Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Subepithelial tumors are diverse and are reflective of the variety of cell types that reside in the subepithelial space.
Systemic processes, such as inflammation or metastatic disease, may produce subepithelial masses.
Lymphoproliferative disease can be associated with systemic lymphoma at the time of diagnosis or may be the harbinger of future systemic lymphoma.
Expert ophthalmic pathology evaluation—including histopathology and immunohistochemistry—is invaluable in the diagnosis and ultimately treatment of subepithelial tumors.
The substantia propria of the conjunctiva is a loose network of connective tissue composed of fibroblasts, blood vessels, nerves, and lymphatics. Rather than arising from mesodermal somites, which are absent in the head and neck region, the cells of this layer arise from migration of the neural crest (mesectoderm or ectomesenchyme). A wide range of tumors may arise from this rich tissue layer. This chapter discusses subepithelial neoplasms of the conjunctiva; for completeness, certain congenital, inflammatory, and degenerative lesions are also mentioned.
While evaluating a patient with a subepithelial lesion, the clinician should obtain a careful history, especially concerning the time of onset, rapidity of growth, history of trauma, and history of systemic disease. A complete ocular examination, including palpation of regional lymph nodes, should be performed. Photographic documentation should be obtained. Ultrasound biomicroscopy, anterior segment ocular coherence tomography, and in vivo confocal microscopy may play an increasingly important role in the diagnosis and management of conjunctival tumors.
Congenital lesions are primarily choristomas, tumor-like growths composed of elements not normally indigenous to the affected area. There are three types of choristomas: dermoids and dermolipomas, ectopic lacrimal glands, and epibulbar osseous and neuroglial choristomas.
Dermoids most commonly involve the inferotemporal limbal corneal and epibulbar region ( Fig. 54.1 ). Bilateral congenital ring dermoids rarely affect 360 degrees of both limbal areas. Small dermoids appear clinically as tan, rather inconspicuous lesions, whereas larger lesions are whiter and may protrude from the ocular surface. Clinically, most dermoids are asymptomatic; however, they may produce significant astigmatism, with secondary amblyopia and ocular irritation due to poor eyelid closure or tear film anomalies. Fine hairs may grow from their surface, producing irritation or trauma. Histologically, dermoids are solid, placoid tumors that arise from the outer third of the sclera and consist of thick dermis-like collagen that may contain hair follicles, sebaceous glands, sweat glands, and adipose tissue. In rare cases, the entire cornea is involved with white corneal opacifications and disorganized stroma with adnexal glands.
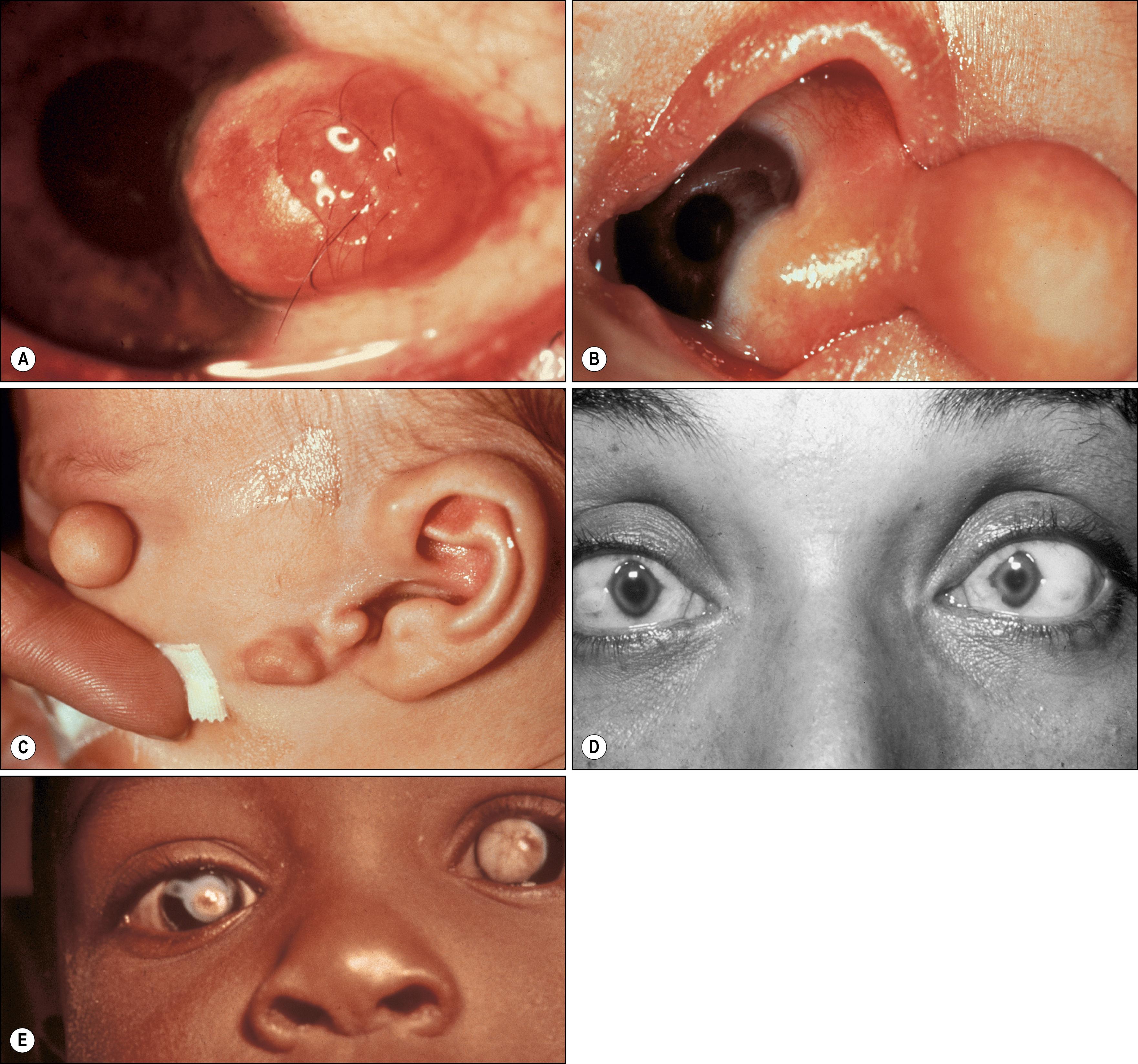
Clinically, dermolipomas are similar to dermoids but are somewhat yellow in color and arise near the insertion of the lateral rectus muscle. The lesion may extend upward to the superior fornix. Because of the location and color of dermolipomas, the differential diagnosis of this lesion includes prolapsed orbital fat ( Fig. 54.2 ), prolapse of the palpebral lobe of the lacrimal gland, and lymphoma. These last three lesions, however, are freely mobile over the underlying sclera, unlike dermolipomas, which are firmly fixed. On computed tomography (CT) or magnetic resonance imaging (MRI), dermolipomas have features that may be indistinguishable from prolapsed orbital fat. Dermolipomas are similar to dermoids histopathologically except that the former usually lack pilosebaceous units and have more mature adipose tissue.
Both dermoids and dermolipomas may occasionally coexist with other systemic malformations including Goldenhar syndrome (with preauricular appendages, hemifacial microsomia, and vertebral body anomalies), Emanuel syndrome, mandibulofacial dysostosis (Treacher Collins syndrome, Franceschetti syndrome), and band-like cutaneous nevus and central nervous system (CNS) dysfunction (Solomon syndrome, linear sebaceous nevus of Jadassohn). Oculoectodermal syndrome (Torjello Lacassie Droste syndrome) is associated with somatic KRAS gene mutations and includes epibulbar dermoids and aplasia cutis as well as various systemic congenital anomalies such as intracranial anomalies and heart defects. , Both lesions are occasionally associated with eyelid colobomas, suggesting that limbal dermoids and dermolipomas may arise from the entrapment of dermal elements within the sclera at the time of eyelid development. Indeed, choristomas have also been associated with microphthalmos; in some of these cases dermal appendages are found within the vitreous cavity. ,
Dermoids and dermolipomas tend to grow with the patient and not to undergo neoplastic transformation. Indications for removal include astigmatism with or without amblyopia, irritation, and cosmetic deformity. Because the outer third of the sclera is often involved, a lamellar excision is usually required. When the cornea is involved exclusively, the surgeon should anticipate lamellar or penetrating keratoplasty. Amniotic membrane transplantation may assist with the closure of large conjunctival defects. Complications of excision include globe penetration, reduced motility from scarring or surgical injury of a rectus muscle, and increased astigmatism. Some dermoids and most dermolipomas extend to the fornices and may become entangled in extraocular muscles and orbital fat. Care should be taken to excise all remnants, since exposed elements may incite a significant inflammatory response. , , Zhong et al. have devised a grading system that correlates lesion location, shape, and size with postexcision visual acuity.
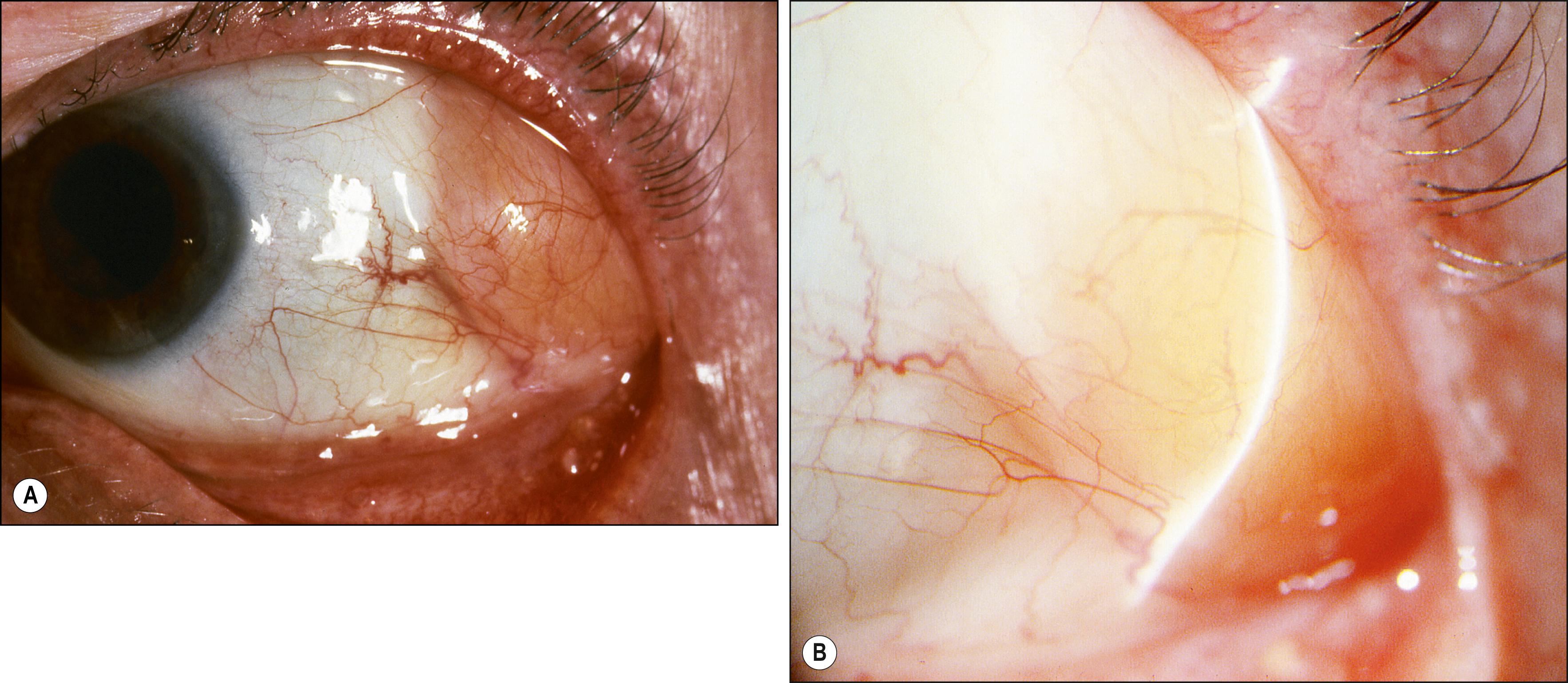
Ectopic lacrimal gland is the second most common choristoma that affects the epibulbar surface ( Fig. 54.3 ). Simple choristomas are composed only of acinar lacrimal gland tissue, whereas complex choristomas also possess other elements, such as smooth muscle, nerve elements, cartilage, sweat glands, or pilosebaceous units. The frequent presence of smooth muscle suggests that the lesions may represent the ectopic palpebral lobe of the lacrimal gland, which is intermixed with the smooth Müller muscle. Clinically, the lesions tend to have a fleshy pink appearance with raised translucent nodules; they are well vascularized. Some lesions may resemble the salmon patch often associated with lymphoid tumors. If the lesion extends onto the corneal surface, associated corneal scarring may be present. Occasionally the palpebral portion of the lacrimal gland may prolapse into the subconjunctival space at the upper temporal bulbar conjunctiva and may consequently be misdiagnosed as ectopic lacrimal gland tissue. Incorrect diagnosis with consequent surgical intervention can jeopardize the excretory ducts of the prolapsed lacrimal gland tissue, leading to dry eye. , Choristomas have been reported in cases of encephalocraniocutaneous lipomatosis, which is associated with hemifacial microsomia, subcutaneous lipomas, and CNS lipomas. , Complex and simple choristomas may slowly grow under the hormonal influence of puberty; however, unlike orbital ectopic lacrimal glands, there is exceedingly rare malignant potential, with only one reported case in the literature. Because the lesion may extend quite deeply into the sclera and cornea, only partial excision may be possible.
Osseous choristomas, the rarest of the epibulbar choristomas, are solitary nodules that resemble conjunctival dermoids , but are much more discrete and spare the underlying cornea, as they are characteristically located 5–10 mm posterior to the limbus ( Fig. 54.4 ). Since the first description by von Graefe in 1863, a total of 66 cases of epibulbar osseous choristoma have been reported. Most frequently, they present as isolated epibulbar lesions in the superotemporal quadrant but may occur in other locations on the surface of the globe or in association with other choristomatous tissue in 10% of cases. Extensive tumors have simulated the extraocular extension of retinoblastoma. Histologically, these lesions are composed of mature, compact bone surrounded by additional choristomatous elements, as outlined previously. The osteocytes appear normal and Haversian canals are present. Alyahya et al. reported a 15-year-old with a cartilaginous choristoma in the medial subconjunctival space. Epibulbar neuroglial choristomas may represent ectopic neural tissue from the anterior lip of the optic cup or vesicle. , Both lesions are stationary, and removal is indicated only for diagnostic and cosmetic purposes.
Unlike choristomas, hamartomas are composed of elements normally found in the affected area. Examples of subepithelial hamartomas that occur rarely include fibrous hamartomas. Neurofibromas are similar lesions but are thought to be neoplastic. Neurofibromas are solid, nodular, tan or gray-white lesions occurring on the bulbar or palpebral conjunctiva. The lesions may be plexiform, solitary, or diffuse types and are almost always associated with neurofibromatosis type 1 or 2. Sometimes diagnosis of the conjunctival lesion may antedate the diagnosis of neurofibromatosis by at least 10 years. , Neurofibromas must be distinguished from neuromas of the conjunctiva, which can occur in association with multiple endocrine neoplasia (MEN). Fibrous hamartomas have been reported in patients with the Proteus syndrome, a rare hamartomatous syndrome associated with epibulbar tumors, skeletal anomalies, plantar or palmar gyriform hyperplasia, subcutaneous tumors, epidermal nevi, and visceral abnormalities. These lesions are isolated, temporal epibulbar lesions that contain abundant mature elastic fibers intermixed with fibrous tissue. , Smooth muscle hamartomas can arise from vascular smooth muscle and from smooth muscle in the eyelid retractor complexes (Müller muscle superiorly and capsulopalpebral muscle inferiorly). Periocular congenital smooth muscle hamartomas have rarely been reported but should be considered in the differential diagnosis of a cystic-appearing conjunctival fornix lesion. ,
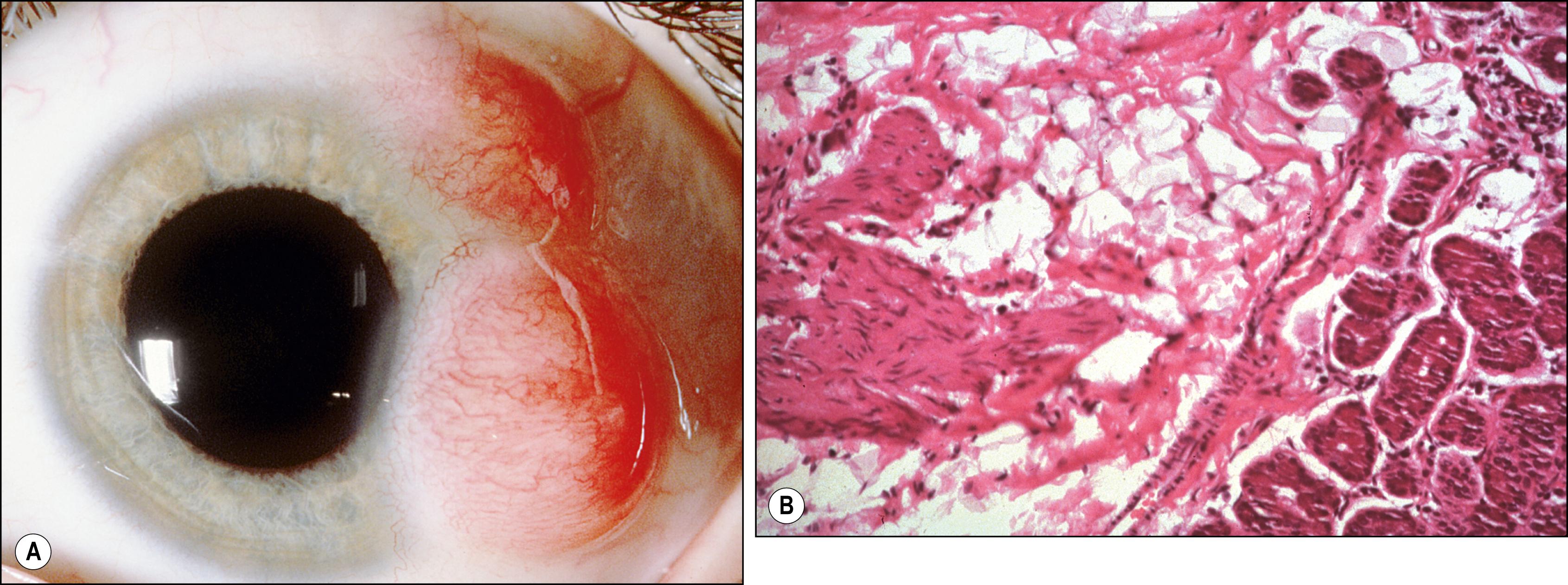
First described by Leber in 1880, hemorrhagic lymphangiectasia may be a developmental anomaly or may occur in association with trauma or inflammation. Unlike lymphangiomas, which are cellular proliferations of lymphatic channel elements, hemorrhagic lymphangiectasias are irregularly dilated periodically hemorrhage-filled lymphatic channels of the bulbar conjunctiva. Surrounding conjunctival edema or subconjunctival hemorrhage may be present ( Fig. 54.5 ). Treatment is local excision or diathermy. The preceding condition must be distinguished from ataxia telangiectasia (Louis-Bar syndrome), Bloom syndrome, and hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease), in which there are epibulbar and interpalpebral telangiectasias of the arteries without an associated lymphatic component. The conjunctival lesions of the Louis-Bar syndrome are a marker for associated cerebellar abnormalities and immunologic derangements (e.g., hypogammaglobulinemia), which are conducive to sinopulmonary infection and lymphoreticular proliferations, particularly T-cell leukemias. Bloom syndrome is an autosomal recessive condition caused by a mutation of the BLM gene on chromosome 15 (15q26.1). Most common in Ashkenazi Jews, it is associated with increased susceptibility to cancer and infections, photosensitivity, and growth retardation. Patients with hereditary hemorrhagic telangiectasia, or Rendu-Osler-Weber disease, have dilated vessels due to abnormalities in vascular wall repair, which increases such patients’ susceptibility to bleeding from the skin and mucous membranes, CNS, lungs, and other vital organs. This is an autosomal dominant disorder produced by an abnormality in one of two genes (chromosomes 9q33–34 and 12q13) involved in vessel wall repair. In these conditions, the epibulbar vascular lesions do not acquire a tumefactive character (hamartia) because they are simple telangiectasias that grow with the patient and the eyeball. Episodic events such as hemorrhage or swelling are also not encountered.
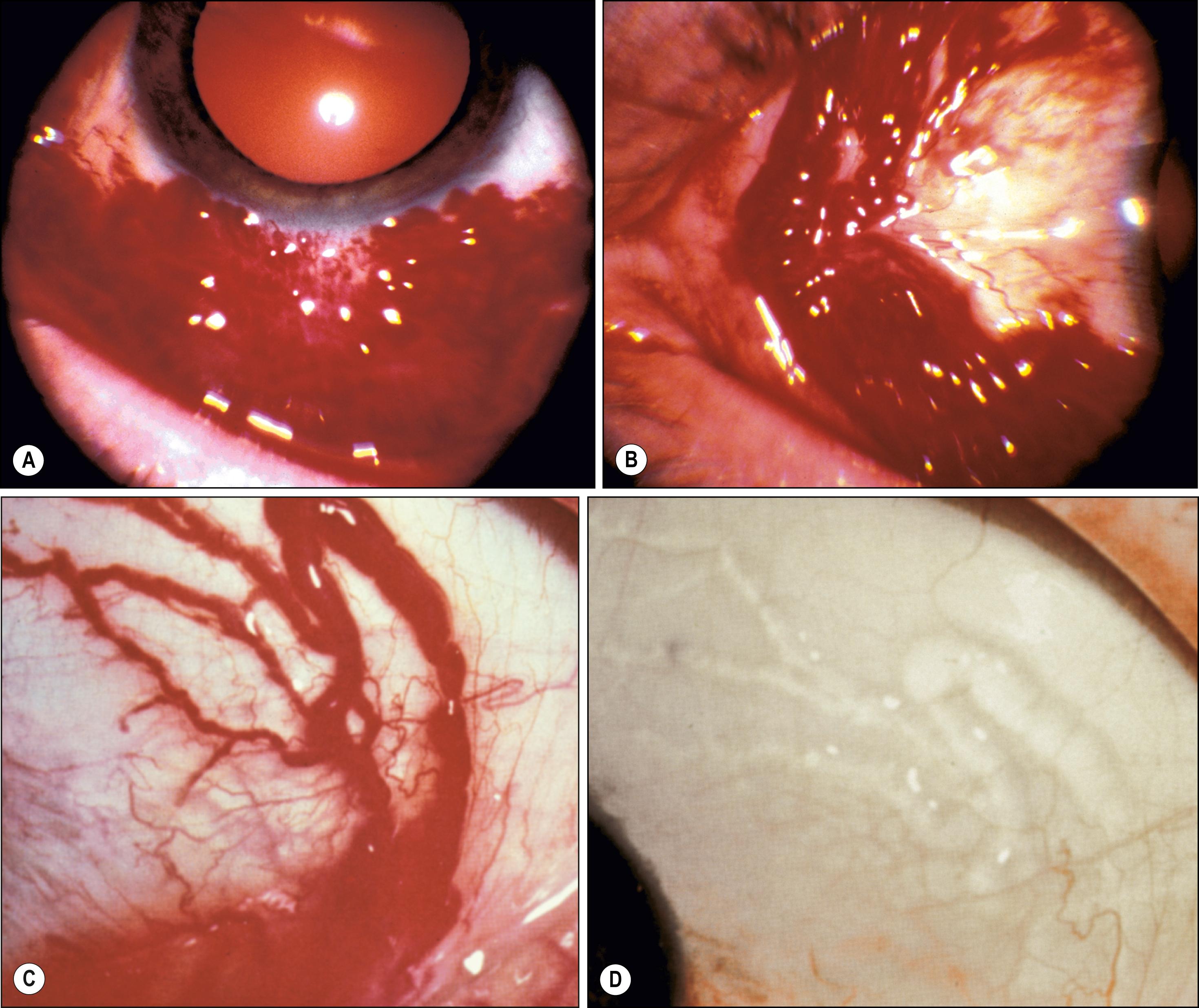
Jakobiec et al. described a congenital purely venous conjunctival malformation that was excised due to symptoms from multifocal thrombosis. On histopathology, compared with lesions of arterial origin, venous lesions have thin walls with a negligible and irregular muscularis and no elastic lamina. Immunohistochemistry of the endothelial cells can assist in the diagnosis in that those of vascular origin are CD31- and CD34-positive, whereas those of lymphatic origin are also D2-40–positive.
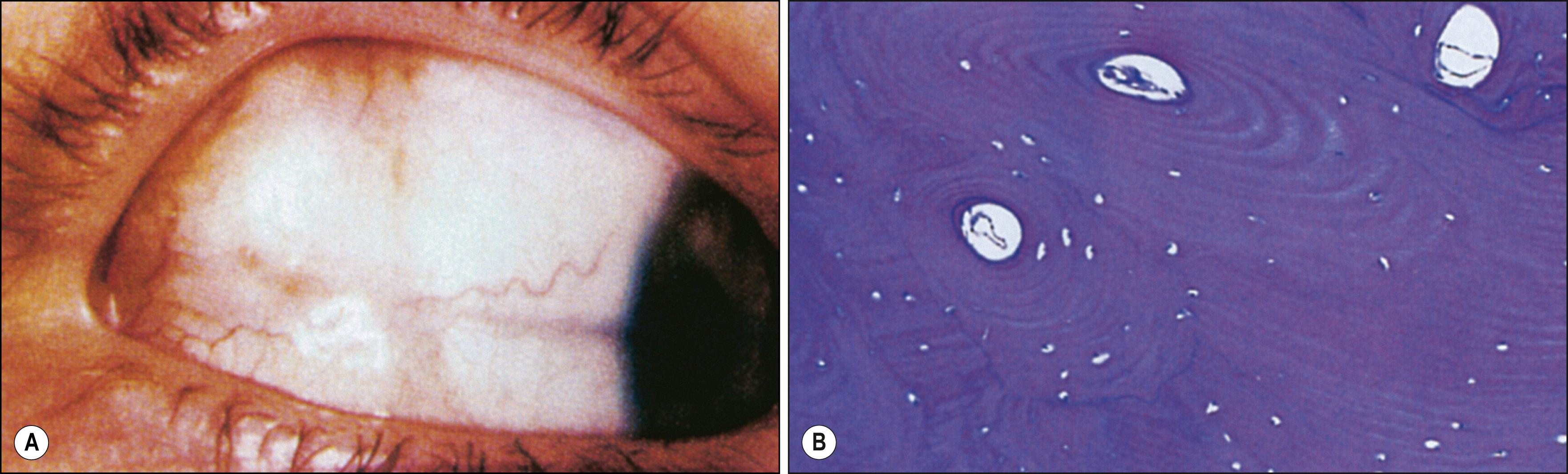
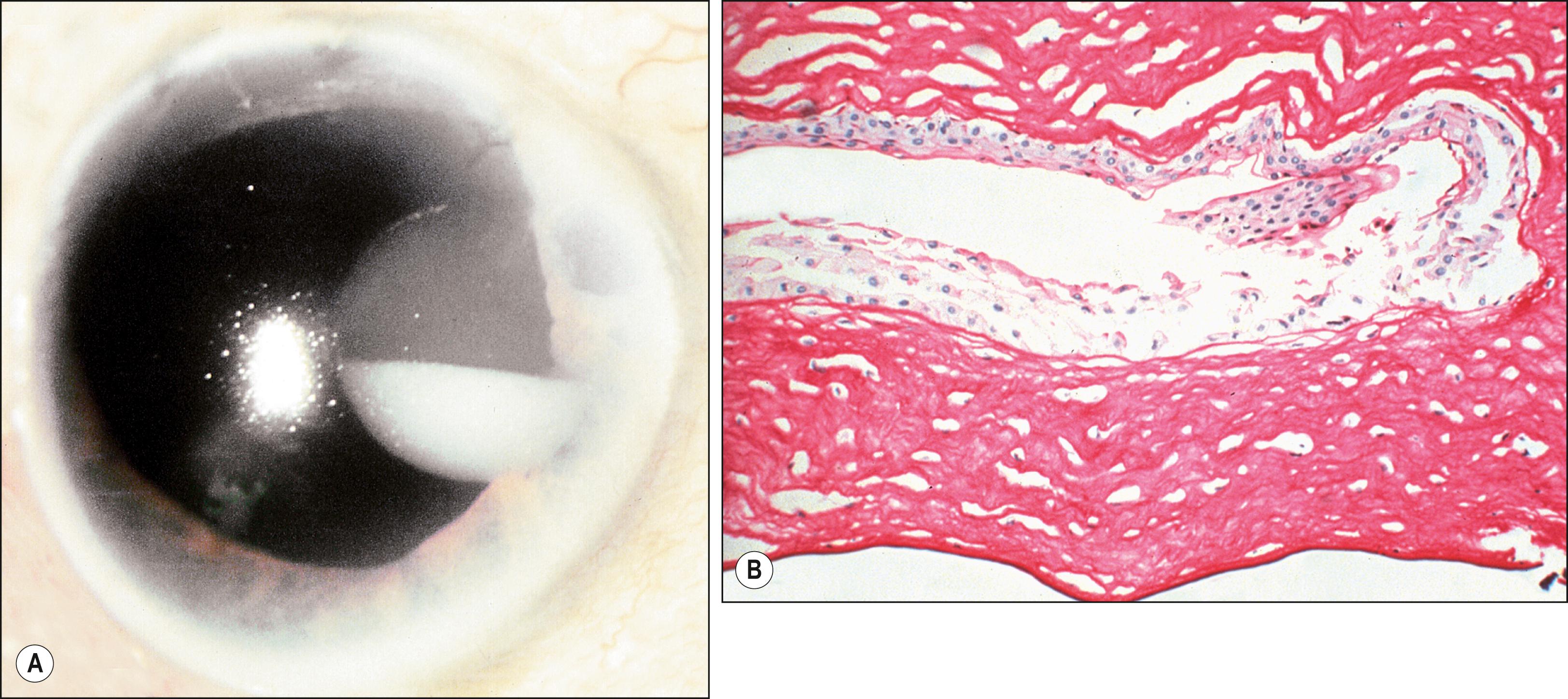
Conjunctival cysts may be congenital or acquired; the latter occur in the setting of inflammation or following inflammatory processes such as vernal conjunctivitis, Stevens-Johnson syndrome, or traumatic or surgical subconjunctival implantation of epithelium; they are much more common than congenital cysts. Usually stable, conjunctival cysts may rarely enlarge or, if intracorneal, cause intracorneal pseudohypopyon. , Histologically, cysts are lined by nonkeratinizing conjunctival epithelium with goblet cells. The center contains cellular debris and inflammatory cells. If double-layer cuboidal epithelium is present, the cyst is of ductal origin. Rarely, corneal intrastromal cysts may result from penetrating or perforating wounds to the cornea ( Fig. 54.6 ). Such wounds implant epithelium within the stroma, where it proliferates between the lamellae. Spontaneous, acquired epibulbar, mucogenic, and subconjunctival cysts have been described. These lesions are freely movable and may arise because of a mucosecretory abnormality of both goblet cells and conjunctival epithelial cells. Rarely, conjunctival cysts may enlarge sufficiently to involve substantial portions of the orbital cavity. , Primary conjunctival cysts of the orbit are most frequently located in the superonasal quadrant of the orbit, whereas dermoid and epidermoid cysts of the orbit are most commonly located superotemporally. Conjunctival cysts can develop after the sequestration of conjunctival epithelium into the orbital soft tissues during embryonic development. The cysts can be treated with simple excision; visualization of the cyst may be enhanced by preoperative injection of a dye such as trypan blue or indocyanine green. In patients who have undergone enucleation, conjunctival cysts of the orbit have been successfully treated with intralesional injections of a 20% trichloroacetic acid solution.
Subconjunctival keratinous and epidermoid cysts have been described. Both contain acellular keratin debris. Significantly, the latter lesion may be associated with Gorlin-Goltz syndrome or nevoid basal cell carcinoma syndrome.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here