Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We are indebted to our colleagues and collaborators who have made major contributions to our research on SGLTs, and grants from the National Institutes of Health that provided financial support.
As in animals, humans need to eat, digest, and absorb food to grow and conduct their daily activities. Glucose contained in the diet and synthesized in the liver and other organs is a central unit of energy currency in the body. The concentration of glucose in plasma is held within narrow limits, 4–10 mM, to ensure a steady supply to the organs of the body where it is primarily used to generate the ATP required to fuel the electrical activity in the brain, muscle contraction, secretion, and absorption in the gastrointestinal (GI) tract and kidney. Surprisingly to many, glucose is not an essential nutrient, as the body can meet its needs from gluconeogenesis. Normally, adult humans consume 100–800 g of carbohydrates each day in the form of starches, di- and monosaccharides, subject to availability and individual preferences. Digestion in the GI tract results in the breakdown of complex carbohydrates to glucose, galactose, and fructose before assimilation can begin. In the 6th edition of this work we focus on the recent advances in glucose, galactose, and fructose absorption across the wall of the small intestine, and readers are referred to other chapters in this and earlier editions for discussions about digestion, sugar absorption and metabolism, and the control of food consumption.
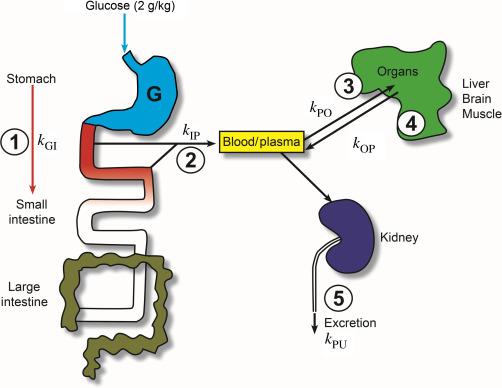
Fig. 46.1 gives an overview of the steps in glucose absorption and distribution after a test meal. The transit time for food to pass from the stomach to the anus is highly variable, but on average the stomach empties in 4–6 h, digestion and absorption in the small intestine takes 6–8 h, and unabsorbed food remains in the colon 1–3 days. Following delivery to the stomach, the sugar empties into the duodenum (1), where it is absorbed into the blood (2). Before entering the general circulation, glucose passes through the liver, where it may be stored and released (3, 4). In this simple scheme, the liver is included with the other organs (brain, muscle), and the rate of release is determined by metabolism, including synthesis and breakdown of glycogen and gluconeogenesis. Finally, glucose may be lost via the urine through the kidneys (5).
Initially, the test used to study glucose absorption in humans involved drinking a solution containing 2 g of glucose per kg and monitoring blood glucose levels over time, the so-called oral glucose tolerance test (OGTT, see Fig. 46.2 ). However, it has become increasingly clear that it is necessary to consider all the above variables to interpret changes in blood glucose levels in an OGTT. The Cobelli group has led in the use of multiple tracer techniques and modeling to describe the rate of appearance of glucose in plasma after a test meal (R a ), after considering endogenous glucose production (EGP), and glucose disposal (R d ). Their approach has been to include [ 13 C]-glucose as a tracer in the test meal, and estimate EGP and R d from intravenous infusions of [6,6- 2 H 2 ]-glucose and/or [6- 3 H]-glucose in dual and/or triple tracer protocols (see, e.g., Refs. ). For a critical evaluation of the dual and triple OGTTs, see Ref. . The bottom line is that it is not trivial to extract the rate of glucose absorption in vivo in human or in experimental animals.
The approach that we have embraced for OGTTs is to use positron emission tomography (PET) with nonmetabolized glucose tracers. The advantages are that one can monitor continuously the distribution of the PET tracer throughout the living subject as a function of time and determine the rates of gastric emptying, glucose absorption, and distribution throughout the body. The obvious disadvantages are that glucose is ingested as a simple sugar, not a test meal, and that metabolism in the intestine, liver, and other organs cannot be evaluated.
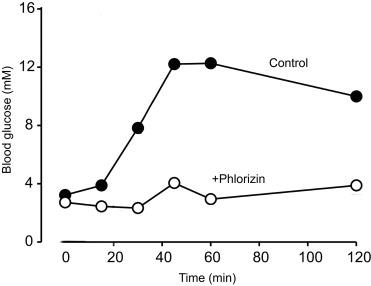
The results of typical OGTTs in mice are shown in Fig. 46.2 . Following an overnight fast, the blood glucose levels were measured before and after gavage of a test meal of 2 g of glucose per kg of mouse body weight. The blood glucose level increased from 3.5 to 12 mM in 40 min and then slowly returned toward the fasting level. When phlorizin, the specific inhibitor of SGLTs, was included in the gavage solution, the rise in blood glucose was blunted. This is taken as evidence for the importance of SGLT1 in glucose absorption in fully conscious mice. Although the primary use of OGTT in the clinic is to evaluate insulin release in response to an oral glucose load, it has limited usefulness in evaluating glucose absorption in the absence of additional information about EGP and R d from double and triple tracers (see above).
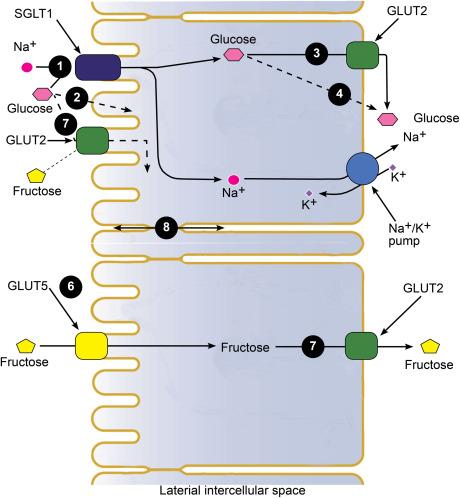
After the digestion of complex carbohydrates by pancreatic enzymes to free monosaccharides, chiefly glucose, galactose, and fructose, the sugars are absorbed into the blood by the mature enterocytes on the upper third of the intestinal villi ( Fig. 46.3 ). It is commonly accepted that the monosaccharides are absorbed by transport proteins embedded in the brush border and basolateral membranes, SGLT1 (1), GLUT2 (7), and GLUT5 (6), and that passive absorption through the plasma membranes and tight junctions (2, 4, and 8) is of minor importance. Glucose (and galactose) is actively transported into the epithelium by the sodium-glucose cotransporter (SGLT1) in the brush border membrane (1), and the sodium gradient across the brush border membrane is maintained by the basolateral Na/K-pump. The sugar selectivity of SGLT1 is quite restrictive in that only hexoses with an equatorial ―OH group at carbon #2 are accepted. Large substitutions of ―OH groups are only accepted at C#1 in the beta-conformation (β-glucopyranosides), and these may be either transported substrates, such as indican, or potent competitive inhibitors, such as phlorizin. Fructose is not a substrate for SGLT1 and the sugar is transported across the brush border membrane by the facilitated fructose transporter GLUT5 (6). Glucose and fructose within the epithelium escape into the blood through the facilitated glucose transporter GLUT2 (3 and 7). The net result of this polarized distribution of sugar transporters and the Na/K-pump is the net absorption of glucose, sodium, and water across the intestine and is the basis of oral glucose therapy (OGT) for secretory diarrhea (see below). It has been proposed that GLUT2 may be present in the brush border membrane and play a role in passive glucose and fructose transport. The hypothesis is controversial but we do not think that it is an important player given that glucose OGTTs are normal in mice and humans lacking GLUT2 ( Glut2 -null mice ; Fanconi-Bickel syndrome ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here