Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pulmonary thromboembolism is a significant cause of morbidity and mortality in the United States and worldwide. The estimated incidence of acute pulmonary embolism is approximately 63 per 100,000 patients in the United States, based on clinical and radiographic data. Acute pulmonary embolism is the cause of approximately 235,000 deaths per year based on autopsy data. Acute pulmonary embolism occurs half as often as acute myocardial infarction and is three times as common as cerebrovascular accident. It is the third most common cause of death (after heart disease and cancer). Estimates of the incidence of acute pulmonary embolism, however, are generally thought to be low, because in 70% to 80% of patients in whom the primary cause of death was pulmonary embolism, the diagnosis was unsuspected premortem.
Of patients who survive an acute pulmonary embolic event, approximately 3.8% will go on to develop chronic pulmonary hypertension (PH). Once PH develops, the prognosis is poor, and this prognosis is worsened in the absence of intracardiac shunt. Patients with PH caused by pulmonary emboli fall into a higher risk category than those with Eisenmenger syndrome, and they encounter a higher mortality rate. In fact, once the mean pulmonary pressure in patients with thromboembolic disease exceeds 50 mm Hg, the 3-year mortality rate approaches 90%. Despite an improved understanding of pathogenesis, diagnosis, and management, pulmonary emboli and their long-term sequelae remain frequent and often fatal disorders.
Pulmonary embolism was first described by Laennec in 1819. It was he who related the condition to deep venous thrombosis, and Virchow later associated the three factors predisposing to venous thrombosis as stasis, hypercoagulability, and vessel wall injury. Virchow distinguished two types of thrombus in the pulmonary arteries of such patients: the embolus that arose as thrombus in a systemic vein and the thrombus that occurred in situ within the pulmonary arteries distal to the occluding embolus as a result of the stagnant blood flow in that segment. To prove that pulmonary emboli arose from the peripheral venous circulation, Virchow inserted pieces of rubber or venous thrombi recovered from humans at autopsy into the jugular or femoral veins of dogs. When the animals were sacrificed, the foreign embolic material was found in the pulmonary arteries. Although pulmonary embolism can be caused by tumors, septic emboli, and foreign bodies, the overwhelming occurrence of pulmonary embolism is due to venous thromboembolism.
The majority of pulmonary thromboembolic episodes are silent, and it is not until the amount of embolic material is substantial that the patient becomes symptomatic. After an acute, major thromboembolic episode, approximately 15% to 20% of patients die within 48 hours. Most of the remaining patients resolve the emboli substantially by a variety of mechanisms. Therefore, it is in the subgroup of patients who have a sudden fatal outcome (≈100,000 annually) that invasive therapy for acute pulmonary embolism might be considered.
Although the role of surgical therapy for the PH resulting from chronic pulmonary emboli is now well established, the appropriate treatment for acute pulmonary embolism remains unclear. There are several reasons for this. Many patients die of massive pulmonary embolism in the terminal phases of another illness, which would make aggressive therapy inappropriate. For patients in whom invasive therapy is potentially indicated, there is substantial difficulty in defining which patients will respond to anticoagulation therapy for an acute massive pulmonary embolism in the limited amount of time available for diagnosis and treatment before death occurs.
The hemodynamic response to a large, sudden pulmonary embolus relates to a variety of factors, most notably the size of the embolus, the degree of obstruction that it produced in the pulmonary vascular bed, and the underlying function of the lung that remains perfused. The degree of vascular obstruction is related to the number of segmental arteries that are occluded and to prior pulmonary vascular capacitance. Thus, the hemodynamic consequences of acute pulmonary embolism are also a reflection of factors, such as the age of the patient and any possible previous thromboembolic events. The preexisting status of the right ventricle that governs the forward flow of blood is also significant in determining the hemodynamic response to pulmonary embolism. Right ventricular function is affected by factors such as the degree of right ventricular hypertrophy or dilation, tricuspid valve regurgitation, and the presence of coronary artery disease.
In addition to the mechanical factor of pulmonary artery obstruction, there are reflex and hormonal factors that can increase pulmonary vascular resistance (PVR) at the time of acute pulmonary embolism. Humoral factors, specifically serotonin, adenosine diphosphate, platelet-derived growth factor, and thromboxane, are released from platelets attached to the thrombi, whereas platelet-activating factor and leukotrienes are secreted by neutrophils. Anoxia and tissue ischemia downstream from emboli inhibit endothelium-derived relaxing factor production and enhance release of superoxide anions by activated neutrophils. The combination of these humoral effects contributes to enhanced pulmonary vasoconstriction. Thus, some patients with a relatively small embolus may have an exaggerated response to the degree of pulmonary vascular obstruction.
In patients without preexisting cardiac or pulmonary disease, an obstruction of less than 20% of the pulmonary vascular bed results in minimal hemodynamic consequences. It is only when the acute pulmonary obstruction exceeds 50% to 60% of the pulmonary vascular bed that cardiac and pulmonary compensatory mechanisms are overcome and cardiac output begins to fall. Right ventricular failure occurs, which is accompanied by systemic hypotension as the amount of blood reaching the left ventricle decreases. The dilated right ventricle causes a shift of the ventricular septum to the left, further compromising left ventricular filling. Although patients with chronic pulmonary artery obstruction can have high pulmonary artery pressure levels that reflect the degree of obstruction, in acute pulmonary embolism the previously normal right ventricle cannot generate these pressures. Therefore, in acute massive pulmonary embolism, pulmonary artery pressures may be normal, and a pulmonary artery systolic pressure of 30-40 mm Hg may represent severe PH.
Acute pulmonary embolism usually presents suddenly. Symptoms and signs vary with the extent of blockage, the magnitude of humoral response, and the preembolus reserve of the cardiac and pulmonary systems of the patient. The clinical diagnosis is often missed or falsely made. Most pulmonary emboli occur without sufficient clinical findings to suggest the diagnosis, and in an autopsy series of proven emboli, only 16% to 38% of patients received a diagnosis while still alive. The acute disease is conveniently stratified into low-risk, submassive, or massive embolism on the basis of hemodynamic stability, arterial blood gases, and lung scan or angiographic assessment of the percentage of blocked pulmonary arteries.
For patients with minor pulmonary embolism, physical examination may reveal tachycardia, rales, low-grade fever, and sometimes a pleural rub. Heart sounds and systemic blood pressure are often normal; sometimes the pulmonary second sound is increased. Less than one third of patients with acute pulmonary embolism have concurrent evidence of clinical deep venous thrombosis. Room air arterial blood gases indicate a Pa o 2 between 65 and 80 torr and a normal PaC o 2 of approximately 35 torr. Pulmonary angiograms typically show less than 30% occlusion of the pulmonary arterial vasculature. Recent studies suggest that normotensive patients who have normal biomarker levels (Brain Natriuretic peptide [BNP], N-terminal pro-BNP, Troponin I, Troponin T) and have no RV dysfunction on echocardiographic imaging have short-term mortality rates approaching 1%.
Submassive pulmonary embolism is defined as an acute pulmonary embolism without systemic hypotension (sytolic blood pressure > 90 mm Hg) but with either right ventricular dysfunction or myocardial necrosis. This form of embolism is associated with dyspnea, tachypnea, dull chest pain, and some degree of cardiovascular changes manifested by tachycardia, mild to moderate hypotension, and elevation of central venous pressure. Some patients may have syncope rather than dyspnea or chest pain. In contrast to massive pulmonary embolism, patients with submassive embolism (at least two lobar pulmonary arteries obstructed) are usually hemodynamically stable and have adequate cardiac output. Room air blood gases reveal moderate hypoxia, with a Pa o 2 between 50 and 60 torr, and mild hypocarbia, with a PaC o 2 no more than 30 torr. Echocardiograms may show right ventricular dilation. Pulmonary angiograms indicate that 30% to 50% of the pulmonary vasculature is blocked; however, in patients with preexisting cardiopulmonary disorders, a lesser degree of vascular obstruction may produce similar symptoms.
Massive pulmonary embolism is life threatening and is defined as a pulmonary embolism that causes hemodynamic instability, requiring inotropic support. It is usually associated with occlusion of more than 50% of the pulmonary vasculature, but it can occur with much smaller occlusions, particularly in patients with preexisting cardiac or pulmonary disease. The diagnosis is clinical, not anatomical. Patients develop acute dyspnea, tachypnea, tachycardia, and diaphoresis and may lose consciousness. Both hypotension and low cardiac output (<1.8 L/min/m 2 ) are present. Cardiac arrest can occur. Neck veins are distended, central venous pressure is elevated, and a right ventricular impulse may be present. Room air blood gases show severe hypoxia (Pa o 2 < 50 torr), hypocarbia (PaC o 2 < 30 torr), and acidosis. Urine output falls, and peripheral pulses and perfusion are poor.
The clinical diagnosis of submassive or massive pulmonary embolism is unreliable and is incorrect in 70% to 80% of patients who have subsequent angiography. The differentiation between submassive or massive pulmonary embolism and acute myocardial infarction, aortic dissection, septic shock, and other catastrophic states can be difficult and costly in time. Although plain chest radiography, electrocardiogram, and insertion of a bedside Swan-Ganz catheter may add confirmatory information, they will not necessarily prove the diagnosis. Routine laboratory test results are usually normal.
The most common electrocardiographic abnormalities of acute pulmonary embolism are tachycardia and nonspecific ST and T wave changes. The major value of the electrocardiogram is the exclusion of a myocardial infarction. A minority of patients with massive embolism may show evidence of cor pulmonale, right axis deviation, or right bundle branch block. Chest radiography may show oligemia (Westermark sign) or linear atelectasis (Fleischner lines), both of which are nonspecific findings. Ventilation–perfusion (V/Q) scans can provide confirmatory evidence, but these studies can be unreliable because pneumonia, atelectasis, previous pulmonary emboli, and other conditions can cause a mismatch in ventilation and perfusion that mimics positive results.
In general, negative V/Q scans exclude the diagnosis of clinically significant pulmonary embolism. V/Q scans are usually interpreted as high, intermediate, or low probability of pulmonary embolism to emphasize the lack of specificity but high sensitivity of the test. Magnetic resonance angiography is an excellent noninvasive method for the diagnosis of pulmonary emboli, and it provides specific information regarding flow within the pulmonary vasculature. Unfortunately, this method is expensive, time consuming, and not widely available. Like catheter-based pulmonary angiography, it is generally not suitable for hemodynamically unstable patients. Transthoracic echocardiography or transesophageal echocardiography with color flow Doppler mapping can provide reliable information about the presence or absence of major thrombi obstructing the main pulmonary artery; however, these techniques are usually inadequate for visualization of the lobar vessels, where the embolic material is often localized. More than 80% of patients with clinically significant pulmonary embolism have abnormalities of right ventricular volume or contractility, often associated with acute tricuspid regurgitation. In a subset of patients, abnormal flow patterns can be discerned in major pulmonary arteries during transesophageal echocardiography.
Although prophylactic measures should be considered and used for all patients undergoing major surgery or who have prolonged immobility, certain other patients also fall into a potentially high-risk group for pulmonary embolism. These patients include those with previous embolism, malignancy, cardiac failure, obesity, or advanced age. The prevalence of deep venous thrombosis of the thigh or pelvis, its strong association with pulmonary embolism, and the identification of the associated risk factors listed earlier provide the basis and rationale for prophylactic anticoagulation for the prevention of acute pulmonary embolism. Simple measures such as compression stockings probably should be prescribed more often and should be used in most nonambulating patients in the hospital. Intermittent pneumatic compression devices are more cumbersome, but also effective. These compression devices are available for the calf or the whole leg. They can provide a range of compression pressures, inflation and deflation duration, and sequential or nonsequential inflation, although a clear difference between these variations has not been demonstrated. Both compression stockings with a compression pressure of 30 to 40 mm Hg at the ankle and pneumatic compression devices reduce the incidence of deep venous thrombosis after general surgery to approximately 40% of control patients. Multiple studies have shown that low-dose subcutaneous heparin or low-molecular-weight heparin given once a day reduces the incidence of deep venous thrombosis with a concomitant reduction in the incidence of pulmonary embolism. This reduction in thrombosis has not been associated with an excessive risk of bleeding. Recent studies suggest that of patients who have deep venous thrombosis diagnosed in the hospital without pulmonary embolism, the probability of clinically diagnosed pulmonary embolism within the next 12 months is 1.7%. If pulmonary embolism occurs, the probability of recurrent pulmonary embolism is 6%.
The majority of patients who die of pulmonary embolism do so within 2 hours of the initial acute event, before the diagnosis can be firmly established and before effective therapy can be instituted. Once the diagnosis is made, however, treatment will be either medical (supportive and thrombolytic therapy) or surgical ( Fig. 102-1 ).
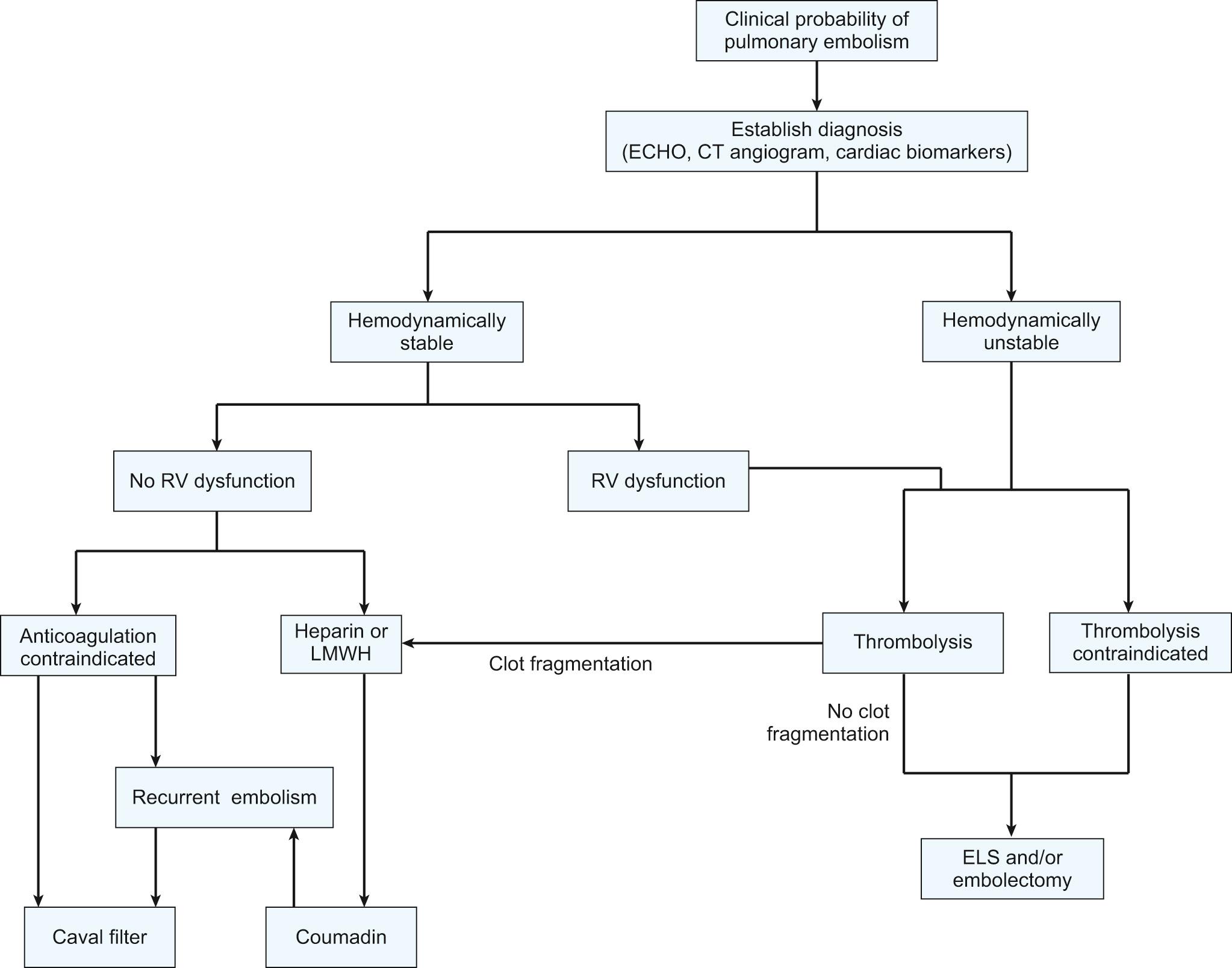
Oxygen should be administered to alleviate hypoxic pulmonary vasoconstriction, and it is likely that a severely affected patient will require intubation and ventilatory support. Pharmacologic agents, including cardiovascular pressors and vasoactive agents, can be used to stabilize the patient's hemodynamics. Once the circulation has been stabilized, arterial and central venous catheters are placed to monitor cardiac output and pulmonary arterial oxygen saturation. There is debate as to whether pulmonary artery catheters, although obviously helpful in management, should be used in the setting of acute pulmonary embolism, because of the risk of dislodging further thromboembolic material. The electrocardiogram is monitored, a Foley catheter is inserted for accurate recording of urine output, and blood gas levels are obtained. Patients with objectively confirmed pulmonary embolism and no contraindications for anticoagulation should receive prompt anticoagulant therapy with subcutaneous low-molecular-weight heparin, intravenous or subcutaneous unfractionated heparin, or subcutaneous fondaparinux (an inhibitor of activated factor Xa). For patients with suspected or confirmed heparin-induced thrombocytopenia, a non–heparin-based anticoagulant, such as lepirudin, argatroban, or bivalirudin should be used. Although each of these individual therapies prevents propagation and formation of new thromboemboli, they rarely dissolve the existing clot. In most cases, the patient's intrinsic fibrinolytic system will lyse fresh thrombi over a period of days to weeks. Intravenous heparin is monitored by measurement of activated partial thromboplastin times, which are maintained between 51 and 70 seconds (roughly twice that of controls) every 6 to 8 hours. The platelet count should be measured every 2 to 3 days to detect the presence of heparin-induced thrombocytopenia. Prothrombin times are also obtained at baseline to prepare for anticoagulation with warfarin later.
Although warfarin is typically used for long-term anticoagulation after acute pulmonary embolism, several new oral anticoagulants have recently come on the market that do not require laboratory monitoring of anticoagulation ( Table 102-1 ). They include factor Xa inhibitors, rivaroxaban (Xarelto), apixaban (Eliquis), and edoxaban (Savaysa), as well as the direct factor IIa (thrombin) inhibitor dabigatran (Pradaxa). All these drugs except edoxaban have been approved by the U.S. Food and Drug Administration (FDA) for the long-term treatment of deep venous thrombosis with or without pulmonary embolism, with edoxaban currently undergoing FDA scrutiny. To date, there are no known clinically validated reversal agents for rivaroxaban, aprixaban, edoxaban, or dabigatran. Based on the strength of available evidence and current American College of Chest Physicians Evidence-Based Guidelines, warfarin continues to be a monitorable, reversible, and effective agent in patients with venous thromboembolism and acute pulmonary embolism, and it should remain the first-line option for long-term (3-6 month) treatment of pulmonary embolism. However, the factor Xa inhibitors rivaroxaban and apixaban, as well as the direct thrombin inhibitor dabigatran, are each viable alternatives in patients with less than optimal INR control (targeted therapeutic range < 60%) or in whom warfarin monitoring and management is not possible. Three months of warfarin anticoagulation is recommended for patients with a first-episode deep venous thrombosis related to a major reversible risk factor (i.e., recent surgery or trauma). Six months of warfarin anticoagulation is recommended for patients who have recurrent or unprovoked deep venous thrombosis with or without pulmonary embolism as prophylaxis against recurrent disease.
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|
| Trade name | Pradaxa | Xarelto | Eliquis | Savaysa |
| Approved by the FDA for treatment of PE | Yes | Yes | Yes | No |
| Target | Factor IIa | Factor Xa | Factor Xa | Factor Xa |
| Onset of action | 2 h | 2.5-4 h | 3 h | 1-5 h |
| Half-life | 12-14 h | 9-13 h | 8-11 h | 8-10 h |
| Renal clearance (%) | 80 | 60 | 25 | 35 |
| Metabolism | P-gp | P-gp/CYP3A4 | P-gp/CYP3A4 | P-gp/CYP3A4 |
| Dosing | b.i.d. | b.i.d., qd | b.i.d. | b.i.d, qd |
The natural history of the clot in survivors of acute embolic events is fragmentation and progressive lysis. It therefore follows that the addition of streptokinase, urokinase, or recombinant tissue plasminogen activator improves survival by increasing the rate of lysis of fresh thrombi in the pulmonary arterial tree. Thromboembolytic agents dissolve thrombi by activating plasminogen to plasmin. Plasmin, when in proximity to a thrombus, degrades fibrin to soluble peptides. Circulating plasmin also degrades soluble fibrinogen and, to variable degrees, factors II, V, and VIII. In addition, increased concentrations of fibrin and fibrinogen degradation products contribute to coagulopathy by both inhibiting the conversion of fibrinogen to fibrin and interfering with fibrin polymerization. The thromboembolytic agents currently approved by the FDA for the treatment of acute pulmonary embolism are streptokinase, urokinase, recombinant tissue plasminogen activator (alteplase), and tissue plasminogen activator. Some new agents (so-called second-generation thrombolytics) such as reteplase, saruplase, staphylokinase, tenecteplase, and anistreplase are undergoing clinical testing.
Although thrombolytic therapy has been shown to increase the rate of lysis of fresh pulmonary clots more than that of heparin alone, there has been little difference measured in the amount of residual thrombus between the two treatments in early studies using small cohorts of patients. However, four registries (MAPPET, ICOPER, RIETE, EMPEROR) comparing thrombolytic agents with intravenous (IV) heparin in acute pulmonary embolism have been large enough to detect a significant difference in the most important endpoint—mortality, particularly in patients with massive pulmonary embolism, presenting with hypotension. More recent experience suggests a trend toward better results with thrombolytic therapy because of a more rapid diminution in right ventricular afterload and dysfunction. Thus, thrombolytic therapy should also be considered in normotensive patients with evidence of severe right ventricular dysfunction on echocardiography. Compared with heparin therapy alone, thrombolytic agents carry a higher risk of bleeding problems, with up to 20% of patients experiencing a significant bleeding complication. In general, thrombolytic therapy is contraindicated in patients with intracranial hemorrhage, fresh surgical wounds, anemia, recent stroke, peptic ulcer, or bleeding dyscrasias.
Percutaneous techniques to recanalize complete and partial occlusions in the pulmonary trunk or major pulmonary arteries are potentially life saving in selected patients with massive or submassive pulmonary embolism. Transcatheter procedures can be performed as an alternative to thrombolysis when there are contraindications or when emergency surgical embolectomy is unavailable or contraindicated. Catheter interventions can also be performed when thrombolysis has failed to improve hemodynamics in the acute setting. The goals of catheter-based therapy include rapidly reducing pulmonary artery pressure, increasing systemic perfusion, and facilitating RV recovery.
There are three general categories of percutaneous intervention for removing emboli and decreasing thrombus burden: aspiration thrombectomy, thrombus fragmentation, and rheolytic thrombectomy. For aspiration thrombectomy, a device is used that consists of a small terminal cup attached to a flexible catheter. Syringe suction is applied to the cup as a thrombus is engaged. The catheter and clot are removed en masse through the venotomy site, and this process can be repeated multiple times. Thrombus fragmentation has been performed with balloon angioplasty, a pigtail rotational catheter, or a more advanced fragmentation device (the Amplatz catheter with an impeller housed in a capsule at the tip) driven by a compact air turbine that homogenizes thrombus by maceration and fragmentation. A metal capsule protects the vessel from the rotating impellar. A third technique, the rheolytic thrombectomy technique, uses a dual-lumen catheter: one small lumen for delivery of pulsatile pressurized saline and a larger effluent or exhaust lumen to drain the thrombus. High-velocity saline jets are injected toward the effluent lumen. This produces an area of low pressure at the tip of the catheter (Venturi effect), thereby fragmenting the thrombus and allowing it to be evacuated through the effluent lumen.
Recently, a new technique of suction thrombectomy with extracorporeal venovenous support (AngioVac, Angiodynamics, Latham, NY) has been described for the removal of proximal pulmonary emboli. With this technique, a drainage cannula with a balloon-actuated, expandable, funnel-shaped distal tip is introduced through a femoral or right internal jugular vein and advanced under fluoroscopy to the site of embolism. The cannula is connected in circuit with an extracorporeal circulation pump attached to a second cannula inserted into the contralateral femoral vein. Venovenous bypass is instituted, and particulate matter suctioned through the inflow cannula is trapped within filters in the circuit, allowing for reinfusion of blood that is free of gross debris. This hybrid procedure is typically performed in an operating room, with thoracic surgeon and cardiologist present. Successful catheter extraction of thrombus with clinically significant reduction in pulmonary arterial pressure for each of the above methods varies between 75% and 88%, with the best results achieved for proximal and main pulmonary artery embolism.
When contraindications preclude thrombolysis, emergency pulmonary thromboembolectomy is indicated for suitable patients with either life-threatening circulatory insufficiency from massive pulmonary embolism or submassive pulmonary embolism. The decision to proceed with catheter-based versus surgical embolectomy requires interdisciplinary teamwork, discussion that involves the surgeon and interventionalist, and an assessment of the local expertise. If a patient has been taken directly to the operating room without a definitive diagnosis, transesophageal or epicardial echocardiography and color Doppler mapping can confirm or refute the diagnosis in the operating room. The primary difficulty with the broad application of operative embolectomy is that it is almost impossible to determine which patients will die without intervention. An emergency pulmonary embolectomy is most feasible (because of time) and most successful in patients who ultimately may not require it, which makes it difficult to establish the efficacy of this operation. To date, no randomized trial has evaluated surgical embolectomy in patients with acute pulmonary embolism. Indications for acute surgical intervention include the following: (1) critical hemodynamic condition, with the patient deemed unlikely to survive; (2) definitive diagnosis of pulmonary embolism in the main or lobar pulmonary arteries with compromise of oxygen gas exchange; (3) unstable patients in whom thrombolytic or anticoagulation therapy is absolutely contraindicated; and (4) the presence of a large clot trapped within the right atrium or ventricle.
Acute pulmonary embolectomy was first described by Trendelenburg in 1908 using pulmonary artery and aortic occlusion, through a transthoracic approach. There were no surviving patients. Sharp performed the first successful open embolectomy, using cardiopulmonary bypass.
For pulmonary embolectomy, a median sternotomy incision is used and cardiopulmonary bypass is instituted. The procedure is best performed on a warm, beating heart, without aortic cross-clamping, cardioplegia, or fibrillatory arrest. Occluding tapes are placed around the superior and inferior vena cavae. Two polypropylene sutures are placed in the mid–pulmonary artery for traction. A longitudinal incision is made between these sutures in the main pulmonary artery trunk 1 to 2 cm distal to the valve. If necessary, the incision can be extended directly into the left pulmonary artery. Extraction is limited to directly visible emboli, which can be accomplished to the level of the lobar and segmental arteries. Fresh clot removal from subsegmental arteries may be attempted, but is often surgically difficult because of the soft, sticky, often fragmental nature of peripheral pulmonary emboli. The emboli are extracted using forceps, suction, and balloon catheters. The right pulmonary artery also can be exposed and opened between the aorta and superior vena cava to allow better exposure in segmental vessels, if necessary. A sterile pediatric bronchoscope can be used to visualize emboli in tertiary or quaternary pulmonary vessels, so that they can be cleared with balloon embolectomy or suction. After cleaning the pulmonary arterial tree lumina, the pleural spaces can be entered, and the lungs are manually compressed to dislodge small distally lodged clots, which can then be suctioned out. The pulmonary arteriotomy is then closed with a 6-0 polypropylene suture. After restarting the heart, the patient is weaned from bypass, decannulated, and closed. The aim of this operation is to remove most of the embolic material, and no attempt is made to perform an endarterectomy.
As a corollary to this operation, some groups recommend either placement of an inferior vena caval filter or caval clipping before chest closure. Greenfield has recommended placement of an inferior vena caval filter under direct visualization before closing the chest. Historically, some European surgeons clipped or plicated the intrapericardial vena cava at the end of the embolectomy to prevent migration of lower body clot into the pulmonary circulation; however, this procedure is associated with stasis in the venous system in the lower body and leg swelling. In most centers that offer emergency pulmonary thromboembolectomy, no caval procedure is performed in the perioperative period, and recurrent deep venous thrombosis or pulmonary embolism are treated by anticoagulation with warfarin for 6 months. Percutaneously placed filters are recommended only for patients with contraindications to anticoagulation or for patients with recurrent pulmonary embolism on therapeutic anticoagulation. The cone-shaped Greenfield filter is the most widely used permanent filter in the United States, and it is associated with a lifetime recurrent embolism rate of 5% and a lifetime patency rate of 97%. The advent of retrievable inferior vena caval (IVC) filters appears to have lowered thresholds for IVC filter placement in the United States; however, there are few data to support or refute this claim. Late complications of permanent and retrievable filters include recurrent deep venous thrombosis (21%), IVC thrombosis (2% to 10%), and IVC penetration (0.3%). IVC filter fractures have also been reported.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here