Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Thoracic incisions traditionally used in adult cardiac and thoracic surgery have been used in children with varying success with respect to exposure, pain, and cosmetic result. Special considerations relevant to pediatric surgery are related to the lack of development and growth of soft tissue structures such as breast tissue and bony structures such as ribs and vertebra. Thus, incisions that fix growing bony structures, such as ribs in a posterior or lateral thoracotomy, may lead to scoliosis. Anterior thoracic incisions may also injure underdeveloped breast tissue and pectoral muscles, resulting in chest wall deformities and sensory loss. Then again, the flexibility of the chest cage and ribs in young children permit the use of limited incisions with adequate exposure of relevant structures, whereas in adults this may not be possible without the risk of rib or sternal fracture or instability of the chest cage. Therefore, these factors should be considered when selecting the optimal approach to intrathoracic structures in children, to optimize exposure and for the safe conduct of the procedure, and to minimize pain and achieve a cosmetically acceptable result.
The child should be positioned so that the surgeon and assistant have a direct view of the relevant anatomic structures and so that the anesthesiologist has access to the airway and major access lines. Because the head size of newborns and infants is significantly larger in proportion to the chest than it is in older children and adults, a shoulder roll should be used when children are placed supine on the table, to elevate the shoulders and relieve some of the pressure from the occiput. Soft padding, such as gel-filled plastic bags, should be placed under all pressure areas. Cooling or heating blankets are used routinely in pediatric cardiac surgery, but these require relatively direct patient contact to optimize heat transfer. Perforated blankets, filled with cold or heated air that is blown through, have improved heat-transfer properties compared with water-filled blankets. Direct skin contact should be avoided, however, with either method, because injury to the skin can result in full-thickness skin loss, particularly in infants. For a lateral thoracotomy, an axillary roll is used to elevate the thorax and relieve pressure on the shoulder and potentially the brachial plexus. As with any thoracotomy incision, care must be taken not to extend the arm and shoulder under tension, even in infants, because this may cause injury to the brachial plexus.
For cosmetic purposes, the skin incision can be placed below the actual entry site into the thorax, whether the incision is a sternotomy or a lateral intercostal approach. Care must be taken, however, to minimize creation of flaps, particularly in infants, because this often leads to breakdown of subcutaneous tissue with fat necrosis, resulting in wound separation. Excessive use of cautery, particularly in the subcutaneous fat in infants, is another cause of fat necrosis and wound separation, often prolonging hospital stay and resulting in a poor cosmetic result.
The most commonly used incision for access to the heart and anterior mediastinal structures is a sternotomy. A vertical skin incision over the sternum, staying below the manubrium, permits full division of the sternum and provides an unobstructed view of all anterior mediastinal structures and direct access from branches of the aortic arch down to the inferior vena cava (IVC) at the level of the diaphragm. This approach is necessary when the surgical procedure requires access to upper and lower mediastinal structures, such as the aortic arch and right atrium, branch pulmonary arteries, and right ventricle. Examples include a first-stage procedure for hypoplastic left heart syndrome, repair of truncus arteriosus, and pulmonary atresia with ventricular septal defect.
The incision in the pericardium is also vertical. If it is placed directly over the cardiac structures to be accessed, it can facilitate exposure, particularly when transatrial procedures to access the ventricles are being performed. By opening the pericardium to the right of midline and retracting the right side of the pericardium to the sternum, the ventricles fall away leftward, aiding exposure. Sutures to suspend the pericardium to the sternum are critical in children, and they should also be used to rotate the heart to facilitate exposure to the chamber or vessels required. When the right atrium and cavae need exposure, suspension of the right-sided pericardial edge to the periosteum of the sternum is often required to visualize the lateral wall of the right atrium and inferior cava. If the left side of the pericardium is left unsuspended and is incised along the edge of the diaphragm to the apex, the entire ventricular mass will rotate away from the surgeon, facilitating exposure of the atrioventricular valves and interventricular septum to the level of the apex. A similar approach can be used to optimize exposure of the right ventricular outflow tract for surgery to correct tetralogy of Fallot. Here, the pericardium at the level of the great vessels is suspended to the periosteum, leaving the pericardial edge at the diaphragm unsuspended, facilitating the view of the ventricular septal defect through the ventriculotomy.
For many procedures, a full sternotomy is not necessary to provide adequate access to all the relevant structures of the heart. This is particularly true in infants and young children because their sternum and rib cage are pliable, which permits retraction with minimal force. Preoperative planning of the procedure includes determining which mediastinal structures will need exposure for the surgical procedure and for cannulation for cardiopulmonary bypass (CPB). With the availability of thin-walled, wire-reinforced, small-diameter cannulas for use in children, cannulation for CPB can often be achieved with minimal extension of the thoracic incision beyond that needed for the repair itself. These approaches usually provide exposure sufficient to use standard techniques for myocardial protection, such as cardioplegia and left ventricular venting. A full sternotomy is unnecessary when, for example, the intracardiac repair is accomplished via a right atriotomy, such as for repair of atrial septal defect, ventricular septal defect, or complete atrioventricular canal defect, and in transatrial repair of tetralogy of Fallot and mitral valve repair.
With the trans-xyphoid mini-sternotomy approach, the skin incision extends from the level of the areola (mid thorax) down to the tip of the xyphoid process ( Fig. 108-1 ). By detaching anterior diaphragm attachments to the cartilaginous segment of the rib cage anteriorly, access is gained to the anterior mediastinum. Before performing the partial sternotomy, blunt dissection is required to detach the pericardium and thymus from the sternum. A partial sternotomy can be performed with a saw, but in infants, heavy bandage scissors are sufficient for dividing the lower sternum. Once the partial sternotomy is completed, a narrow-blade retractor such as an army-navy retractor is used to lift the sternum anteriorly and cephalad to provide exposure to the ascending aorta for cannulation. The retractor should not be placed on the skin and subcutaneous tissue, because adequate exposure of the upper mediastinum will not be achieved and necrosis of the skin could occur from prolonged retraction. To expose the aorta, however, caudal and anterior traction must be placed on the pericardium. This maneuver requires that thymus attachments to the pericardium be divided, or mobility of the pericardium and aorta will be inadequate. The pericardial incision must be made to the right of midline, and the pericardium should be pinned to the right edge of the divided sternum to expose the right atrium and cavae. In most cases, cannulation of the ascending aorta and both cavae can be easily achieved with this approach. Insertion of the inferior caval cannula through a separate skin incision keeps the cannula out of the way, and the incision can later be used for tube thoracostomy ( Fig. 108-2 ).

When the right ventricular outflow tract or aortic root must be accessed, the partial sternotomy approach may still be used, but usually the skin and sternal incisions need to be extended superiorly 1 or 2 cm above the level of the areola. In this case, traction sutures on the pericardium at the level of the pulmonary and aortic roots suspend these structures into view, permitting adequate exposure. To assist with caval cannulation, traction sutures should be placed only on the right side of the pericardium, lifting both cavae toward the right side of the sternotomy. The cardiac structures are allowed to rotate and shift leftward, enhancing exposure to the right atrium, the left atrium via the right upper pulmonary veins, and the aortic root.
An anterior thoracotomy has been advocated for surgical repair of atrial septal defect and occasionally ventricular septal defects. A meta-analysis of six case control trials found that intubation time and hospital length of stay were shorter using an anterolateral mini-thoracotomy over a median sternotomy, whereas cardiopulmonary and cross-clamp times were longer. Axillary incision have also been advocated for simple heart lesions using CPB.
For the anterolateral thoracotomy, the incision is made in the anterior fourth intercostal space (ICS), and in females great care must be taken to incise well below breast tissue. Dissection of the pericardial attachments to the sternum and thymus greatly facilitates exposure by permitting retraction of the pericardium down toward the diaphragm and bringing the ascending aorta closer into view. Direct cannulation of the ascending aorta is preferable to peripheral cannulation via the femoral or axillary artery because these vessels are small in children and stenosis at the cannulation site can result in claudication with exercise. Recently, more flexible cannulas, available in all sizes for pediatric use, have facilitated aortic cannulation. Some arterial cannulas can be introduced over a guidewire, which makes insertion easier and safer. The cavae can be cannulated directly, although the superior cava cannula is often best introduced via the right atrial appendage and directed retrograde into the superior vena cava (SVC). Aortic clamping to achieve cardiac arrest can be difficult with conventional arterial clamps, which were designed for application via a sternotomy. In small children, a bulldog clamp is sufficient for aortic occlusion, and for larger children and teenagers, flexible clamps are available. Cardioplegia can be delivered via a small flexible cannula inserted into the aortic root; some have advocated transthoracic insertion of a needle into the aortic root.
Noncardiac thoracic surgery for the treatment of congenital cardiac defects or complications of cardiac surgery most often requires exposure to the posterior mediastinum. Exceptions include procedures to plicate the diaphragm, or for unifocalization of aortopulmonary collaterals, which requires dissection of the hilum of the lung. A posterolateral incision extending from just anterior to the tip of the scapula to the mid-posterior scapula provides access, through the fourth, fifth, or sixth ICS, to posterior mediastinal structures as well as the hilum of the lung, the pericardium, and the diaphragm ( Fig. 108-3 ). If the upper half of the mediastinum needs exposure, an incision through the fourth ICS is optimal. For access to the hilum of the lung, the fifth ICS is optimal, and, for access to the thoracic duct at the level of the diaphragm, or to the central tendon of the diaphragm, a sixth ICS incision is optimal. The most common procedures in which a thoracotomy is performed routinely are operations on the thoracic aorta or branches, such as surgery for repair of coarctation of the aorta or for ligation of the patent ductus. Care should be taken to ensure that the appropriate interspace is entered, because exposure to the upper thorax can be very difficult in small children if the fifth interspace, or lower, is entered. Although counting ribs starting at the second rib, which has attachments of the anterior scalene muscle, can be done, external landmarks can also be used. A useful landmark is the position of the areola when the arm is extended over the head. With the arm extended, the interspace below the areola is usually the fourth ICS, and this landmark can confirm a particular interspace identified by other techniques. This method for identification of interspace is particularly useful in neonates and premature infants. Extensive division of the intercostal muscle is usually unnecessary in infants and small children. Adequate exposure can be obtained by separating the ribs, which are more flexible in this age group and entail less risk for fracture.

This same approach can be used for exposure of intrapericardial structures such as the pulmonary trunk for pulmonary artery banding and even for intracardiac procedures. Exposure to the IVC for cannulation through a posterolateral thoracotomy can be difficult, however, and may require division of one rib posteriorly. Closure of the thoracotomy incision, as in adults, should be done in layers, approximating the serratus muscle and fascia separate from the latissimus dorsi muscle and subcutaneous tissue. This approach minimizes distortion of the chest wall muscles and provides the best cosmetic results as well.
A transaxillary approach has been described for ligation of the patent ductus, access to the transverse aortic arch and descending aorta, and, from the right axilla, closure of atrial septal defects. Either a transverse incision is made over the third interspace between the fold of the pectoralis muscle and scapula or, as some have described, a vertical incision is made from the axilla down to the fourth ICS. The third interspace is then entered, which provides direct access to the distal aortic arch and arterial duct. However, exposure is limited and extension of the incision, if more exposure is required, is difficult. In experienced hands, however, this approach is adequate for ductus ligation, even in small infants, with a less visible incision.
Because most cardiac procedures in children require CPB and intracardiac repair, port access for reconstruction has been applied almost exclusively to adult patients or, rarely, adult-size teenagers. Thoracoscopic procedures in children have been, for the most part, confined to approaches to noncardiac structures or to the pericardium. Examples include ligation of the patent ductus, division of vascular rings, creation of a pericardial window, and, more recently, insertion of pacer leads. More recently, total thoracoscopic approaches for repair of atrial and ventricular septal defects have been described. Much of the instrumentation has been adapted from other surgical applications, and the thoracoscopic procedures have involved primarily dissection and ligation or division with little reconstruction or suturing. Using induced electromyography to establish the location and local course of the recurrent laryngeal nerve may be of some benefit in infants and small children undergoing patent ductus arteriosus ligation or vascular ring procedures.
Positioning and location of port incisions follow the principles of thoracoscopy or thoracotomy in adults. For access to the distal transverse aortic arch and descending aorta, the patient should be in a full lateral decubitus position. For access to anterior mediastinal structures, such as the anterior pericardium or thymus, a partial decubitus position with the thorax tilted toward a supine position is optimal. Usually, four incisions are required, two for the surgeon's instruments for the dissection, one for the scope and camera, and the fourth for the assistant to introduce lung retractors or occasionally a grasper or suction. As with any thoracoscopic procedure, the central port is used for the camera and the instrument ports are to each side of the camera port, separated by sufficient distance to prevent the scope from interfering with instrument movement. When a surgical robot is used to assist, the same port position is used, but the port for the lung retractor and suction is placed at the midaxillary line at the sixth or seventh ICS ( Fig. 108-4 ).
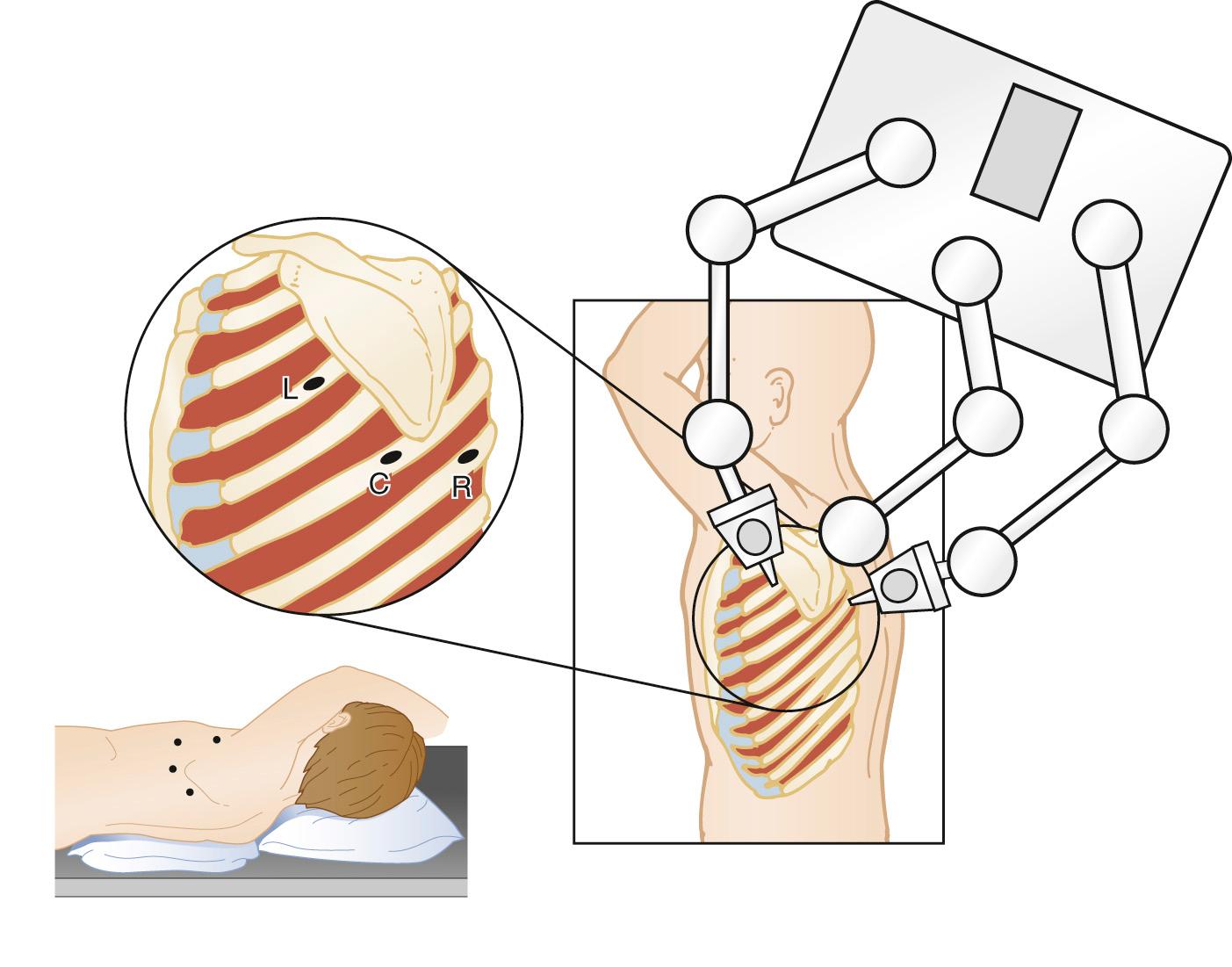
For the approach to anterior mediastinal structures, the same arrangement is used with respect to the camera and instrument ports. In cases such as dissection of the right lobe of the thymus for thoracoscopic innominate artery suspension to relieve tracheal compression, the central port for the scope is placed at the anterior axillary line in the fourth ICS, and the two instrument ports are placed two or three interspaces to each side and 2 to 3 cm more anterior. A fourth port can be used for lung retraction and should be placed one or two ICSs lower toward the diaphragm so as not to interfere with instrument motion. For the anterior pericardium, the three ports are placed more inferiorly on the chest using the fourth or fifth ICS for the scope and camera, and the instrument ports one or two interspaces on either side.
The use of extracorporeal circulatory techniques in the repair of congenital cardiac defects began in the 1950s, shortly after the concept of CPB appeared and a heart-lung machine was constructed. Gibbon repaired an atrial septal defect using the first heart-lung machine, which required 12 to 14 units of blood prime, in 1953. At approximately the same time, Lillihei and colleagues began using cross-circulation to repair a variety of defects in relatively young infants and children, including ventricular septal defects, atrioventricular canal defects, and tetralogy of Fallot, achieving a remarkable overall survival of greater than 60%. Shortly thereafter, Kirklin developed a pump oxygenator derived from Gibbon's earlier efforts. This device required approximately half of the original amount of fresh blood prime but also extreme care to prevent severe foaming of blood, which was lethal; nonetheless, the survival rate was 50%. These early reports prompted many subsequent investigations aimed at developing the scientific and technologic knowledge needed to successfully undertake extracorporeal circulation in infants and children. In spite of these efforts, the morbidity and mortality associated with the use of CPB remained high throughout the 1960s.
The next major advance occurred in the early 1970s, when Castaneda and colleagues and Barratt-Boyes described the use of deep hypothermic circulatory arrest (DHCA) in infants. These techniques relied primarily on surface cooling, with exposure to the CPB circuit limited to a brief period of core cooling and rewarming, so that total CPB time was typically kept under 20 to 30 minutes. Progressive advances in the design of circuit components and perfusion techniques for infants and small children occurred throughout the 1980s and into the early 1990s. As a result, the “toxicity” associated with the use of CPB in infants declined significantly. Currently, lengthy and complex repairs, such as the arterial switch procedure for transposition of the great arteries and primary repair of tetralogy of Fallot, can be undertaken using CPB in neonates and very young infants and result in an overall mortality rate of less than 5%. Nonetheless, the morbidity associated with the use of CPB in infants and children is still widely held to be a major limitation to completely successful outcomes.
There are many significant differences in circuit technology and the physiologic effects of CPB in neonates, infants, and small children compared with adults. The surface area and volume of the CPB circuit relative to patient size and blood volume is much greater for neonates and infants. Arterial and venous cannulas are smaller but more likely to deform or obstruct the aorta or venae cavae. The placement of these cannulas can be different and more variable than in adults—for example, separate superior and inferior vena caval cannulas or initial placement of the aortic cannula in the pulmonary artery (with retrograde systemic perfusion via the ductus arteriosus) during stage I repair of hypoplastic left heart syndrome. To minimize hemodilution, the sizes of various circuit components and tubing diameters are kept as small as possible. Nonetheless, hemodilution that is equivalent to one to two blood volumes from the circuit prime and cardioplegia is fairly common in neonates and small infants (see Table 108-1 ).
| Oxygenator | Optimal Body Surface Area (m 2 ) † | Estimated Total Prime Volume ‡ (mL) | Membrane Surface Area (m 2 ) | Heat Exchange Surface Area (m 2 ) | Manufactured Recommendation Maximal Flow Rate (mL/min) |
|---|---|---|---|---|---|
| Dideco Kids D100 | <0.23 | 240-265 | 0.22 | 0.03 | 700 |
| Terumo Baby RX | 0.3-0.4 | 290-320 | 0.5 | 0.035 | 1500 |
| Terumo RX15-30 | 0.5-0.7 | 590-655 | 1.5 | 0.14 | 4000 |
| Terumo RX15-30 Small Adult/KVAD | 1.0-1.3 | 990-1075 | 1.5 | 0.14 | 5000 |
| Terumo RX25 Adult/KVAD | >1.34 | 1200-1275 | 2.5 | 0.2 | 7000 |
* Used under standard configuration.
† Assuming maximum 3 liter/min/m 2 flow rate.
‡ Assuming use of standard configuration and usual tubing size and length for weight.
DHCA, although used much less frequently than even a few years ago, is still used occasionally. Overall, pump flow rates can range from no flow (i.e., circulatory arrest) to more than 200 mL/min; mean arterial pressures can vary from 10 to 20 mm Hg during low-flow CPB to more than 50 mm Hg at full or high flow. Temperatures are typically lower in CPB for infants (core temperatures of 15° to 18° C for deep hypothermia; 22° to 25° C is used by some for many other complex repairs), and different blood pH management strategies may be used (i.e., alpha-stat versus pH-stat). In part because of these differences, the magnitude of neuroendocrine stress responses and systemic inflammatory responses to CPB, as well as their consequences, are generally believed to be more profound in neonates and infants than in adults.
Patient-specific variables and the diverse pathophysiology associated with specific congenital cardiac defects further complicate CPB in neonates, infants, and small children. It is likely that neonates, in general, and particularly those who are premature or weigh less than 1.8 to 2.0 kg, comprise a high-risk group because of immature organ function and coexisting diseases such as sepsis, respiratory distress syndrome, and other congenital anomalies. The immature myocardium may be similarly prone to CPB-related dysfunction for several reasons, including its relatively deficient (compared with in adults) contractile protein mass and organization of contractile proteins, the presence of fetal contractile protein isoforms, immature calcium cycling (which occurs primarily via the sarcolemmal membrane as opposed to the sarcoplasmic reticulum, which is less abundant and less well organized), and fewer mitochondria.
Various aspects of congenital heart disease can mean additional complicating features. Hypertrophic and cyanotic myocardium is more likely to be injured by ischemia-reperfusion and other consequences of CPB. Aortopulmonary collaterals, which can be particularly significant in various cyanotic lesions, may promote pulmonary dysfunction as a result of high flow on CPB, whereas steal from systemic perfusion can compromise the function of other organs, and collaterals to the coronary circulation can wash out cardioplegia and thereby hinder effective myocardial preservation. Pulmonary dysfunction after CPB may be more prevalent in infants with other routes of high pulmonary blood flow (e.g., truncus arteriosus, hypoplastic left heart syndrome, transposition of the great arteries) and in cyanotic infants. Diffuse organ dysfunction is likely to be more common in patients who were severely cyanotic and hypoperfused at the time of delivery or who required complex surgery in the early neonatal period. In the neonate who was compromised at the time of delivery or thereafter, most centers have found it beneficial to allow a period of stabilization of the circulation and recovery of organ function prior to undertaking CPB and cardiac surgery, using lesion-appropriate interventions such as prostaglandin E1, inotropic support, ventilatory strategies to balance systemic and pulmonary blood flow, and even extracorporeal circulatory support (see later). Post-CPB organ dysfunction (e.g., kidney, liver) can also be a source of morbidity in older (i.e., adult-age) patients with various forms of congenital heart disease complicated by long-standing cyanosis, low cardiac output, or high systemic venous pressures.
A summary of the components of different-sized CPB circuits is shown in Table 108-1 .
Oxygenator systems for infants and children must function over a wide range of pump flow rates (maximal flow rates range between 800 and 4000 mL/min, and they must be efficient over a range of flows equivalent to 0 to 250 mL/kg), temperatures (10° to 38° C), hematocrits (15% to 40%), and line pressures (because of different sizes of cannulas and tubing).
Virtually all current pediatric CPB applications use membrane-type oxygenators. The two main types are microporous (hollow-fiber or folded-membrane) and nonporous membrane oxygenators. The major advantage of microporous-type membranes is their ability to effect gas exchange with a relatively modest membrane surface area, typically in the range of 0.2 to 1.5 m 2 , depending on the specific oxygenator and configuration. Major disadvantages include some blood-gas contact at the start of CPB (until protein accumulation blocks the 0.05- to 0.25-µm pores) and protein leakage across the membrane, along with the potential for gas embolization if negative pressure develops on the blood side of the artificial membrane. Nonporous oxygenator membranes, typically of the folded-sheet silicone-membrane variety, require a larger surface area to achieve gas exchange but do not accumulate or leak protein as readily and are therefore more often selected for longer-term circulatory support applications (e.g., extracorporeal membrane oxygenation).
There is a real need to minimize circuit priming volume to minimize hemodilution, blood product exposure, and the potential for fluid overload and edema. Priming volume is usually defined as the volume of the membrane compartment plus the minimal amount required for the venous reservoir. Typical priming volumes of commercial membrane oxygenators range from approximately 225 to 375 mL when used in the open configuration. The Dideco Lilliput hollow-fiber membrane oxygenator (Dideco, Mirandola, Italy) is an example of one with a smaller prime volume (≈70 mL), but it is not available in an open-system configuration. More recently, oxygenators and arterial filters have been combined into one unit, further reducing priming volume (e.g., Quadrox-I Neonatal and Pediatric with integrated arterial filter, Maquet, Rastatt, Germany; or Capiox FX05, Terumo Corporation, Tokyo, Japan). Various schema for achieving low priming volume and thereby reducing or avoiding the need for blood products as a component of the priming solution have been described.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here