Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors acknowledge support provided by the American Heart Association (SDG 17SDG33410716 to BL) and the National Institutes of Health (grants RO1 HL074045, HL063043, and HL138579 to S.G.; R00 HL127299 and AHA 19TPA34910191 to P.B.R.).
The sarcoplasmic reticulum (SR) is a membrane-delimited intracellular organelle that spans the sarcomere and wraps up the contractile myofilaments in the striated muscle of almost all species. The SR is not continuous with the external membrane, but recent data indicate that it is continuous with the nuclear envelope. In striated muscle, the main function of the SR is to provide the majority of Ca 2+ ions required to activate the myofilaments and to resequester Ca 2+ from the myoplasm to allow for relaxation. The compartmentalization of muscle fibers into small (∼2-μm) structural–functional units (sarcomeres) and the enveloping of sarcomeres by SR both ensure that Ca 2+ diffusion from and reuptake into the SR is not a limiting step for the muscle contractile cycle.
The SR is composed of two regions: junctional SR (jSR), which directly faces invaginations of the surface membrane, called transverse tubules (T-tubules), and extrajunctional free SR (fSR), which is situated near the myofibrils. jSR forms extended, flattened cisternae with an average diameter of around 0.6 μm. Each cisterna carries sets of closely grouped structures (“feet”) that represent the cardiac SR Ca 2+ release channels, also known as ryanodine receptors (RyR2s), and contains electron-dense material, which is formed by the Ca 2+ -binding protein, calsequestrin-2 (CASQ2). On the other hand, the fSR is devoid of CASQ2, and its external surface exhibits densely distributed particles corresponding to the SR Ca 2+ pump (SERCA2a).
Electrical excitation of cardiac muscle relies on activation of voltage-dependent Na + channels (Na V 1.5) preferentially localized to the cell-to-cell contact sites called intercalated discs (IDs). During the action potential (AP), the opening of L-type Ca 2+ channels (LCC; Cav1.2) or dihydropyridine receptors on the T-tubules, allows Ca 2+ influx into the cell that in turn activates RyR2s on the SR membrane, triggering Ca 2+ -induced Ca 2+ release (CICR) from the SR. The SR-derived Ca 2+ contributes to the Ca 2+ transient responsible for the excitation–contraction coupling (ECC) process underlying the activation of the myofibrils and cardiac muscle shortening. Cardiac ECC is structurally organized into discrete units called couplons. A couplon consists of a group of LCCs within a T-tubule that juxtaposes a larger cluster of RyR2s on the surface of the jSR. Within a couplon the LCCs and the RyR2s are separated by a narrow gap called the junctional cleft (∼12 nm). This gap forms a restricted domain (“fuzzy” space) such that local elevation of Ca 2+ derived from LCCs results in opening of RyR2s. The resulting local Ca 2+ signals (“sparks”) then sum to form a global Ca 2+ transient. Of about 100 RyR2s identified by electron microscopy in a cluster, only about 4 to 8 open to release Ca 2+ during a spark. The role of the functionally silent majority of RyR2 is presently unknown.
In addition to LCCs, the Na + /Ca 2+ exchanger (NCX), distributed throughout the surface membrane, plays a major role modulating CICR in the jSR of the cardiac muscle. Outside of being the dominant Ca 2+ efflux mechanism, NCX affects Ca 2+ concentration near jSR couplons. Specifically, in response to Na + influx during the systolic AP, NCX primes the jSR couplon cleft with Ca 2+ , increasing the ECC gain. Rather than being mediated by the cardiac Na V 1.5 Na channels, this local Na + influx mainly occurs through a special type of Na + channel, predominantly expressed in neurons, i.e., neuronal Na + (nNa + ) channels (e.g., Na V 1.1, Na V 1.3, and Na V 1.6). In cardiac myocytes, these nNa + channels are strategically localized in the vicinity of NCX and RyR2 in the T-tubules, in which they form a local Na + -Ca 2+ signaling nanodomain.
After its activation, SR Ca 2+ release terminates, and the release mechanism becomes temporally disabled (i.e., refractory), thus containing potentially self-regenerating CICR. This allows the SERCA pump to recycle Ca 2+ from the cytosol to the SR, and, consequently, contracted myocytes to relax, while preventing CICR from spontaneous activation during the diastolic phase. Impaired Ca 2+ signaling refractoriness is associated with uncontrollable, aberrant SR Ca 2+ release and cardiac disease (see later). The specific mechanisms responsible for SR Ca 2+ release termination and refractoriness are yet to be fully elucidated. Inhibitory effects of Ca 2+ changes on RyR2 on the cytosolic and luminal side of the channel appear to be important factors in controlling and containment of SR Ca 2+ release.
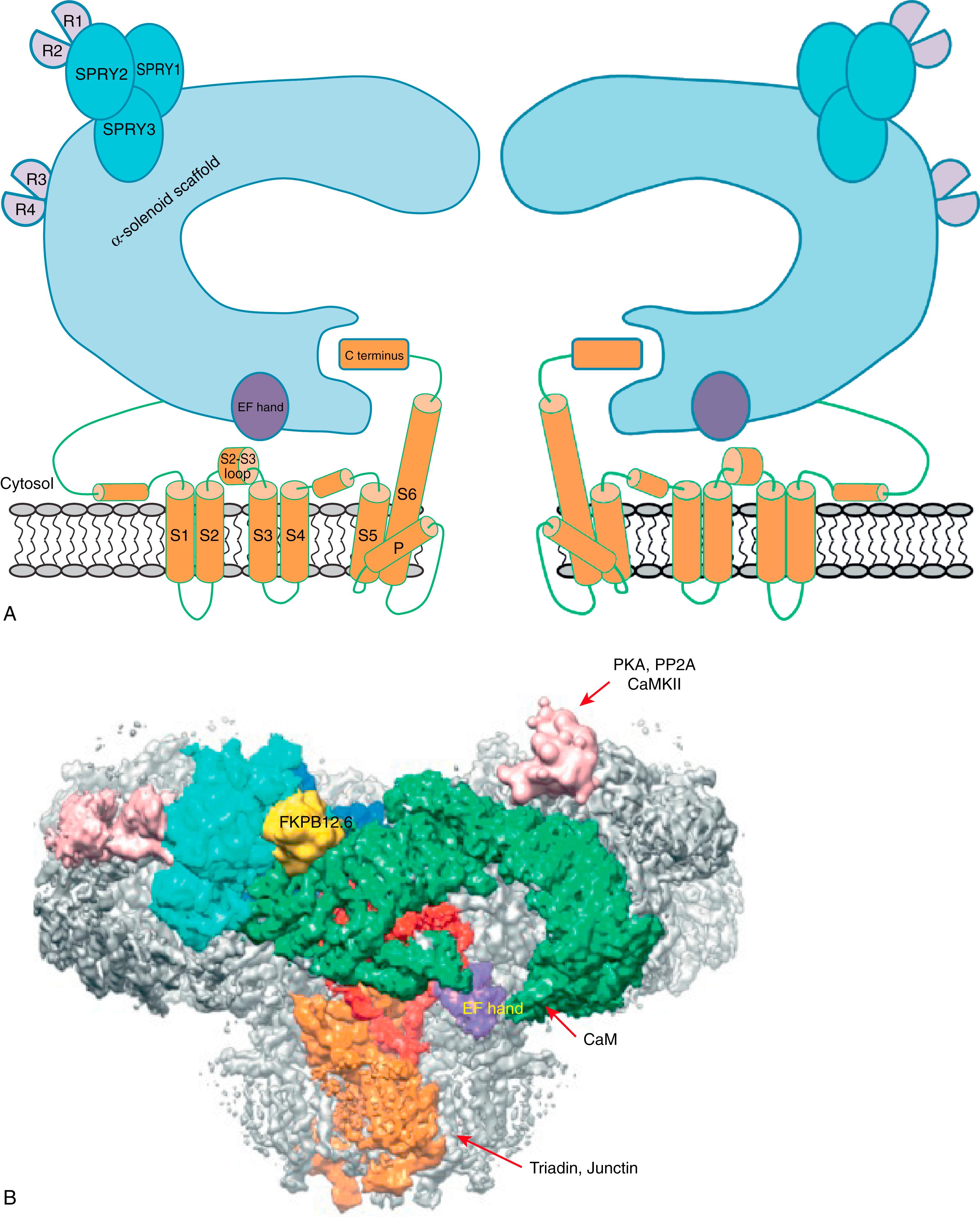
Three isoforms of RyR have been identified in mammals, including RyR1 and RyR2, which are expressed mainly in skeletal and cardiac muscle, respectively, and RyR3, which is found in several cell types but remains the least understood. All the isoforms are highly homologous with up to 70% amino acid sequence identity. The homotetrameric RyR complex of more than 2.2 million Da is one of the largest ion channels known. Deciphering the molecular structure of this channel has long been a challenge, given the enormous size of the protein complex. Early studies have revealed the overall RyR structure as a fourfold symmetrical mushroom-like shape, with the cap of the mushroom (80% of the mass) residing in the cytosol and the stalk embedded in the SR membranes. Recent advances in single-particle cryoelectron microscopy (EM) lead to a significant progress in determining the molecular structure of both the skeletal and cardiac RyRs, reaching an unprecedented resolution of 3.8 Å. These studies confirmed the existence of six transmembrane segments (S1–S6) in each subunit of the channel tetramer and determined that the transmembrane region is divided into two parts: the ion conducting pore, which is formed by S5, S6, the pore helix (p-helix), and the canonical pore loop (P-loop), and the pseudo voltage–sensor domain (pVSD), which makes up S1–S4 and interacts with the cytosolic domain. Notably, the cytosolic part of the RyR protomer is built around an extended α-solenoid scaffold, which interacts with regulatory proteins and transmits conformational changes to the pore. These studies also defined the locations of the phosphorylation domains (also known as repeat 3–4 of RyR ), the Ca 2+ -binding EF hand, and the three SPRY domains, which probably serve as a structural scaffold for the repeat domain ( Fig. 6.1 ). RyR1 and RyR2 share a similar overall structure and the organization of the structural domains, although portions of the C terminus of helical domain 2 were unresolved in RyR2. Groundbreaking work by Yan and colleagues on the closed and open structure of RyR2 also provides a foundation to understanding the gating mechanism of this channel. Specifically, the channel opening is attributable to the outward tilting of the S6 bundle, which shifts the location of the pore constriction site between the closed and open conformations of RyR2 (from Ile4868 to Gln4864, respectively). Of note, the central domain is identified as the key transducer of conformational changes that control RyR2 gating, caused by intra- and interdomain structural shifts. Despite substantial progress, important questions remain unanswered, particularly with regard to modulation of RyR2 by both cytoplasmic and luminal auxiliary proteins and ligands.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here