Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
MILLIONS OF CHILDREN UNDERGO SURGERY with anesthesia every year. During the perioperative period they are exposed to a multitude of stressors capable of interfering with normal brain development. Pain, stress, inflammation, hypoxia, and ischemia have all been shown to adversely affect the immature central nervous system (CNS). However, recent findings from animal studies have indicated that sedatives and anesthetics—the very drugs used to reduce pain and stress—may themselves undesirably influence brain development by triggering structural and functional abnormalities. In fact, this phenomenon is currently one of the most intensely investigated laboratory research fields in anesthesiology and a passionately debated topic ( Fig. 25.1 ). To date, more than 400 animal studies have addressed the effects of anesthetics on the developing brain. However, translating these laboratory findings to humans in clinical settings has been complicated.
To begin with, human epidemiologic studies have found mixed evidence for an association between surgery with general anesthesia in childhood and subsequent neurodevelopmental abnormalities. Some studies identified more frequently occurring learning disabilities in children after surgery with general anesthesia early in life, whereas others have not. Although surgery or comorbidities may affect the developing brain, the mounting laboratory data demonstrating deleterious effects of anesthetic exposure without surgery have forced clinicians to consider the possibility that anesthetics may also play a role in this phenomenon. However, similar to the uncertainties surrounding animal studies, the interpretation of the human data is fraught with substantial limitations.
Any discussion of the long-term effects of anesthetics on the developing brain is further complicated by the fact that anesthetic drugs may be organ protective under certain conditions, and may indeed mitigate brain damage that is due to inflammatory responses, hypoxia-ischemia, or other insults that might occur during the perioperative period.
It is currently impossible to provide any definitive statements about the effects of anesthesia on human neurodevelopment. This chapter provides an overview of the current laboratory and clinical data concerning the affect of sedatives, anesthetics, and analgesics on the immature brain.
About 170 years ago, William T.G. Morton conducted the first successful public demonstration in the Western hemisphere of a drug-induced, reversible coma during a surgical procedure at the Massachusetts General Hospital. After witnessing Morton's demonstration, Oliver Wendell Holmes called the phenomenon “anesthesia,” from the Greek words an- (without) and aisthēsis (sensibility), and it instantaneously revolutionized the field of surgery. The inscription on Morton's tombstone, “Inventor and Revealer of Inhalation Anesthesia: Before Whom, in All Time, Surgery was Agony; By Whom, Pain in Surgery was Averted and Annulled; Since Whom, Science has Control of Pain,” represents a powerful testament to the tremendously positive impact that anesthesiology has made on the field of medicine. The use of general anesthetics to facilitate surgery quickly spread around the world and according to recent estimates, anesthesia currently allows more than 230 million patients of all ages worldwide to undergo major surgical procedures every year. This positive impact is now tempered by the possibility that anesthetics may adversely affect normal brain development. During the first century of their use, general anesthetics were regarded with serious concern because they were combustible and carried substantial hemodynamic and respiratory adverse effects. Accordingly, up until 25 to 30 years ago, general anesthesia was rarely used in critically ill neonates because of the fear of myocardial depression and hemodynamic instability. Perioperative drug regimens were often limited to neuromuscular blocking agents and nitrous oxide. However, with the realization that unopposed pain exerts deleterious effects on the developing brain, that dramatic stress responses to painful stimulation are detectable even in preterm infants, and that modern anesthetics and analgesics can abolish these responses without substantial hemodynamic compromise, pediatric anesthesia, for the past three decades, has afforded critically ill neonates the benefits of amnesia, analgesia, and immobility during increasingly invasive surgeries. These surgical interventions have helped to save countless lives and preserve quality of life in this vulnerable population. All the while, the powerful coma induced by general anesthetics has been thought to be temporary and devoid of serious long-term adverse effects after emergence. This notion of reversibility is now being seriously questioned because of structural abnormalities detected in neonatal animals during and immediately after anesthetic exposure and long-term cognitive abnormalities reported in animals and some children exposed to anesthesia at a young age.
To put the effects of anesthetic exposures into context of brain development, the natural developmental processes must be taken into consideration. The human brain undergoes a complex and extended process of enormous growth in cell number, synapses, and connections during the perinatal period and beyond. Combined with this expansion of cells and connections early in life, subsequently massive regressive processes also occur during normal brain development. These expanding and regressive processes allow the brain to fully develop and eventually execute tasks, such as talking, walking, reading, writing, calculating, acquiring social skills, perfecting fine motor dexterity, and accomplishing complex functions, including learning, abstract thinking, as well as planning and executing long-term objectives.
To accommodate these functions, the human brain initially undergoes a rapid growth in size and cell number both in utero and during the early postnatal period, and is then pared back to achieve an efficient network of about 100 billion neurons in the adult brain. At birth, the size of the immature brain is one-third that of the adult brain, doubling in weight within the first year of life, and reaching 90% of its adult size by 6 years of age. This dramatic growth spurt coincides with a remarkable overabundance of neurons and neuronal connections. In fact, less than half the neurons generated during development survive into adulthood. Superfluous neurons that lose in the competition for a limited amount of trophic factors are removed by programmed cell death.
After their rapid growth in number during early brain development, immature neurons subsequently form an excess of connections via synapses. Depending on the brain region, synaptic densities are maximum in infants and young toddlers between 3 and 15 months of age and will undergo a progressive reduction by about half during adolescence and into adulthood. Connections that are active with continued electrical and chemical signals are sustained, whereas those with little or no activity are lost. Axons are myelinated by oligodendrocytes, leading to maturation of the CNS.
In summary, brain architecture changes rapidly and dramatically throughout early life. Neuronal density is greatest during fetal life, and excess neurons are eliminated via apoptosis or programmed cell death, predominantly in utero, during the neonatal period and throughout infancy. Rapid growth of dendrites and synaptic connections occur during infancy and early childhood, and unneeded dendrites and synapses are trimmed back, predominantly during later childhood and adolescence.
While these processes occur at slightly different stages of development for different regions of the brain, the first several years after birth represent a critical period for the entire developing CNS. Recent findings in animals suggest that exposure to anesthetics or sedatives during this period may interfere with proper neuronal development, brain architecture, and subsequent function. Although the exact molecular mechanisms by which anesthetics afford their therapeutic properties of amnesia, analgesia, and immobility are incompletely understood, their interaction with a wide variety of ion channels, such as sodium, calcium, and potassium channels, as well as several cell membrane proteins, including γ-aminobutyric acid (GABA), glycine, glutamate ( N -methyl- d -aspartate [NMDA]), acetylcholine, and serotonin receptor systems, make it conceivable that anesthetics could create permanent abnormalities by interfering with important processes during critical windows of brain development. In fact, both GABA and NMDA receptors play critical roles in trophic signaling and in the regulation of neuronal maturation and programmed cell death. During early brain development, for example, GABA directs cell proliferation, neuroblast migration, and dendritic maturation. In turn, developmental NMDA receptor stimulation directly fosters survival and maturation of some neurons. It is therefore plausible that, by acting as modulators of these receptors, anesthetics might interfere with these critical developmental processes.
Concerns regarding neurologic abnormalities after general anesthesia in young children were first raised more than half a century ago, when postoperative behavioral changes were observed after administration of vinyl ether, cyclopropane, or ethyl chloride for otolaryngologic surgery. However, these abnormalities were regarded as psychological in nature because they were alleviated by the timely administration of preoperative sedative drugs. Approximately two decades later the focus of research into the long-term effects of anesthetics shifted to animal models representing occupational exposure in pregnant health care workers. Delayed synaptogenesis and behavioral abnormalities were observed in neonatal rats born to rodent dams that were chronically exposed to subanesthetic doses of halothane during their entire pregnancy. However, interest in the effects of anesthetics on brain development in children was not elicited until a seminal study observed widespread neuronal degeneration after a prolonged exposure to ketamine in neonatal rat pups. This initial discovery led to numerous editorials and reviews, and more than 400 original articles (see Fig. 25.1 ) into the brain structural and/or functional effects of almost every sedative and anesthetic in current clinical use in a wide variety of immature animal species. However, no discussion about the effects of drug exposure of the developing brain would be complete without examining the effects of opioid analgesics in the developing brain. Accordingly, this chapter examines the specific cellular effects that sedatives, anesthetics, and analgesics trigger in the immature brain.
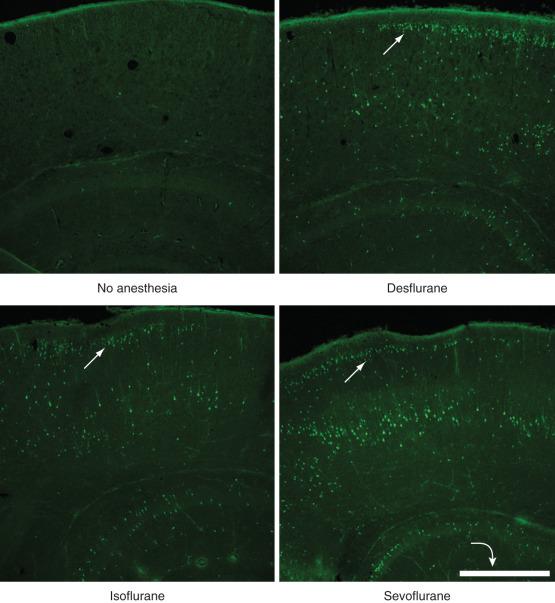
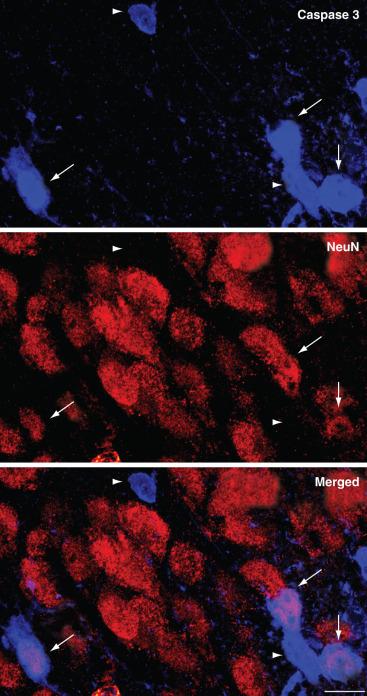
The most widely studied deleterious structural consequence of exposure to sedatives or anesthetics in immature animals is widespread apoptosis. Although neuronal apoptosis eliminates approximately 50% to 70% of neurons throughout the brain during the entire developmental period, this natural process affects only a small fraction of cells at any particular time point. Exposure to anesthetics or sedatives briefly, but dramatically, increases the number of apoptotic neurons ( Fig. 25.2 ). Some studies demonstrated up to a 68-fold increase in the density of degenerating neurons after a combination of anesthetics in newborn rats compared with control animals, although it remained unclear what fraction of the entire neuronal population these degenerating neurons represented. In newborn mice, a 6-hour exposure to a clinically relevant dose of isoflurane triggers apoptotic cell death in 2% of neurons in the superficial cortex, a substantially affected brain region at this age. Under normal conditions, less than 0.1% of neurons undergo physiologic apoptosis in this region in unanesthetized littermates. The exact mechanism and selectivity of the cell death process remains unknown as dying neurons are located immediately adjacent to seemingly unaffected neighboring cells ( Fig. 25.3 ). Increased neuroapoptosis has now been observed after in vitro and in vivo exposures to a wide variety of sedatives and anesthetics, including chloral hydrate, clonazepam, diazepam, midazolam, nitrous oxide, desflurane, enflurane, halothane, isoflurane, sevoflurane, ketamine, pentobarbital, phenobarbital, propofol, and xenon, as well as opioid receptor agonists in a wide variety of species, including fruit flies, nematodes, chicks, mice, rats, guinea pigs, piglets, and rhesus monkeys ( E-Table 25.1 ). Selective stains such as cupric silver and Fluoro-Jade (EMD Millipore, Billerica, MA) have confirmed the cellular demise in neurons that positively stained for activated caspase 3, the central executioner enzyme of the apoptotic cascade.
| Anesthetic Agent | Dose and Duration | Species, Age | Pathology | Study |
|---|---|---|---|---|
| Buprenorphine | 1.5 mg/kg per day to dam | Rat, E7–E21 or P10 | Decrease in striatal nerve growth factor | Wu et al. |
| Chloral hydrate | 50–300 mg/kg × 1 | Mouse, P5 | Increased neuroapoptosis, ameliorated by lithium | Cattano et al. |
| Clonazepam | 0.5–4 mg/kg × 1 | Rat, P7 | Increased neurodegeneration | Bittigau et al. |
| Clonazepam | 0.5–4 mg/kg × 1 | Rat, P7 | Increased neurodegeneration | Ikonomidou et al. |
| Desflurane | 7% for 30–120 minutes | Rat, P16 | No increase in neurodegeneration or gross changes in dendritic arborization, increase in number of dendritic spines | Briner et al. |
| Desflurane | 12% for 6 hours | Mouse, cortical neuronal culture | No increase in expression of proapoptotic factors, no accumulation of reactive oxygen species, no activation of apoptosis pathways | Zhang et al. |
| Desflurane | 7.4% for 6 hours | Mouse, P7–P8 | Increased neuroapoptosis compared with no anesthesia, but similar to equianesthetic doses of isoflurane or sevoflurane | Istaphanous et al. |
| Desflurane | 8% for 6 hours | Mouse, P6 | Increased neuroapoptosis, compared with sevoflurane and isoflurane, and long-term impairment in memory function | Kodama et al. |
| Desflurane | 9% for 2 hours/day × 3 days | Mouse, P6–P8 | No increase in inflammatory markers or learning impairment in adulthood | Shen et al. |
| Dexmedetomidine | 1–75 µg/kg × 3 | Rat, P7 | No increase in neuroapoptosis | Sanders et al. |
| Dexmedetomidine | 25 µg/g every 2 hours × 3 | Rat, P7 | No increase in cortical neuroapoptosis | Sanders et al. |
| Dexmedetomidine | 75 µg/kg IP × 1 or 25 µg/kg every 2 hours × 3 | Rat, P7 | No increase in neuroapoptosis in hippocampus, dexmedetomidine ameliorated isoflurane-induced neuroapoptosis | Li et al. |
| Dexmedetomidine | 5 or 10 µg/kg ×1 | Rat, P7 | Unaltered hippocampal synaptic function in young adulthood | Tachibana et al. |
| Dexmedetomidine | 3 or 30 µg/kg per hour for 12 hours to dam | Cynomolgus monkey, E120 | Minimal neuroapoptosis in frontal cortex at both doses | Koo et al. |
| Dexmedetomidine | 25 µg/kg daily × 3 | Rat, P7–9 | No increase in apoptotic neurons in hippocampus, learning and memory in adulthood unaltered | Duan et al. |
| Dexmedetomidine | 75 µg/kg × 1 or 25 µg/kg × 3 |
Rat, P7 | No increase in apoptosis observed in hippocampus | Li et al. |
| Dexmedetomidine | 75 µg/kg × 1 | Rat, P7 | No significant increase in neuroapoptosis in hippocampus | Liao et al. |
| Dexmedetomidine | 30 or 45 µg/kg every 90 minutes × 6 | Rat, P7 | Cellular degeneration and apoptosis in somatosensory cortex and thalamus, but not limbic thalamus | Pancaro et al. |
| Diazepam | 10–30 mg/kg × 1 | Rat, P7 | Increased neurodegeneration | Ikonomidou et al. |
| Diazepam | 5–30 mg/kg × 1 | Rat, P7 | Increased neurodegeneration above 10 mg/kg, prevented by flumazenil | Bittigau et al. |
| Diazepam | 5 mg/kg × 1 | Mouse, P10 | No increased neurodegeneration in cortex, but increased in thalamus, no subsequent behavioral or learning deficits | Fredriksson et al. |
| Diazepam | 20 mg/kg on P6 and on P8 | Rat, P6 and P8 | Inhibition of neurogenesis | Stefovska et al. |
| Enflurane | 2%–4% for 0.5 hour | Mouse, prenatal E6–E17 | Learning impairment in adulthood | Chalon et al. |
| Fentanyl | 50 µg/kg per hour for 72 hours | Rat, P14 | Diminished morphine antinociceptive sensitivity in juvenile and adult animals | Thornton et al. |
| Fentanyl | 0.1, 1, or 10 µg/kg × 3 | Mouse, P4 and P5 | Significant exacerbation of ibotenate-induced white-matter brain lesions at highest dose | Laudenbach et al. |
| Fentanyl | 30 µg/kg + 15 µg/kg per hour for 4 hours | Swine, P5 | Slightly increased neuronal apoptosis, not statistically significantly different from control | Rizzi et al. |
| Fentanyl | 90 µg/kg × 1 | Male rat, P14 | Long-term anxiolytic-like behavior when tested in young adulthood | Medeiros et al. |
| Halothane | 10 ppm for 40 hours/week | Rat, conception to P60 | Learning deficits and decrease in synaptic density | Quimby et al. |
| Halothane | 10 ppm for 40 hours/week | Rat, conception to P60 | Defects in visual and spatial learning tasks | Quimby et al. |
| Halothane | 2.5% for 2 hours | Rat, prenatal E3, E10, or E17 | More errors in maze task in adult animals exposed on E3 and E10, but not at E17 | Smith et al. |
| Halothane | 50–200 ppm continuously | Rat, conception to P28 | Dose-dependent decrease in cerebral synapse density | Uemura et al. |
| Halothane | 25–100 ppm continuously | Rat, conception to P60 | Decrease in apical and basal dendritic length and numbers | Uemura et al. |
| Halothane | 25–100 ppm continuously | Rat, conception to P60 | Decrease in synaptic density, no learning impairment | Uemura et al. |
| Halothane | 1%–2% for 0.5 hour | Mouse, prenatal E6–E17 | Learning impairment in adulthood | Chalon et al. |
| Heroin | 10 mg/kg per day to dam | Mouse, E8–E18 | Hyperactivity and spatial learning deficits in adulthood | Yanai et al. |
| Heroin | 10 mg/kg per day to dam | Mouse, E9–E18 | Increased neuronal apoptosis and impaired spatial learning and memory in juvenile animals | Wang et al. |
| Heroin | 500 µg/mL | Rat, E16–E17 cortical neuronal cultures | Decrease in neuronal viability, increase in apoptosis and DNA fragmentation | Cunha-Oliveira et al. |
| Isoflurane | 0.2–0.3 mmol/L | Mouse, P14 and P60 hippocampal slices | Block of synaptic transmission more pronounced in P14 slices compared with older animals | Simon et al. |
| Isoflurane + nitrous oxide + midazolam | 0.75% + 75% + 9 mg/kg for 6 hours | Rat, P7 | Increased neuroapoptosis and neurodegeneration, adult learning impairment | Jevtovic-Todorovic et al. |
| Isoflurane | 0.75%–1.5% for 6 hours | Rat, P7 | Increased neurodegeneration | Jevtovic-Todorovic et al. |
| Isoflurane + midazolam + thiopental | 1.5% for 4 hours + 1 mg/kg + 7 mg/kg to ewe | Sheep, prenatal E120 | No increase in neuroapoptosis, as assessed 6 days postanesthesia | McClaine et al. |
| Isoflurane + nitrous oxide + midazolam | 0.75% + 75% + 9 mg/kg for 2–6 hours | Rat, P1–P14 | Increased neuroapoptosis following exposure on P7, not P1–P3 or P10–P14 | Yon et al. |
| Isoflurane + nitrous oxide + midazolam | 0.55% + 75% + 1 mg/kg for 4 hours | Swine, P5 | Increased neurodegeneration in neocortex | Rizzi et al. |
| Isoflurane | 1.5% for 1–5 hours | Rat, organotypic slice culture | Increased neurodegeneration only after 5 hours of exposure | Wise-Faberowski et al. |
| Isoflurane | 2.4% for 24 hours | Rat, primary cortical neuron culture | Increased apoptosis | Wei et al. |
| Isoflurane + nitrous oxide + midazolam | 0.75% + 75% + 9 mg/kg for 2–6 hours | Rat, P7 | Increased neuroapoptosis, ameliorated by estradiol | Lu et al. |
| Isoflurane + nitrous oxide + midazolam | 0.75% + 75% + 9 mg/kg for 6 hours | Rat, P7 | Increased neuroapoptosis, ameliorated by melatonin | Yon et al. |
| Isoflurane | 0.75% for 4 hours | Mouse, P5 | Increased neuroapoptosis, ameliorated by pilocarpine | Olney et al. |
| Isoflurane | 0.75% for 6 hours | Rat, P7, and mouse organotypic hippocampal slices | Increased neuroapoptosis, exacerbated by nitrous oxide and ameliorated by xenon | Ma et al. |
| Isoflurane | 2.4% for 24 hours | Rat, primary cortical neuron culture | Increased cellular degeneration, blocked by isoflurane or halothane preconditioning | Wei et al. |
| Isoflurane | 1.3% for 6 hours | Rat, prenatal E21 | Decrease in neuroapoptosis, no impaired learning, improved memory retention in juvenile animals | Li et al. |
| Isoflurane + nitrous oxide + midazolam | 0.75% + 75% + 9 mg/kg for 6 hours | Rat, P7 | Decreased neuronal density in multiple brain areas in adulthood | Nikizad et al. |
| Isoflurane + nitrous oxide + midazolam | 0.55% + 75% + 1 mg/kg for 4 hours | Guinea pig, prenatal E20–E50 | Increased neuroapoptosis between E35 and E40 and decreased adult neuronal density, not after E50 or in fentanyl control group | Rizzi et al. |
| Isoflurane | 0.75% for 4 hours, 1.5% for 2 hours, or 2% for 1 hour | Mouse, P5–P7 | Increased neuroapoptosis | Johnson et al. |
| Isoflurane + nitrous oxide | 0.75% + 75% for 6 hours | Rat, P7 | Increased neuroapoptosis in spinal cord | Sanders et al. |
| Isoflurane | Dose not specified | Mouse, P4 | Increased neuroapoptosis, ameliorated by hypothermia | Olney et al. |
| Isoflurane + nitrous oxide | 0.55% + 75% or individually for 2–8 hours | Rat, P7 | Increased neuroapoptosis and neurodegeneration after exposure of 6 hours or longer, ameliorated by l -carnitine, no increase by either agent administered individually | Zou et al. |
| Isoflurane | 1%–2% for 10 minutes | Mouse, P0 | Neonatal and adolescent impairment in tests of body coordination and adult learning; decreased hippocampal cellularity and volume, more pronounced in males | Rothstein et al. |
| Isoflurane | 1.2% for 12 hours | Rat, primary cortical neuron culture | Increased cytotoxicity, ameliorated by xestospongin C | Wang et al. |
| Isoflurane | 0.75% for 6 hours | Rat, P7 | Increased neuroapoptosis, no effect on short-term, but impairment of long-term memory, ameliorated by dexmedetomidine | Sanders et al. |
| Isoflurane | 0.75% for 6 hours | Mouse, P8 organotypic hippocampal slices | Increased neuroapoptosis, ameliorated by dexmedetomidine, not reversed by gabazine | Sanders et al. |
| Isoflurane | 0.75% for 6 hours | Rat, P7 | Increased cortical neuroapoptosis, reduced by dexmedetomidine coadministration | Sanders et al. |
| Isoflurane | 1.5% for 6 hours | Mouse, P7 | Increased neurodegeneration early after exposure, no adult learning impairment, no decreased adult neuronal density | Loepke et al. |
| Isoflurane | 1 MAC for 1–4 hours | Rat, P7 | Widespread brain cell death following exposure for >2 hours, not 1 hour; adult neurocognitive deficits only after 4-hour exposure | Stratmann et al. |
| Isoflurane | 1 MAC for 4 hours | Rat, P7 | Decreased progenitor proliferation, deficits in fear conditioning and memory impairment in adulthood | Stratmann et al. |
| Isoflurane | 3.4% for 4 hours | Rat, neuronal progenitor culture, harvest P2, 2–12 weeks in culture | No neurodegeneration or activation of caspases 3 or 7, decrease in caspase 9, inhibition of proliferation, preferential differentiation to neurons | Stratmann et al. |
| Isoflurane | 1.4% | Rat, cortical and hippocampal neuron culture, harvest P1, DIC 5 | Increased neuroapoptosis, reduction in dendritic spines and number of synapses, ameliorated by administration of tPA or inhibition of p75 NTR | Head et al. |
| Isoflurane + nitrous oxide + midazolam | 0.75% + 75% + 9 mg/kg for 6 hours | Rat, P7 | Decreased synaptic density in subiculum 2 weeks after treatment | Lunardi et al. |
| Isoflurane | 1.6% for 5 hours | Rhesus monkey, P5 | Increase in apoptotic cell death of neurons in neocortex and immature oligodendrocytes in white matter | Olney et al. |
| Isoflurane | 1.3% or 3% for 1 hour to dam | Rat, E21 | No increase in neuroapoptosis or S100β blood levels following lower dose, but both increased following higher dose | Wang et al. |
| Isoflurane | 1.7% for 35 minutes × 4 days | Rat, P14 and mouse, P14 | No increased immediate hippocampal neuronal cell death, no difference in adult learning of easy task, impairment in adult learning of difficult learning and recognition tasks | Zhu et al. |
| Isoflurane | 1.6% for 5 hours | Rhesus monkey, P5 | Increase in neocortical apoptotic cell death | Brambrink et al. |
| Isoflurane | 1.5% for 30–120 minutes | Rat, P16 | No increase in neurodegeneration or gross changes in dendritic arborization, increase in number of dendritic spines | Briner et al. |
| Isoflurane | 0.75% for 6 hours | Mouse, P7 | Increased neuroapoptosis and serum levels of S100β immediately following exposure, no long-term impairment in learning and memory tests | Liang et al. |
| Isoflurane | 2% for 2 hours and/or caffeine 80 mg/kg IP | Mouse, P4 | Increased neuroapoptosis following caffeine, worse than following isoflurane exposure, demonstrating potentiation following combination exposure | Yuede et al. |
| Isoflurane | 1.5% for 6 hours | Mouse, P7–P8 | Increased neuroapoptosis compared with no anesthesia, but similar to equianesthetic doses of desflurane or sevoflurane | Istaphanous et al. |
| Isoflurane | 2% for 90 minutes for each time point | Mouse, P10, P20, P30, P60, and P90 | No behavioral or cognitive abnormalities in adulthood | Kulak et al. |
| Isoflurane + nitrous oxide | 0.75% isoflurane + 70% nitrous oxide for 6 hours | Rat, P7 | Increased neuroapoptosis and subsequent altered cognitive function, which were ameliorated by xenon pretreatment | Shu et al. |
| Isoflurane + nitrous oxide | 0.75% isoflurane + 70% nitrous oxide for 6 hours | Rat, organotypic hippocampal slice culture | Increased neuroapoptosis, which was ameliorated by xenon pretreatment | Shu et al. |
| Isoflurane + nitrous oxide + midazolam | 0.55% + 75% + 1 mg/kg for 4 hours | Swine, P5 | Increased neuroapoptosis in cerebral cortex compared with control or a fentanyl-treated comparison group | Rizzi et al. |
| Isoflurane | 3% for 24 hours | Rat, astroglial culture | Impairment in growth of immature glia, delay in glial maturation, without effects on total actin or GFAP levels | Lunardi et al. |
| Isoflurane | 1.5% for induction + 1% for 4 hours | Mouse, 1 month | Transient decrease in dendritic filopodia elimination in somatosensory cortex, without effects on dendritic spine density | Yang et al. |
| Isoflurane | 0.7%, 1.4%, or 2.8% for 6 hours | Rat, embryonic neuronal stem cells | No effect on neural stem cell viability. Two higher concentrations inhibited cell proliferation | Culley et al. |
| Isoflurane | 1.5% or 5% for 6–12 hours | Mouse, cortical neuronal culture | No cytotoxicity observed, even when adding clinical doses of nitrous oxide or ketamine | Campbell et al. |
| Isoflurane | 1.4% from 15 minutes to 4 hours | Mouse, primary neuronal culture and hippocampal slice culture | Increased apoptosis and cytoskeletal destabilization | Lemkuil et al. |
| Isoflurane + intrathecal bupivacaine | 1% for 1 or 6 hours | Rat, P7, P14, P21 | Neuroapoptosis in brain and spinal cord after 6 hours of exposure, but not 1 hour; intact spontaneous motor performance in adulthood | Yahalom et al. |
| Isoflurane and/or nitrous oxide | 1% and/or 70% for 8 hours | Rhesus monkey, P5–P6 | No neuroapoptosis observed following either agent alone, neuroapoptosis increased following anesthetic combination | Zou et al. |
| Isoflurane + nitrous oxide + midazolam | 0.75% + 75% + 9 mg/kg for 6 hours | Rat, P7 | Enlargement and impairment of structural integrity of mitochondria and decreased density in subiculum, as well as increased autophagic activity | Sanchez et al. |
| Isoflurane + nitrous oxide | 0.75% isoflurane + 70% nitrous oxide for 6 hours | Rat, P7 | Increased neuroapoptosis and subsequent altered cognitive function, which were ameliorated by xenon pretreatment | Shu et al. |
| Isoflurane | Titrated between 0.7% and 1.5% isoflurane for 5 hours | Rhesus monkey, P6 | Widespread apoptotic cell death of neurons and oligodendrocytes (6.3% of all myelinating oligodendrocytes in forebrain) | Brambrink et al. |
| Isoflurane | Isoflurane 1.5% for 6 hours | Mouse, P7 | Quantification of apoptotic neuronal cell death in superficial layers of visual cortex finds almost 3% of neurons affected following exposure, compared with less than 0.1% of neurons undergoing natural apoptosis in control animals | Istaphanous et al. |
| Isoflurane | 2% for 6 hours | Mouse, P6 | Increased neuroapoptosis, but no long-term impairment in memory function | Kodama et al. |
| Isoflurane | 1.5% for 6 hours | Mouse, P7, P21, or P49 | Increased apoptosis in olfactory bulb at all ages and dentate gyrus at the two older time points; neurons younger than 15 days of age particularly vulnerable | Hofacer et al. |
| Isoflurane | Titrated between 1% and 1.5% for 5 hours to dam | Rhesus monkey, E120 | Increased neuroapoptosis, predominantly in cerebellum, caudate, putamen, and amygdala, as well as diffuse apoptotic oligodendrocyte death | Creeley et al. |
| Isoflurane | 0.75% for 6 hours | Rat, P7 | Neuronal apoptosis in hippocampus, dose-dependently inhibited by dexmedetomidine | Li et al. |
| Isoflurane | 0.75% for 6 hours | Rat, P7 | Increased neuroapoptosis in hippocampus, inhibited by dexmedetomidine coadministration | Liao et al. |
| Isoflurane | 1.5% for 6 hours | Mouse, P7, P21, or P49 | Differential degrees of neuroapoptosis among brain regions dependent on age during exposure; sustained vulnerability into adulthood in regions with continued neurogenesis | Deng et al. |
| Isoflurane | 1.5% for 6 hours | Rats, P7 | Apoptotic marker caspase 3 increase in cortex and hippocampus was ameliorated by preconditioning with a 30-minute exposure to 1.5% isoflurane on P6 | Peng et al. |
| Isoflurane | 2% for 1 hour | Mouse, P7 | Apoptotic cell death increased in inner nuclear layer of retina | Cheng et al. |
| Isoflurane | Titrated between 1.5% and 3% for 5 hours | Rhesus monkey, P6 | Increased neuroapoptosis and oligodendrocytic apoptosis was reduced by lithium coadministration | Noguchi et al. |
| Ketamine | 20 mg/kg × 7 | Rat, P7 | Increased neurodegeneration | Ikonomidou et al. |
| Ketamine | 25–75 mg/kg × 1–7 | Rat, P7 | Increased neurodegeneration only after 7 repeated doses of 25 mg/kg | Hayashi et al. |
| Ketamine | 50 mg/kg × 1 | Mouse, P10 | Cortical neurodegeneration; hypo- and hyperactivity, abnormal habituation in adulthood | Fredriksson et al. |
| Ketamine | 50 mg/kg × 1 | Mouse, P10 | Neurodegeneration, worsened by coadministration of diazepam | Fredriksson et al. |
| Ketamine | 50 mg/kg × 1 | Mouse, P10 | Neurodegeneration and impairment in memory tasks in adulthood | Fredriksson et al. |
| Ketamine | 10–25 mg/kg × 1–7 | Rat, P7 | Neurodegeneration observed after highest dose, plasma levels 7 times higher than during human anesthesia | Scallet et al. |
| Ketamine | 1.25–40 mg/kg × 1 | Mouse, P7 | Neurodegeneration after doses of 5 mg/kg or higher, no gross neurobehavioral abnormalities 7 days after treatment | Rudin et al. |
| Ketamine | 0.1–20 µmol/L for 6–48 hours | Rat, forebrain neuron culture | Increased DNA fragmentation after higher doses for longer periods of time | Wang et al. |
| Ketamine | 10–40 mg/kg × 1 | Mouse, P7 | Increased neurodegeneration following doses of 20 mg/kg or higher, worsened by midazolam coadministration | Young et al. |
| Ketamine | 1–20 µmol/L for 2–24 hours | Rhesus monkey, forebrain neuron culture | Increased DNA fragmentation and decreased mitochondrial function after higher doses for longer periods of time | Wang et al. |
| Ketamine | 0.01–40 µg/mL for 1–48 hours | Rat, GABAergic neuron culture | Neuronal cell loss and decrease in dendritic length and branching with higher concentrations for longer exposure times | Vutskits et al. |
| Ketamine | 100 µmol/L for 48 hours | Rat, cortical neuron culture | Increased apoptosis, ameliorated by NMDA, IGF-1, alsterpaullone, or iodoindirubin | Takadera et al. |
| Ketamine | 0.1–30 µmol/L for 24 hours | Rat, cortical neuron culture | Decreased cell viability and increased DNA fragmentation, ameliorated by erythropoietin | Shang et al. |
| Ketamine | 20–50 mg/kg per hour × 24 hours | Rhesus monkey, E122, P5, or P35 | Increased neurodegeneration in two younger age groups, ketamine plasma levels higher than in humans | Slikker et al. |
| Ketamine | 10–10 µg/mL for 1–24 hours | Rat, GABAergic neuron culture | Neuronal cell loss with higher concentrations or longer exposure times | Vutskits et al. |
| Ketamine | 2.5 mg/kg × 2 for 4 days | Rat, P1–P4 | Minimal neurodegeneration, but ketamine ameliorated pain-induced neurodegeneration and subsequent learning abnormalities | Anand et al. |
| Ketamine | 25 mg/kg × 1 | Mouse, P10 | No increase in neurodegeneration, but subsequent learning impairment, worsened by coadministration of thiopental or propofol | Fredriksson et al. |
| Ketamine | 1–20 µmol/L for 24 hours | Rat, forebrain neuron culture | Increased DNA fragmentation after higher doses, blocked by nitroindazole | Wang et al. |
| Ketamine | 5–25 mg/kg × 1 | Mouse, P10 | Dose-dependent alterations in levels of developmentally expressed proteins and adult behavioral abnormalities | Viberg et al. |
| Ketamine | 2.5 mg/kg × 2 for 4 days | Rat, P1–P4 | No increase in neuronal cell death, no change in adult learning and exploratory behavior and ameliorated pain-induced neurodegeneration and behavioral abnormalities | Rovnaghi et al. |
| Ketamine | 20 mg/kg × 6 | Rat, P7 | Decreased body weight 1–3 days after exposure, no abnormal spontaneous behavior observed during first 4 days | Boctor et al. |
| Ketamine | 30 mg/kg + 15 mg/kg every 90 minutes × 2 | Mouse, P15–P90 | No increase in neurodegeneration, impairment in dendritic spine development following exposure on P20 or younger | Vutskits et al. |
| Ketamine | 40 mg/kg | Mouse, P5 | Increased neuroapoptosis, ameliorated by lithium coadministration | Straiko et al. |
| Ketamine | 5–20 mg/kg × 1–6 | Rat, P7 | Increased apoptosis and neurodegeneration only observed following 6 repeated doses of 20 mg/kg | Zou et al. |
| Ketamine | 20 mg/kg × 7 | Rat, P7 | Increased apoptosis and neurodegeneration, decreased weight gain, increase in BDNF and TrkB cDNA | Ibla et al. |
| Ketamine | 20–50 mg/kg per hour for 3–25 hours | Rhesus monkey, P5–P6 | Neuronal degeneration only in neocortex following 9-hour exposure or longer, no degeneration in deeper brain areas | Zou et al. |
| Ketamine | 30 mg/kg + 15 mg/kg every 90 minutes × 2 | Mouse, P15 | Increase in dendritic spine density and decrease in spine head diameter in the somatosensory cortex and hippocampus | De Roo et al. |
| Ketamine | 100 mg/kg per day × 5 days | Mouse, P8–P12 | Impaired dendritic spine maturation immediately following exposure, which subsided within 2 weeks | Tan et al. |
| Ketamine or ( S )-ketamine | 1–8 mmol/L or 0.6–4 mmol/L for 24 hours | Human, neuroblastoma cells | Increased apoptosis, but up to 80% less cell death following ( S )-ketamine | Braun et al. |
| Ketamine | 5–20 mg/kg × 5 | Rat, P7 | Increased neuroapoptosis in neocortex and thalamus, increase in cell cycle proteins | Soriano et al. |
| Ketamine | 0.1–1000 µmol/L × 6–48 hours | Rat, cortical neuron culture | Increase in expression of caspase 3 and cell cycle proteins | Soriano et al. |
| Ketamine | 20 mg/kg every 2 hours × 6 | Rat, P7 | Increased neurodegeneration in frontal cortex | Shi et al. |
| Ketamine | 3–15 mg/kg intrathecal L4-6 | Rat, P3, P7, or P21 | Increased neuronal cell death and subsequent altered spinal cord function after injection at P3, but not at P7 or P21 | Walker et al. |
| Ketamine | 20 mg/kg bolus + 20–50 mg/kg per hour for 24 hours | Rhesus monkey, P5–P6 | Decreased performance of the National Center for Toxicological Research Operant Testing Battery and motivation | Paule et al. |
| Ketamine + fentanyl | 20 mg/kg + 90 µg/kg | Rat, P14 | Long-term hyperactivity and behavioral changes indicating lower levels of anxiety | Madeiros et al. |
| Ketamine | 20 mg/kg every 2 hours × 6 | Rat, P7 | Greater accumulation of a radioactive tracer and more cell death in frontal cortex in adulthood | Zhang et al. |
| Ketamine + xylazine | 42.5 + 4.3 mg/kg every 90 minutes × 3 | Mouse, 1 month | Transient increase in dendritic filopodia formation in somatosensory cortex, without effects on dendritic spine density | Yang et al. |
| Ketamine | 100 µmol/L, 1 mmol/L, or 3 mmol/L | Mouse, cortical neuronal culture | Cytotoxicity only observed in doses of 1 mmol/L or higher | Campbell et al. |
| Ketamine | 18.4–86.5 mg/kg per hour for 5 hours to dam | Rhesus monkey, E120 | Increased neuroapoptosis, especially in cerebellum, caudate-putamen, and nucleus accumbens | Brambrink et al. |
| Ketamine | 18.4–56.0 mg/kg per hour for 5 hours | Rhesus monkey, P6 | Increased neuroapoptosis, especially in basal ganglia and thalamus | Brambrink et al. |
| Ketamine | 75 mg/kg daily × 3 | Rat, P7–9 | Increase in apoptotic neurons in hippocampus, impaired learning and memory in adulthood, both effects ameliorated by coadministration of dexmedetomidine | Duan et al. |
| Ketamine | 20–50 mg/kg per hour for 12 hours to dam | Cynomolgus monkey, E120 | Neuroapoptosis in frontal cortex | Koo et al. |
| Ketamine | 1–500 µM for 24 hours | Rat, neural stem cell culture | No oxidative DNA damage, mitochondrial toxicity, or impaired proliferation observed following exposure to clinically relevant concentration. However, number of differentiated neurons reduced | Slikker et al. |
| Ketamine | 20 mg/kg every 90 minutes × 6 | Rat, P7 | Significant cellular degeneration and apoptosis in limbic thalamus, but not somatosensory cortex or thalamus | Pancaro et al. |
| Methadone | 10 mg/kg per day to dam | Rat, E5–E21 | Decreased brain monoamines in juveniles and hyperresponsiveness in adulthood | Rech et al. |
| Methadone | 10–15 mg/kg per day to dam | Rat, E8–E21 | Increased excitability tested in juvenile animals using the acoustic startle reflex | Hutchings et al. |
| Methadone | 9 mg/kg per day to dam | Rat, E7–E21 | Increased noradrenergic activity in hippocampus of male juveniles | Robinson et al. |
| Methadone | 9 mg/kg per day | Rat, P1–P10 | Reduced activity of dopaminergic neurons in frontal cortex of juvenile males | Robinson et al. |
| Methadone | 9 mg/kg per day to dam | Rat, E7–E21 | Reduction in nerve growth factor, disruption of cholinergic neuronal activity | Robinson et al. |
| Methadone | 9 mg/kg per day to dam | Rat, E7–E21 or P10 | Reduction in nerve growth factor in striatum | Wu et al. |
| Midazolam | 9 mg/kg × 1 | Rat, P7 | No increase in neurodegeneration | Jevtovic-Todorovic et al. |
| Midazolam | 9 mg/kg × 1 | Mouse, P7 | Increased neuroapoptosis | Young et al. |
| Midazolam | 9 mg/kg × 1 | Mouse, P5 | Increased neuroapoptosis | Olney et al. |
| Midazolam | 3–9 mg/kg × 1 | Rat, P1–P14 | No increase in neuroapoptosis | Yon et al. |
| Midazolam | 0.25–25 µg/mL | Rat, GABAergic neuron culture | No effect on neuronal survival | Vutskits et al. |
| Midazolam | 25 mg/kg + 15 mg/kg every 90 minutes × 2 | Mouse, P15–P90 | No increase in neurodegeneration, impairment in dendritic spine development following exposure on P20 or younger | Vutskits et al. |
| Midazolam | 25 mg/kg + 15 mg/kg every 90 minutes × 2 | Mouse, P15 | Increase in dendritic spine density and decrease in spine head diameter in the somatosensory cortex and hippocampus | De Roo et al. |
| Midazolam | 50 mg/kg | Mouse, P10 | No impairment of learning and memory in adulthood | Xu et al. |
| Midazolam | 50 mg/kg per day × 5 days | Mouse, P8–P12 | Impaired dendritic spine maturation immediately following exposure, which subsided within 2 weeks | Tan et al. |
| Midazolam | 25 mg/kg IP injections for 6 hours | Rats, P5 or P15 | Increased neuroapoptosis in medial prefrontal cortex at both age points without long-term measurable diminution in neuronal density | Osterop et al. |
| Morphine | 10 mg/kg per day | Rat, E12–P5 | Gender- and region-specific changes in µ-opioid receptor density, decrease in neuronal density, and reduction in dendritic growth, partially reversed by naltrexone | Hammer et al. |
| Morphine | 5 mg/kg per day | Rat, P1–P4 | Decreased µ-opioid receptor density in striatum following exposure | Tempel el al. |
| Morphine | 20 mg/kg per day to dam, except for E11: 10 mg/kg per day |
Rat, E11–E18 | Decreased µ-opioid receptor density in adulthood | Rimanoczy et al. |
| Morphine | 1 µmol/L for 1–2 days | Mouse, P5–P6 cerebellar neuronal precursor culture | Marked decrease in DNA synthesis, without effect on cell survival | Hauser et al. |
| Morphine | 5 mg/kg × 3 doses, then 10 mg/kg twice a day to dam | Rat, E11–E18 | Impairment in spatial learning in adulthood | Slamberova et al. |
| Morphine | 20 mg/kg | Mouse, P4–P5 | Suppression of DNA synthesis in astrocytes | Stiene-Martin et al. |
| Morphine | 1–100 µmol/L for 5 days | Human, GW16–22 fetal microglial, astrocyte, and neuronal cultures | Progressive increase in apoptosis in neurons after 2-day exposure, or microglia after 3 days, but not in astrocytes. Apoptosis blocked by naloxone | Hu et al. |
| Morphine | 2 mg/kg per day to dam, progressively increased by 2 mg/kg per day | Rat, E0–P21 | Impairment in spatial learning in young adulthood | Yang et al. |
| Morphine | 20 mg/kg per day | Chick, E12–E16 | Impairment in long-term memory formation | Che et al. |
| Morphine | 2 mg/kg per day | Rat, P3–P7 | Impairment in adult cognitive function | McPherson et al. |
| Morphine | 0.5, 1, or 3 mg/kg | Rat, P7 | Increased pain behavior to a pain challenge several days after morphine injection | Zissen et al. |
| Morphine | 15 µmol/L for 7 days | Mouse, E16 hippocampal neuronal culture | Increased apoptosis, decreased neuronal density | Svensson et al. |
| Morphine | 4 mg/kg per day | Mouse, P5–P9 | Impairment in reward-mediated learning in adulthood | Boasen et al. |
| Morphine | 5 mg/kg × 3 doses, then 10 mg/kg twice a day to dam | Rat, E11–E18 | Decreased synaptic plasticity and impaired learning in juveniles | Niu et al. |
| Morphine | 2 mg/kg per day to dam, progressively increased by 2 mg/kg per day, maximum 14 mg/kg per day | Rat, E0–P21 | Decreased performance in learning tasks in adulthood | Lin et al. |
| Morphine | 5 µg/kg per day × 8 days | Rat, P8–P14 | Increased nociceptive response throughout adulthood, which could be blocked by ketamine | Rozisky et al. |
| Morphine | 10 mg/kg × 1 | Rat, P7 or P15 | No increase in neuronal apoptosis in deeper layers of medial prefrontal cerebral cortex | Massa et al. |
| Morphine | 10 mg/kg twice daily × 14 days | Rat, P1–14 | Long-term decrease in threshold for thermal stimulation and temporary learning impairment | Craig et al. |
| Nitrous oxide (hyperbaric) | 50%–150% for 2–6 hours | Rat, P7 | No increase in neurodegeneration | Jevtovic-Todorovic et al. |
| Nitrous oxide (hyperbaric) | 50%–150% for 2–6 hours | Rat, P1–P14 | No increase in neuroapoptosis | Yon et al. |
| Nitrous oxide | 75% for 6 hours | Rat, P7, and mouse organotypic hippocampal slices | No increase in neuroapoptosis in rats, increased neuroapoptosis in mice | Ma et al. |
| Pentobarbital | 5–10 mg/kg × 1 | Rat, P7 | Increased neurodegeneration | Bittigau et al. |
| Pentobarbital | 0.5, 5, or 50 µg/mL | Rat, PC12 pheochromocytoma cell culture | Dose-dependent inhibition of apoptotic cell death triggered by oxygen-glucose deprivation | Morimoto et al. |
| Pentobarbital | 10–20 mg/kg | Rat, P7 | Impairment in spatial learning; however, hypercarbia and hypoxia observed during exposure | Tachibana et al. |
| Phenobarbital | 40–100 mg/kg | Rat, P7 | Increased neuroapoptosis, ameliorated by β-estradiol | Bittigau et al. |
| Phenobarbital | 50 mg/kg | Rat, P7 | Increased neuroapoptosis, ameliorated by β-estradiol | Asimiadou et al. |
| Phenobarbital | 30 mg/kg × 2 | Mouse, P6 | Long-term alterations in brain protein expression | Kim et al. |
| Phenobarbital | 75 mg/kg | Rat, P7–P8 | Increased neuroapoptosis, enhanced by lamotrigine administration | Katz et al. |
| Phenobarbital | 25 mg/kg | Mouse, P0 | Neonatal and adolescent impairment in tests of body coordination and adult learning; decreased hippocampal cellularity and volume, more pronounced in males | Rothstein et al. |
| Phenobarbital | 50 mg/kg (P6), 40 mg/kg (P8) | Rat, P6 and P8 | Inhibition of neurogenesis | Stefovska et al. |
| Propofol | 0.5–10 µg/mL for 8 hours | Rat, GABAergic neuron culture | Dose-dependent decrease in GABAergic enzyme GAD after higher doses | Honegger et al. |
| Propofol | 10–100 µg/mL for 2 hours to 10 days | Rat, dissociated neuronal culture P1 or P7 | Increased cell death in P1 neurons following higher doses, no evidence for neurotoxic effects in organotypic hippocampal culture on P7 | Spahr-Schöpfer et al. |
| Propofol | 1–50 µg/mL | Rat GABAergic neuron culture | Increased neuronal cell death following highest dose, altered dendritic development following lowest dose | Vutskits et al. |
| Propofol | 5–500 µmol/L for 2–24 hours | Chick, neuron explant culture | Dose-dependent neurite growth cone collapse | Al-Jahdari et al. |
| Propofol | 10–60 mg/kg × 1 | Mouse, P10 | Increased neurodegeneration following highest dose | Fredriksson et al. |
| Propofol | 25–300 mg/kg × 1 | Mouse, P5–P7 | Increased neuroapoptosis following doses >50 mg/kg | Cattano et al. |
| Propofol + fentanyl | 6 mg/kg per hour + 10 µg/kg per hour for 24 hours | Swine, P0 | No increased neurodegeneration | Gressens et al. |
| Propofol | 5 µmol/L for 5 hours | Rat, hippocampal neuron culture | Increased neuroapoptosis | Kahraman et al. |
| Propofol | 50 mg/kg + 25 mg/kg every 90 minutes × 2 | Mouse, P15–P90 | No increase in neurodegeneration, impairment in dendritic spine development following exposure on P20 or younger | Vutskits et al. |
| Propofol | 25 mg/kg | Rat, P7 | Increased neuroapoptosis, decrease in nerve growth factor | Pesic et al. |
| Propofol | 50–100 mg/kg | Mouse, P5 | Increased neuroapoptosis, ameliorated by lithium coadministration | Straiko et al. |
| Propofol | 0.01–1 mg/mL for 3–48 hours | Rat, primary cortical neuron culture, harvested E18 | Highest concentration improved cell viability after up to 6 hours of exposure, but reduced cell viability after more than 12 hours | Berns et al. |
| Propofol | 30 mg/kg every 90 minutes × 3 | Rat, P6 | Increase in neurodegeneration in some brain areas, especially in parts of thalamus and subiculum, subtle neurologic impairment in adult animals | Bercker et al. |
| Propofol | 50 mg/kg + 25 mg/kg every 90 minutes × 2 | Mouse, P15 | Increase in dendritic spine density and decrease in spine head diameter in the somatosensory cortex and hippocampus | De Roo et al. |
| Propofol | 40 mg/kg + 20 mg/kg per hour for 6 hours | Rat, P5, P10, P15, P20, or P30 | Decrease in dendritic spine density following exposure between P5 and P10, but increased density at P15–P30 | Briner et al. |
| Propofol | 10 or 60 mg/kg | Mouse, P10 | Reduction in BDNF, no changes in adult spontaneous behavior, but reduced sedative effect of diazepam in adulthood | Pontén et al. |
| Propofol | 50 mg/kg × 7 days | Rat, P7–P14 | No increase in neuroapoptosis, only in hypoxic condition | Tu et al. |
| Propofol | 100 mg/kg × 1 | Mouse, P5 | Increased neuroapoptosis in hippocampus | Pearn et al. |
| Propofol | 3 µmol/L for 6 hours | Mouse, neuron culture, harvested P1–P3, DIC 5–7 | Increased neuroapoptosis, mediated by p75 neurotrophin receptor activation | Pearn et al. |
| Propofol | 300–450 µg/kg per minute for 5 hours | Rhesus monkey, E120 or P6 | Apoptotic cell death of oligodendrocytes and neurons; neuroapoptosis predominantly in subcortical and caudal regions during fetal exposure and in neocortex during neonatal exposure | Creeley et al. |
| Propofol | 0.9–50 µg/mL for 1–48 hours | Rat, hippocampal astrocyte culture | Dose-dependent increase in apoptosis and reduction in astrocytic viability | Sun et al. |
| Propofol | Rat, E20 | Increased neuroapoptosis and subsequent learning impairment, attenuated by dexmedetomidine coadministration | Li et al. | |
| Sevoflurane | 4% for 24 hours | Rat, primary cortical neuron culture | No increase in apoptosis | Wei et al. |
| Sevoflurane | 2% for 12 hours | Rat, primary cortical neuron culture | No increase in cytotoxicity | Wang et al. |
| Sevoflurane | 1.7% for 2 hours | Mouse, P7 | Increased neuroapoptosis | Zhang et al. |
| Sevoflurane | 3% for 6 hours | Mouse, P6 | Increased neuroapoptosis, impairments in fear conditioning and social interaction in adulthood | Satomoto et al. |
| Sevoflurane | 4% or 8% for 12 or 6 hours, respectively | Rat, primary cortical neuron culture, E18 | No difference in cell viability compared with untreated controls | Berns et al. |
| Sevoflurane | 3%–5% for 6 hours | Rat, P6 | No increase in neurodegeneration immediately after exposure, no long-term neurologic impairment | Bercker et al. |
| Sevoflurane | 2.1% for 0.5–6 hours | Rat, P4–P8 | Seizure activity during exposure and neuroapoptosis partially blocked by administration of bumetanide | Edwards et al. |
| Sevoflurane | 2.5% for 30–120 minutes | Rat, P16 | No increase in neurodegeneration or gross changes in dendritic arborization, increase in number of dendritic spines | Briner et al. |
| Sevoflurane | 1.1% for 6 hours | Mouse, P7 | Increased neuroapoptosis immediately following exposure, no long-term impairment in learning and memory tests | Liang et al. |
| Sevoflurane | 2.1% or 3% for 2 hours or 6 hours | Mouse, P6 | Increased neuroapoptosis following the longer, but not the shorter, exposure time. Transgenic Alzheimer disease mice more susceptible | Lu et al. |
| Sevoflurane | 2.9% for 6 hours | Mouse, P7–P8 | Increased neuroapoptosis compared with no anesthesia, but similar to equianesthetic doses of desflurane or isoflurane | Istaphanous et al. |
| Sevoflurane | 3.8% for 6 hours | Mouse, P6 | Increased neuroapoptosis, less than with desflurane and isoflurane, and long-term impairment in memory function | Kodama et al. |
| Sevoflurane | 1 MAC × 4 hours | Rat, P7 | Memory impairment, alleviated by environmental enrichment | Shih et al. |
| Sevoflurane | 3% for 2 hours daily × 3 | Mouse, P6–8 | Immediate increase in neuroinflammation and subsequent cognitive impairment, ameliorated by environmental enrichment or antiinflammatory treatment (ketorolac) | Shen et al. |
| Sevoflurane | 1.2% for 6 hours | Rat, P7 | No increased neuronal apoptosis detected in hippocampus | Li et al. |
| Sevoflurane | 2.5%–3.5% for 6 hours | Mouse, P6–7 | Neuroapoptosis immediately following exposure and long-term memory impairment; normal subsequent social behavior, anxiety level, and vocalization | Chung et al. |
| Sevoflurane | 2%–2.5% for 4 hours × 3 | Rhesus monkey, between P6 and P28 | Exaggerated anxiety behavior in juveniles following repeated anesthetic exposures in infancy | Raper et al. |
| Sevoflurane | 2.5% for 9 hours | Rhesus monkey, P5–6 | Increased neuronal degeneration | Liu et al. |
| Sevoflurane | 2%–2.5% for 4 hours × 3 | Rhesus monkey, 3 exposures during first 6 postnatal weeks | No differences in maternal behavior towards infants observed | Raper et al. |
| Sufentanil | 2 µg/kg × 3 | Mouse, P4 and P5 | No exacerbation of ibotenate-induced white-matter brain lesions | Laudenbach et al. |
| Thiopental | 5–25 mg/kg × 1 | Mouse, P10 | No increase in neurodegeneration by itself, but exacerbation and learning abnormalities in conjunction with ketamine | Fredriksson et al. |
| Xenon | 75% for 6 hours | Rat, P7 and mouse organotypic hippocampal slices | No increase in neuroapoptosis | Ma et al. |
| Xenon | 70% for 4 hours | Mouse, P7 | Increased neuroapoptosis, but decreased isoflurane-induced neuroapoptosis | Cattano et al. |
| Xenon | 50% for 24 hours + fentanyl infusions | Swine, P0 | No neuroapoptosis detected | Sabir et al. |
| Xenon | 0.75, 1, or 2 MAC for 6 hours | Rat, hippocampal slice cultures | Increased cell death following two higher doses in CA1, CA3, and dentate | Brosnan et al. |
Apoptosis represents an inherent, energy-consuming process using a cascade of enzymes called caspases. Apoptosis is highly conserved among species and culminates in self-destruction and elimination of cells, even under physiologic conditions, when these cells are functionally redundant or potentially detrimental to the organism. It involves an orderly breakdown of the cell that includes chromatin aggregation, nuclear and cytoplasmic condensation, and partitioning of cytoplasmic and nuclear material into apoptotic bodies for subsequent phagocytosis, without an extensive inflammatory response. That contrasts with features observed during necrosis, for example during ischemia, which includes energy failure, cellular swelling, membrane rupture, and release of cytoplasmic content into the extracellular compartment, followed by an inflammatory response. However, there seems to exist substantial overlap and common pathways between cell death processes, such as apoptosis, necrosis, and autophagy, which have previously been thought to be entirely separate. Apoptosis, which has also been termed cellular suicide or programmed cell death, is extensively used during tissue homeostasis, endocrine-dependent tissue atrophy, and normal embryogenesis (e.g., cardiac sculpting, ablation of tail tissue as part of tadpole metamorphosis in amphibians, or elimination of interdigital mesenchymal tissue of fingers and toes). Similarly, brain cells are produced in excess during normal brain development and are eliminated in large number during normal brain maturation in rodents, nonhuman primates, and humans. This physiologic apoptotic cell death is critical to establish proper brain structure and function, and any disruption of this process can lead to massive brain malformations and intrauterine demise. In the developing brain, apoptotic cell death can also be triggered by pathologic, extrinsic factors, such as hypoxia and ischemia. It currently remains unclear whether anesthesia-induced neuroapoptosis accelerates physiologic programmed cell death or whether it eliminates cells not destined to die, as in pathologic apoptosis.
Animal studies have initially identified a narrow window of susceptibility to neuronal cell death induced by several anesthetic drugs, such as the NMDA antagonist ketamine, the GABA agonist isoflurane, or ethanol (a combined NMDA antagonist and GABA agonist). Ketamine-induced neuronal demise is most pronounced during exposure between 5 and 7 days of age in neonatal rodents or before 6 days of age in monkeys. Similarly, a dramatic increase in neuronal apoptosis was detected in the cortex, thalamus, and amygdala of 3- to 10 day-old rodents after prolonged exposure to isoflurane, but minimal cell death was detected in 1-day-old animals or those older than 10 days of age. However, data from the laboratory of one of the authors challenge the notion that anesthetic-induced neuroapoptosis is limited to a narrow age range. In mice, neuronal apoptosis was similarly observed in the cortex and hippocampus of neonatal animals after a prolonged exposure to isoflurane; however, vulnerability extended into adulthood in brain regions with ongoing neurogenesis, such as the dentate and olfactory bulb. This finding could be explained by the fact that neurons were found particularly vulnerable to isoflurane-induced apoptosis before 14 days of cellular age and therefore would be expected to occur throughout the life of the animal in neurogenic niches containing neurons of this particular age. Surprisingly, however, other studies suggest that intrauterine exposure to clinical doses of isoflurane in prenatal rats may actually decrease physiologic apoptosis and improve subsequent memory retention, whereas only supraclinical doses of isoflurane induced neuroapoptosis in this setting. Whereas chronic opioid exposure in immature animal models can lead to long-term neurodegeneration, a recent study found no neuronal cell death immediately after a brief exposure to morphine in newborn rats. Additional research is needed to elucidate the timing of exposure on cellular demise and the differential effects of anesthetics and analgesics on brain structural integrity.
To answer the important question of whether anesthetics simply hasten natural apoptosis or whether they induce pathologic apoptosis, long-term neuronal density and neurologic function have to be assessed in adult animals exposed to anesthesia as neonates. If exposure to anesthetics or analgesics only temporarily accelerated physiologic apoptosis, one would expect normal cellular density and function in adulthood. Conversely, permanent neuronal cell loss and long-term neurocognitive impairment after anesthetic exposure early in life would suggest that anesthesia-induced neuronal apoptosis may be pathologic in nature and that the organism was unable to compensate for the neonatal cell loss by postexposure neuronal plasticity and repair. To address these questions, several studies measured neurologic function, assessed behavior, and/or determined neuronal density in adult animals after anesthetic exposure in the neonatal period. Results from these studies, however, are conflicting. A number of studies reported long-term neurocognitive or behavioral abnormalities after neonatal exposure to enflurane, halothane, isoflurane, sevoflurane, propofol, or ketamine, or to a combination of isoflurane, nitrous oxide, and midazolam. a
a References .
Importantly, however, many of these studies only observed abnormalities in very specific tests or subsets of neurocognitive batteries, whereas many other neurobehavioral domains remained intact. For example, a 6-hour exposure to midazolam, isoflurane, and nitrous oxide in neonatal rats transiently impaired learning in a water maze task in young adulthood and in older animals, whereas in the same animals, several other tests of behavior and learning, including acoustic startle response, sensorimotor tests, spontaneous behavior in an open field, and learning and memory in the radial arms maze, remained unimpaired. Similarly, after a 4-hour exposure to one minimum alveolar concentration (1 MAC) of isoflurane in 7-day-old rats, long-term memory retention was abnormal at two time points, whereas performance at several other time points, as well as in other tests of learning and memory, remained intact. Accordingly, the relevance of these temporary and limited learning deficits remains unclear. Similar to humans, the performance of rodents and primates in learning tasks depends to a great extent on maternal behavior and rearing conditions, making them strong confounders during neurocognitive testing. Moreover, another obvious and important factor in neurocognitive testing is the verification of similar degrees of motivation when comparing separate groups of animals. For example, a 24-hour exposure to ketamine sedation early in life impaired subsequent performance of rhesus monkeys in learning and memory tests, in addition to decreasing their motivation to perform these tasks.
Studies of prolonged opioid administration in immature animals have also found evidence for long-term impairment in learning tasks, as well as altered pain responses in adult animals after exposure to morphine, fentanyl, heroin, or methadone early in life. a
a References .
Conversely, several other investigations have observed no neurologic abnormalities after administration of midazolam, isoflurane, sevoflurane, or ketamine, even when using complex neurologic tests in neonatal animals. b
b References .
It remains to be determined whether these differential findings are attributable to the anesthetic doses, exposure times, species, or are related to the specific type of neurologic test used or the timing of the exposure or the assessment. Interestingly, escalating exposure times of isoflurane in neonatal rats caused neuronal apoptosis beginning at 2 hours of anesthesia, but no evidence of long-term neurologic abnormalities until 4 hours of anesthesia. In another study, a 6-hour exposure to isoflurane caused significant apoptosis immediately after exposure in neonatal mice but resulted in no measurable long-term deficits in performance of complex neurologic tests as adult animals. Moreover, in this study, brain neuronal density in adulthood was not diminished in regions significantly affected by anesthesia-induced neuroapoptosis compared with unanesthetized littermates. Studies in mice exposed to 6 hours of isoflurane at 21 days of age did not observe subsequent diminution in dentate granule cells, despite considerable apoptotic cell death immediately after exposure. These findings could either suggest that isoflurane may only accelerate physiologic apoptosis or that the developing brain's plasticity and capacity for repair can compensate for a pathologic insult early in life. Conversely, a study in similarly aged rats observed a permanent elimination of neurons, as well as neurologic abnormalities in adult animals, after exposure to isoflurane, nitrous oxide, and midazolam as neonates, suggesting that either the specific combination of anesthetic drugs (isoflurane alone vs. the combination exposure) or species differences (rats vs. mice) could affect relationships between neonatal neuroapoptosis and long-term function and neuronal density. Alternatively, these conflicting results may be explained by the dissimilar testing environment because neurocognitive tests are not easily transferable among laboratories. Yet another explanation holds that neonatal apoptosis may not be causally linked to adult neurocognitive performance at all, as evidenced by substantial apoptotic cell death observed immediately after carbon dioxide–induced hypercarbia in unanesthetized neonatal rats, which lacked long-term neurologic sequelae.
The generation of new neurons, or neurogenesis, as well as new astrocytes—gliogenesis—is most active in the immature brain in utero or soon after birth. Accordingly, several studies have investigated the effects of anesthetic exposure, primarily isoflurane, on progenitor viability and rates of neurogenesis. Although isoflurane did not kill neural progenitor cells in vitro, 3.4% isoflurane for 4 hours decreased the rate of neuronal proliferation and increased neuronal fate selection. These findings have been confirmed as 2.8% isoflurane for 6 hours had no effect on neural stem cell viability, whereas larger doses inhibited cell proliferation. In vivo studies in newborn mice failed to find significant cell death in radial glial-type progenitor cells in the dentate gyrus immediately after a 6-hour isoflurane exposure, although more mature neuroblasts made up a substantial number of cells affected by anesthesia-induced apoptotic cell death. Morphine, on the other hand, did not affect cell survival in a murine cerebellar neuronal precursor culture, although, exposure decreased DNA synthesis.
Isoflurane also impairs the growth of cultured immature astrocytes and delays their maturation after a 24-hour administration of 3% isoflurane, although even this extreme exposure showed no effect on cell viability. Morphine, although increasing apoptosis in neurons and microglia, did not affect astrocytes in a study involving human fetal cell cultures.
The immature brain accumulates an overabundance of neuronal connections in infancy, and the number of dendrites and synapses dramatically decreases after the first year of life. Several studies have examined the effects of a wide variety of anesthetics, such as propofol, isoflurane, sevoflurane, desflurane, midazolam, and ketamine on dendritic arborization and synaptic architecture. c
c References .
A common theme in these studies is that anesthetics can affect dendritic arborization and synaptic density, and that the direction of any change, either an increase or a decrease in the number of dendritic spines, depends on the age at which the animals were exposed to anesthetics and therefore the developmental state of the brain. During the first 2 weeks of life, anesthetic exposure can lead to a decrease in synaptic and dendritic spine density in small rodents, whereas exposure beyond this age can lead to an increase in the number of dendrites. Other studies suggest that this differential effect may depend on the maturational switch of GABA from excitation to inhibition, which is related to the switch from the immature form of the potassium-chloride-cotransporter NKCC1 to the mature KCC2. However, the permanence of these dendritic changes remains controversial because some studies have observed only a transient effect after ketamine, midazolam, or isoflurane anesthesia exposure early in life.
Isoflurane- or propofol-based anesthesia in neonatal animals has been associated with a decrease in brain-derived neurotrophic factor (BDNF), a protein integral to neuronal survival, growth, and differentiation. The cellular mechanism involves a reduction in tissue plasminogen activator and plasmin, which converts proBDNF to BDNF. Accordingly, isoflurane has been found to trigger proBDNF/p75 NTR (p75 neurotrophin receptor) complex–mediated apoptosis in neonatal mice. Similarly, prolonged exposure to opioid receptor agonists early in life alters nerve growth factors in the immature brain.
Ultrastructural morphologic abnormalities have been reported in mitochondria of pyramidal neurons in the subiculum of 7 day-old rats following 6 hours of isoflurane, nitrous oxide, and midazolam. A morphometric analysis demonstrated mitochondrial enlargement, impaired structural integrity, and decreased mitochondrial density, indicating protracted mitochondrial injury after drug exposure. Moreover, an ultrastructural examination with electron microscopy revealed increased autophagy, a form of cell death.
Ketamine induces reentry of postmitotic neurons into the cell cycle in immature rats. This could be one trigger for apoptotic cell death, since postmitotic neurons lose the ability of neuronal progenitor cells to enter the cell cycle during proliferation and are committing programmed cell death when forced to reenter the cell cycle.
Structural integrity of the cellular cytoskeleton is critical for proper neuronal morphology and function. Actin is one of the major components of the cytoskeleton of all eukaryotic cells and participates in important cellular processes, including cell signaling, motility, and division. It is also essential for the formation of dendritic spines. Isoflurane has been found to depolymerize actin in neurons and astrocytes, initiating cytoskeletal destabilization, impairment of astrocyte morphologic differentiation and maturation, as well as neuronal apoptosis.
Most animal studies have focused on the effects of general anesthetics and sedatives on the developing brain. However, it is important to also consider the developmental impact of anesthetics on the spinal cord. One study observed increased neuroapoptosis in the lumbar spinal cord of 7-day-old rats after 6 hours of 0.75% isoflurane with 75% nitrous oxide. Increased neurodegeneration was also documented in brain and spinal cord after 6 hours of 1% isoflurane in a similar model, but not after 1 hour of anesthesia or following spinal administration of bupivacaine. Intrathecal ketamine can also cause neuroapoptosis in the developing spinal cord of 3-day-old rats, but not of 7-day-old rats. Preservative-free ketamine caused long-term alterations in spinal cord function and gait disturbances, whereas in a separate study, even high-dose intrathecal morphine produced no signs of spinal cord toxicity.
The exact mechanisms that trigger the previously listed effects of anesthetics and sedatives on the immature brain and spinal cord remain unresolved. Elucidating these mechanisms will be critical in establishing the relevance of these findings for pediatric anesthesia and neonatal critical care medicine, as well as for developing mitigating interventions, if necessary. The current, overarching hypothesis is that anesthetics and sedatives interfere with normal GABA A and NMDA receptor–mediated activity, which are the putative targets for unconsciousness, amnesia, and immobility, and at the same time are essential for mammalian CNS development. Administering GABA A -receptor agonists and/or NMDA-receptor antagonists could potentially cause abnormal neuronal inhibition during a vulnerable period in neuronal development, triggering apoptosis in susceptible neurons, which in turn leads to neurocognitive impairment and decreased neuronal density into adulthood. Other lines of evidence suggest that the NMDA receptor–blocking properties of ketamine may upregulate NMDA receptors, rendering neurons more susceptible to excitotoxic injury caused by endogenous glutamate action on the increased number of receptors immediately after withdrawal of the anesthetic such as proposed for ketamine. However, several observations partly contradict both hypotheses; neuronal cell death has been reported during exposure to anesthetics and not only after their discontinuation. Moreover, several anesthetics with minimal NMDA-receptor interaction, such as propofol and barbiturates, have demonstrated robust neurotoxic properties, whereas the neurotoxic potency of the NMDA-antagonist xenon has been found to be limited, therefore casting doubt on receptor upregulation as the sole mechanism for anesthetic neurotoxicity. In terms of abnormal neuronal inhibition being the main trigger for apoptosis in developing neurons, GABA A -receptor stimulation indeed decreases neuronal activity in the mature brain; however, it also causes excitation in developing neurons, thereby contradicting the inhibition hypothesis. In immature neurons, intracellular chloride (Cl − ) concentration is high; thus GABA-induced opening of Cl − channels allows this anion to exit the cell, leading to membrane depolarization. On the other hand, the intracellular Cl − concentration is low in mature neurons. When anesthetics open Cl − channels in mature neurons, ions enter the cell, thereby hyperpolarizing the membrane. This reversal of the cellular Cl − gradient occurs as a result of a switch from the immature Na + -K + -2Cl − cotransporter 1 (NKCC1) to the mature brain form, K + -Cl − cotransporter 2 (KCC2). Along these lines, studies in neonatal rats demonstrated excitatory properties in the brain and episodes of epileptic seizures during sevoflurane anesthesia. Isoflurane has also been shown to cause an excessive release of Ca 2+ from the endoplasmic reticulum via overactivation of inositol 1,4,5-trisphosphate receptors (InsP3Rs) in neonatal rats in vivo and in vitro. A similar mechanism may be linked to the production of Alzheimer-associated increases in β-amyloid protein levels after anesthesia. Although xenon and hypothermia cause neuronal inhibition, they do not exacerbate isoflurane-induced neuronal cell death as expected by the cumulative inhibition of neurons, but rather significantly reduce it.
Importantly, evidence indicates that equianesthetic concentrations of the three contemporary inhalational anesthetics cause similar degrees of neuroapoptosis, suggesting that it is the anesthetic depth and not the specific doses or end-tidal concentrations of the anesthetics that determines cytotoxic potency. However, other studies have failed to link the anesthetic and the apoptotic mechanisms. Specifically, although racemic ketamine and ( S )-ketamine both elicit their anesthetic effects via NMDA-receptor blockade, ( S )-ketamine induced up to 80% less cell death in vitro compared with equipotent doses of racemic ketamine. Moreover, concomitant administration of the GABA A -receptor antagonist gabazine failed to attenuate neuroapoptosis induced by the GABA agonist isoflurane, whereas administration of the α 2 -agonist dexmedetomidine did. Decreases in anesthetic-induced neuronal activity may therefore be less important than the disruption of the neuronal balance of excitation and inhibition, as demonstrated by studies that examined anesthesia-induced dendritic morphologic changes in mice. In a mechanistic study of brain development, simultaneous blockade of excitatory and inhibitory activity with tetrodotoxin did not lead to structural changes during synaptogenesis that would have been expected from a causative relationship between neuronal inhibition and structural abnormalities; the administration of either GABA A -agonistic or NMDA-antagonistic compounds alone did alter synaptogenesis.
It is not entirely clear at this time whether cytotoxicity is a direct effect of the anesthetic itself, of any anesthetic by-products, or if it is related to physiologic derangements observed during anesthesia in small rodents. Hypercarbia can trigger widespread neuroapoptosis, even in unanesthetized neonatal rats exposed to increased partial pressures of carbon dioxide. Whereas apoptotic cell death was quantitatively indistinguishable from neurodegeneration in isoflurane-treated litter mates, which were also hypercarbic; neurocognitive impairment in adults was observed only in the isoflurane-treated animals. However, widespread apoptotic neurodegeneration observed in anesthetized nonhuman primates, where carbon dioxide tensions were controlled by tracheal intubation and ventilation, suggests that metabolic derangements may not be sufficient to explain the structural abnormalities observed in immature animal species. Lastly, experimental models of neurodegeneration have implicated reentry of postmitotic neurons into the cell cycle, leading to cell death. Ketamine exposure has been found to induce aberrant cell cycle reentry, leading to apoptotic cell death in the developing rat brain. However, a causative link between neuronal degeneration immediately after exposure and subsequent cognitive abnormalities observed into adulthood have yet to be firmly established.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here