling rational dosing.
Side effects are more predictable.
Understanding of target and pathway increases options for the subsequent development of therapeutics with improved efficacy.
The mechanisms of resistance may, in some cases, be more readily discovered and interdicted with second-generation therapeutics or specific combinations.
Rational pathway-based combination regimens can be tested.
Improved patient selection is enabled.
Drug efficacy and target inhibition are linked, enabling rational dosing.
Side effects are more predictable.
Understanding of target and pathway increase options for the subsequent development of therapeutics with improved efficacy.
The mechanisms of resistance may, in some cases, be more readily discovered and interdicted with second-generation therapeutics or specific combinations.
Rational pathway-based combination regimens can be tested.
By way of example, hormonal therapy has become standard of care for patients with estrogen receptor (ER)–positive breast cancer. In this case there is a lineage-dependent target in the malignant cell, the ER, and antiestrogen agents, such as tamoxifen, have been developed to inhibit competitive binding of estrogen to the ER. Numerous studies exploring estrogen antagonist therapy with tamoxifen for patients with ER-positive versus ER-negative disease demonstrate benefit only in the ER-positive patients. In conjunction with diagnostic tools to identify hormonal receptor status, patients are now rationally chosen for therapy, thus eliminating exposure to drug toxicity for patients not likely to respond.
A second advantage of targeted therapy is the strong link between the target inhibition and efficacy. The dosing goal is achievement of complete target inhibition, not MTD. Thus there is no need to suffer undue toxicity in the achievement of an MTD. In fact, studies have shown that tamoxifen treatment responses do not correlate with serum levels and that doses greater than 20 mg daily do not increase efficacy compared with that of this dose. A related third advantage of targeted therapy is that the side effects can generally be anticipated and serve as a marker for on-target inhibition. For example, the side effects of tamoxifen are predominantly related to the antiestrogen effects of this drug: vaginal dryness, hot flashes, and irregular menses. Because this drug also has proestrogen effects, thromboses and endometrial cancer can be seen.
A fourth advantage of targeted therapy is that understanding the target and the pathway increases the possibilities for therapeutic design. In the case of modifying ER signaling, there are several methods to accomplish this other than direct ER antagonism. One method is to decrease ovarian production of estrogen with luteinizing hormone–releasing factor agonists, which induce a medical ovarian ablation. A second method of interfering with ER signaling is to decrease estrogen production through the inhibition of aromatase activity. Aromatase is the final enzyme in estrogen synthesis converting the androgens androstenedione and testosterone to the estrogens estrone and estradiol. Aromatase inhibitors work by decreasing this conversion and thus decreasing circulating estrogens. Aromatase inhibitors have improved efficacy over tamoxifen and have the advantage of fewer side effects in terms of hot flashes, endometrial disease, and ischemic cerebrovascular effects. Because of the clear dependence of some breast cancers on ER and detailed knowledge regarding estrogen biosynthesis and signaling, multiple agents could be developed to inhibit this pathway.
The fifth advantage of targeted therapeutics is the ability to more readily understand the molecular mechanisms associated with resistance. The success of targeted inhibition of BCR-ABL in CML and of epidermal growth factor receptor (EGFR) in lung cancer was rapidly followed by the specific elucidation of direct resistance mutations in the target leading to decreased activity of the relevant small-molecule inhibitors, imatinib and erlotinib or gefitinib, respectively. In the case of CML, new agents were developed in short order to treat resistance in patients by more efficient inhibition of the mutant BCR-ABL proteins.
The final advantage of targeted therapies may be the ability to generate rational and novel combination regimens. Currently, the vast majority of combination strategies derive from combining a novel agent with components of existing standards of care without regard to mechanism. This strategy is largely based on clinical expedience, existing clinical efficacy (for the standard of care) and tolerability. The difficulty of empirically discovering new synergistic combinations stems from the large number of permutations required to test all possible combinations against multiple in vitro and in vivo models or multiple cancer types. Targeted therapeutics are often related to one another in the context of a pathway. This allows testing of specific pathway-related hypotheses. For example, combinations might target upstream and downstream components of the same pathway to defeat feedback mechanisms, target components of parallel pathways to defeat pathway redundancy, or target distinct antiproliferative and prosurvival pathways.
Much of current cancer therapy development focuses on specific changes that alter the malignant cell, setting it apart from its normal counterpart and creating distinct molecular or pathway dependence for cell survival and replication. These may be genetic mutations, gene rearrangements, epigenetic modifications such as altered patterns of methylation or acetylation, lineage legacies, or other metabolic liabilities. Genes whose expression and activation have been increased, resulting in a cancer-dependent state, are prime targets for therapy development. On the basis of cancer dependencies, cancer targets can be categorized in four classes: genetic, lineage, host, and synthetic lethal or empiric ( Box 44-4 ). Although some targets may cross over from one class to another, this classification may be useful in the design of clinical trials and elucidation of the parallel biomarker strategy.
Genetics track
Lineage track
Host track
Synthetic lethal or empiric track
The genetics track relies on the idea that a cancer develops from a finite number of genetic alterations, that these alterations give the malignant cell a selective advantage, and that these genetic changes are selected for during malignant cell replication. Hence the malignant cell becomes dependent on a set of these changes for continued survival, a process sometimes referred to as oncogene addiction. This cancer cell dependence or addiction should provide a window for drug specificity for the malignant cell compared with normal cells. Targeted therapeutics taking advantage of genetic abnormalities should, in theory, be more injurious to the malignant cell than to the normal cellular counterpart. Advances in cytogenetic studies, high-throughput gene sequencing, detection of copy number alteration, whole genome expression profiling, and proteomic approaches have revolutionized candidate genetic target discovery. Now, tens of thousands of genes in hundreds of tumor samples can be analyzed simultaneously. These candidate targets must then be carefully credentialed for their suitability as a drug target. Is there cancer cell dependence on the genetic abnormality, and can the target be pharmacologically manipulated? Imatinib is one of a number of examples of clinically validated targeted therapeutics exploiting a genetic dependence, in this case the dependence of CML on the activity of the Abelson kinase (ABL). The very existence of the cancer-dependent state is dramatically illustrated by the emergence of resistance mechanisms that largely appear to restore Abelson activity (see further discussion later in this chapter).
Cancer cells often maintain developmental features of the lineage from which they were derived. This is well supported with gene expression studies that report tumor cells more closely resembling the normal lineage counterpart than tumors of a different cell type. The notion that lineage or “legacy” features of a tumor cell may be exploited with targeted therapy is known as lineage addiction . One of the earliest examples of the success of this approach has been the antagonism of ER and its signaling pathway in patients with ER-positive breast cancer. Similarly, the vast majority of patients with prostate cancer derive significant benefit from antagonism of the androgen receptor (AR). In both ER-positive breast cancer and in prostate cancer (virtually all forms of which express AR), the emergence of hormone-refractory disease largely appears to rely on mechanisms that restore steroid receptor signaling. Thus as in the case of imatinib resistance, the dependent state is illustrated by these resistance mechanisms. In many cases lineage dependence may also derive from the normal hierarchy of differentiation beginning with the stem cell.
The importance of tumor cell environment to the development and maintenance of the malignant state has become increasingly recognized and represents another potential inroad to targeted therapy. It is possible that tumors, by evolving in a particular environmental context, may become dependent on certain growth factors or neighboring cells for survival in that niche. Preclinical evidence suggests that many tumors require de novo blood vessel development for their growth and maintenance. The identification of these angiogenesis factors has enabled the development of modifiers of the angiogenesis process, leading to the successful clinical application of bevacizumab, an anti–vascular endothelial growth factor (VEGF) antibody, in patients with colorectal cancer and the approval of kinase insert dependent receptor inhibitors (sunitinib and sorafenib) in renal cell carcinoma (see further discussion later in this chapter). The interaction of tumors with the bone microenvironment has also been targeted using inhibitors of osteoclastogenesis. Here, multiple bisphosphonate agents are approved for the prevention of cancer-related fractures.
A number of highly efficacious anticancer agents do not conveniently map to the first three categories. Admittedly, we can say that the understanding of the mechanisms of efficacy of such agents remains opaque. Nonetheless, it is quite likely that such agents, including many of the cytotoxic agents described previously, take advantage of aspects of cancer cell biology that remain unknown but may be broadly termed as synthetic lethal interactions. Synthetic lethal genetic interactions are defined as mutations that are lethal in combination but that do not produce a lethal phenotype as a single event. In a similar way, gain-of-fitness alterations in a malignant cell, which give the cell a survival advantage, can sensitize the cell to other stresses that have no consequence in a normal state but may be lethal with the malignancy promoting alteration. Thus cancer-promoting mutations give the malignant cell a survival advantage, but they may also incur a distinct liability that can be exploited therapeutically. Rather than two synthetic lethal genetic events, the clinician can also apply such synthetic lethal concepts to a preceding cancer-related molecular alteration followed by the application of a therapeutic. For example, a striking synthetic lethal relationship was discovered between loss of BRCA1 or BRCA2 function and inhibition of the poly(ADP-ribose) polymerase I enzyme (PARP). Here, loss of PARP1 was found to induce double-strand DNA breaks that required the recruitment of the RAD51 homologous recombination repair complex to the site of these breaks for effective repair. Because it was known that BRCA1 and 2 were required for RAD51 recruitment to such complexes, it was hypothesized that loss of BRCA1 or BRCA2 would create sensitivity to PARP inhibition. This was demonstrated both with siRNA and small-molecule inhibitors of PARP. Moreover, clinical activity of PARP inhibitors has now been seen in patients with BRCA1 and BRCA2 mutations with ovarian or breast cancer.
By the strictest of measures, a drug target is not validated until a selective inhibitor of the target of interest has proven clinical activity and when resistance emerges through specific alterations in the direct target. Although very few cancer targets have achieved this degree of validation, we can envision preclinical experimental methods that will allow the researcher to credential a target for further drug discovery efforts. In this effort, the goals are to build data sets that either prove or disprove certain target-based hypotheses and to use such data sets to prioritize target selection for future drug discovery efforts. We can consider three key questions regarding the relevance of any given target to anticancer therapy:
Is the activity of the target required for the development and maintenance of the cancer-dependent state?
Is it possible to identify a strategy for developing a therapeutic for the target of interest?
Is there a clinical application for the eventual therapeutic?
In the subsequent sections we will focus on the approaches relevant to addressing the role of a given target in cancer dependence. In this specific regard there are two general sources of information: the study of a target in human cancers (target epidemiology) and the functional study of a target in model systems of tumor biology (functional validation) ( Box 44-5 ).
Target epidemiology: study of a target in human cancers
Target expression
Genetic alteration
Gain-of-function
Translocation
Activating point mutations
Copy number gain
Loss-of-function
Translocation
Inactivating point mutations
Deletion
Homozygous
Heterozygous
Loss of heterozygosity
Protein Epidemiology
Functional validation: study of a target in a model system of tumor biology
Gain-of-function: address sufficiency of target
Loss-of-function: address necessity of target
Preclinical models of cancer are limited in both number and in their qualitative relationship to human cancers. Comprehensive collection of human tumor specimens could, in theory, be used to study a given target in a large array of samples broadly covering tissue distribution and stage distribution (primary versus metastatic sites). In practice, however, there are limited sets of comprehensive tumor collections, and generally the largest tumor-type specific collections are limited to fixed, paraffin-embedded specimens. The availability of tissue samples paired with robust clinical data remains limited. Furthermore, the vast majority of human cancer collections come from surgical resection samples, which do not necessarily reflect the disease state in which a therapeutic will be initially tested. Despite these limitations, molecular epidemiology studies in tissue samples, enabled by tissue microarrays, single nucleotide polymorphism (SNP) arrays, expression microarrays, reverse protein arrays, and next-generation sequencing technologies remain critical to building a case for the cancer relevance of a given target.
The desire to discover genes “selectively” expressed in cancer led first to the development of differential expression methods, including the subtractive hybridization cDNA libraries, differential display, and serial analysis of gene expression (SAGE) as examples. Following the elucidation of the human genome sequence and the development of DNA-based microarrays, comparative expression profiling largely replaced difference methods, and more recently, RNA sequencing has become a commonly used tool. In all cases the basic premise has been to identify genes overexpressed in cancer with the assumption that overexpression will equate with increased sensitivity to target inhibition and hence serve to improve the therapeutic index. This basic idea, however, must be challenged and carefully considered for each target, for the simple reason that resistance to therapeutics can likewise be engendered through overexpression. Indeed, on the basis of standard biochemical principles, increased target expression should necessitate a higher drug concentration to achieve target inhibition. This is clearly the case in the amplification of dihydrofolate reductase (DHFR) in generating resistance to methotrexate. Likewise, data suggest that increased expression of AR can lead to resistance to AR blockade. Thus caution is required before proceeding with target selection based solely on expression data in the absence of a demonstrated causal or functional link to the maintenance of a cancer state.
As illustrated by the success of ATRA, trastuzumab, and imatinib, direct genetic alteration leading to gene activation of a given drug target remains perhaps the best evidence for the causal role of a given gene in carcinogenesis. Genetic alterations that are highly frequent in a given tumor type and are present in the majority of cells are a strong indicator of causality. In the era of large-scale resequencing projects, it is critical that one distinguishes “driver” mutations (causal mutations) from so-called passenger mutations. In addition, driver mutations that arise late in the course of disease, are present in only a minor fraction of the tumors, or are present in only a fraction of tumor cells in a given single tumor must be given less weight. These findings would suggest a reduced likelihood of this lesion possessing a strong causal link to the initiation of the disease state, although they may pose an eventual mechanism of drug resistance. The types of genetic alterations (e.g., point mutations, focal amplifications, broad amplifications, and translocations) are also distinguished with respect to the extent to which they can be used to establish causality or to infer dependence.
Gain-of-function genetic alterations include translocations, activating point mutations, and increases in gene copy number. Translocations were among the first discovered genetic alterations initially described using chromosome spreads. Approaches to the discovery of chromosomal rearrangements include exon microarrays, which can potentially detect imbalanced or amplified translocations, cancer outlier profile analysis (COPA), paired-end resequencing of BAC clones from a genomic library (giving a structural map of a cancer genome), and newer methods of single-molecule sequencing (discussed later). Translocations are highly informative with respect to causality because they typically alter only two genes, and because background or random translocation events are relatively rare, with chromothripsis (massive DNA rearrangements affecting one or a few chromosomes that occur as a result of one catastrophic event) as a notable exception.
Amplifications are now routinely detected by methods of DNA copy number analyses: comparative genomic hybridization (CGH) array, high-density SNP arrays, and whole exome or whole genome sequencing. There are two general types of increases in gene copy: focal amplifications and broad copy number increases covering an entire chromosome or chromosome arm. With sufficient sample numbers it is possible to discern one to several genes contained within a focal amplicon. Typically, the minimal region of amplification is a guide. However, methods for detecting copy number alterations (e.g., genomic identification of significant targets in cancer [GISTIC]) also use the amplitude of amplification as an important parameter. Although it is not definitive proof, amplification lends evidence to a causal relationship between a given gene within an amplicon and a cancer state. In some amplicons, multiple genes remain strong candidates for oncogenes targeted by the amplification. One notable example is the 11q13 amplification with genes including Cyclin D1 , FGF3 , and FGF4 .
Larger-scale chromosomal alterations leading to trisomy or gain of an entire chromosome arm are frequent yet are far more difficult to study. As such, identifying single genes (or drug targets) within such broad regions remains a challenge. In this case the number of genes implicated in such large-scale alterations is so large that almost no importance can be ascribed to the cancer relevance of a gene contained within such broad chromosomal-level alterations. Outlier analysis may begin to allow the elucidation of multigene targeting by such broad regions and is illustrated by the analysis of trisomy 7 in glioma revealing a dependence on cMET and its ligand HGF, both located on chromosome 7.
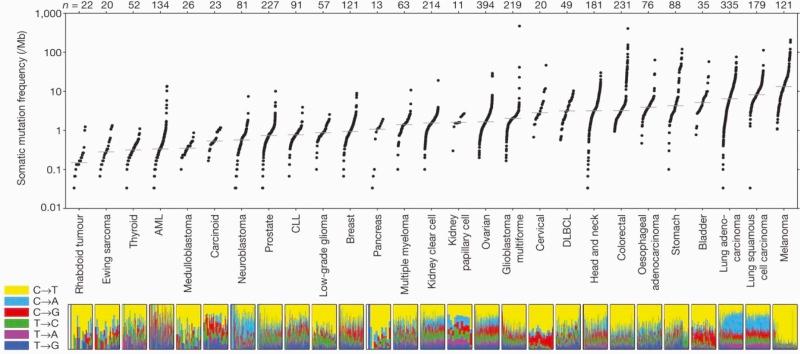
Subtle nucleic acid level alterations, including point mutations and small insertions and deletions, are an important mechanism for the activation of oncogenes. Point mutations leading to the constitutive guanosine triphosphate (GTP)–bound and hence activated form of N-RAS and K-RAS, as well as genetic events leading to activation of the BRAF kinase in melanoma, thyroid cancer, colorectal cancer, pediatric pilocytic astrocytoma, and Langerhans cell histiocytosis are exemplars of this mechanism. Large-scale sequencing projects that unveiled new mutations activating oncogenes such as EGFR and PIK3CA have led to national and international cancer genome sequencing projects. From these studies it has become apparent that the detection of passenger mutations, constituting the background rate of mutation, can pose a significant challenge. These passenger mutations must be separated from the more prevalent and recurrent driver mutations. Such passenger mutations may be exceedingly high in certain types of tumors, particularly those bearing mutations in mismatch repair genes. Indeed, in one case inactivating mutations in the mismatch repair gene MSH6 were discovered in glioma tumors recurring after treatment with temozolomide. Here the sequencing of MSH6 was triggered by the very high rate of observed passenger mutations in treated versus untreated tumors. These data suggest that cautious interpretation of low-frequency events is warranted when considering mutation analysis from larger-scale modalities. A framework for context-specific mutation analysis has been provided by a systematic study of mutation data across many cancer types in The Cancer Genome Atlas (TCGA) project and others, and these studies are revealing remarkably simple genomes in many pediatric cancers ( Fig. 44-1 ).
Loss-of-function alterations do not typically directly indicate or point to a specific gene as a candidate drug target. However, such alterations may predict sensitivity to agents acting against protein targets contained within downstream or parallel pathways. For example, clinical trials are under way testing inhibitors of mammalian target of rapamycin (mTOR) function against sporadic tumors or hereditable cancer predisposition syndromes resulting from loss of the tumor suppressors PTEN and TSC1 or TSC2. In both cases loss-of-function mutations in these tumor-suppressor genes are thought to lead to constitutive activation and hence constitutive dependence on mTOR (discussed later in this chapter).
Similarly, in the hedgehog pathway, a key development pathway regulating cell patterning, deletions or mutations in the gene encoding the Patched receptor (PTCH) are associated with hereditary and sporadic basal cell carcinomas and sporadic medulloblastoma. Loss of PTCH, a receptor for the ligands in the hedgehog family, leads to constitutive activation of the atypical G protein–coupled receptor (GPCR)–like protein Smoothened (Smo). Cyclopamine, a naturally derived inhibitor of Smo, and other, newer small-molecule inhibitors, have had significant activity in preclinical models lacking PTCH gene function and in clinical trials. These examples illustrate that identification of loss-of-function mutations can reveal downstream target molecules in critical cancer pathways.
Translocations are typically thought of as gain-of-function genetic events in that they act dominantly. However, they can functionally inactivate endogenous genes through the creation of dominant-negative protein fusions. For example, the PML-RARα fusion protein is believed to act, at least in part, as a dominant-negative inhibitor of endogenous RARα function. This type of molecular action can be difficult to elucidate in functional experiments. Nonetheless, it is worth remembering this possibility when evaluating proteins or genes as drug targets when they are involved in new or existing translocations.
Chromosomal-level alterations leading to loss-of-function events include homozygous deletions, heterozygous deletions, and loss of heterozygosity. Homozygous deletions are typically highly focal in their nature. When the boundaries are defined with large sample sets, in many cases, only a few genes are found in the overlapping region. For example, in the case of focal deletions on 9p, the typical minimal deleted regions in tumors such as melanoma contain only CDKN2A and CDKN2B . Heterozygous deletions are typically larger and usually involve entire chromosomes or chromosome arms. As in the case of amplifications, such large-scale broad genetic alterations make it difficult to ascertain the cancer relevance of any single gene contained within a larger region. Loss of heterozygosity (LOH) can occur as an event associated with heterozygous deletion leaving only one parental chromosome behind, which is thus homozygous. LOH can also occur when duplication of the remaining parental chromosome creates a situation known as uniparental disomy. This is significant because it may indicate preservation of a mutated allele, leading to two mutant alleles of a given gene. In the case of the tumor suppressor gene p53 , copy-neutral LOH is common.
Inactivating point mutations are a common means of gene inactivation. In this case stop codons are the most efficient mechanism for disrupting gene function. As a result, tumor suppressor gene mutations often are enriched for stop or truncating mutations. This creates certain technical and cost difficulties in sequencing genes for inactivating mutations because it is necessary to sequence all coding exons, consider sequencing of the regulatory elements governing splicing, and consider the detection of promoter mutations. As a result of these difficulties, precise clinical analysis of tumor suppressor gene mutations remains challenging even with the application of next-generation sequencing technologies.
It is important to understand the relationship between the expression or activity of a gene product and patient outcome or other clinicopathologic data. Although establishing a relationship between protein expression and clinical data (e.g., survival) does not provide a causal link to cancer, such linkage to patient outcome likely indicates a nonrandom association with cancer behavior. Here the clinician should be careful to consider the relationship between protein expression and the state of differentiation of the cancer. Because the degree of cancer differentiation state (usually referred to as grade ) is typically already linked to patient prognosis, candidate drug targets whose protein levels vary with differentiation state may be secondarily linked to patient outcome. Thus an independent association between a protein's expression and patient outcome should be sought in multivariate analysis. In the case of the targeted disruption of HER2 in patients with breast cancer, the association of HER2 amplification with poor outcome provided a significant motivation for the development of trastuzumab, an anti-HER2 monoclonal antibody.
The ability to perform larger-scale epidemiology analyses typically relies on archived tissue for which longer-term clinical follow-up is available. This largely dictates that target epidemiology be carried out in paraffin-embedded, fixed tissue. Here RNA and DNA can be examined by in situ hybridization (ISH), fluorescence in situ hybridization (FISH), or next-generation sequencing approaches. Historically, the most common method for interrogating human tissue samples is protein-based immunohistochemistry (IHC). These studies have been made higher throughput by the advent of tissue microarrays. Such arrays align hundreds of tumor samples on a single slide, facilitating the experimental procedures. IHC is primarily limited by the quality of the antibody reagents that are used and the care with which such reagents are subject to rigorous validation. Too often, poorly described or unvalidated antibodies are used in IHC studies after a quality assessment based solely on the observed staining pattern in tissue sections. In all cases where IHC is used, the controls should include experimental, fixed cell blocks derived from negative control cells or tissues known to lack the antigen of interest and positive control cells or tissue known to harbor the antigen of interest. These controls can typically be obtained from blocks created from cell lines where the negative controls can be generated by using short hairpin RNA (shRNA). With the development of protocols for next-generation sequencing from paraffin-embedded tissues, the use of such approaches has become more commonplace for the interrogation of archived primary patient tissues.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here