Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Spinal cord stroke presents with painless paraparesis with urinary retention.
The majority of spinal cord stroke can be attributed to diseases of the aorta.
Spinal cord infarction occurs most frequently as a complication of aortic dissection and surgery for diseases in the aorta.
Prevention of spinal cord infarction may occur with lumbar drainage at the time of aortic surgery.
Immobility and urinary retention are the principal sources of morbidity after spinal cord infarction.
Vascular syndromes of the spinal cord, including stroke and vascular malformations, are significantly less common than those occurring in the brain and retina. Because of the tight arrangement of the pathways in the cord, small infarcts are usually associated with more obvious symptoms and signs than similar lesions in the brain. These diseases are rarely studied outside of the cardiovascular and neurologic surgery literature, where significant attention has been given to causes and prevention strategies. In the neurology literature many of the discussions stem from small case series and case reports. The incidence of vascular syndromes in the spinal cord is unknown, and large epidemiologic stroke studies have generally not included spinal cord stroke. Advances in imaging techniques, including magnetic resonance imaging (MRI), have led to improved diagnosis of vascular syndromes in the spinal cord and updated recognition of the underlying causes. With improved diagnosis, prevention and acute treatment strategies may eventually emerge. Until that time the cornerstone of management is prevention of the associated medical complications that arise from paraplegia and urinary bladder dysfunction.
The clinical syndrome of paraparesis with urinary retention due to disease in the aorta was known by the late 19th and early 20th centuries, with the initial cases describing atherosclerotic and syphilitic diseases of the aorta as the predominant causes. , In the early 20th century diseases of the spinal cord venous system were also first described, notably the syndrome of Foix-Alajouanine with progressive necrotizing myelopathy due to venous occlusion. For much of the past century the literature on vascular diseases of the spinal cord was primarily in the form of case reports and short case series describing a wide range of presumed etiologies. In the last two to three decades substantial literature has emerged on spinal cord stroke, particularly in relation to surgery in diseases of the aorta, and on the diagnosis and management of vascular malformations.
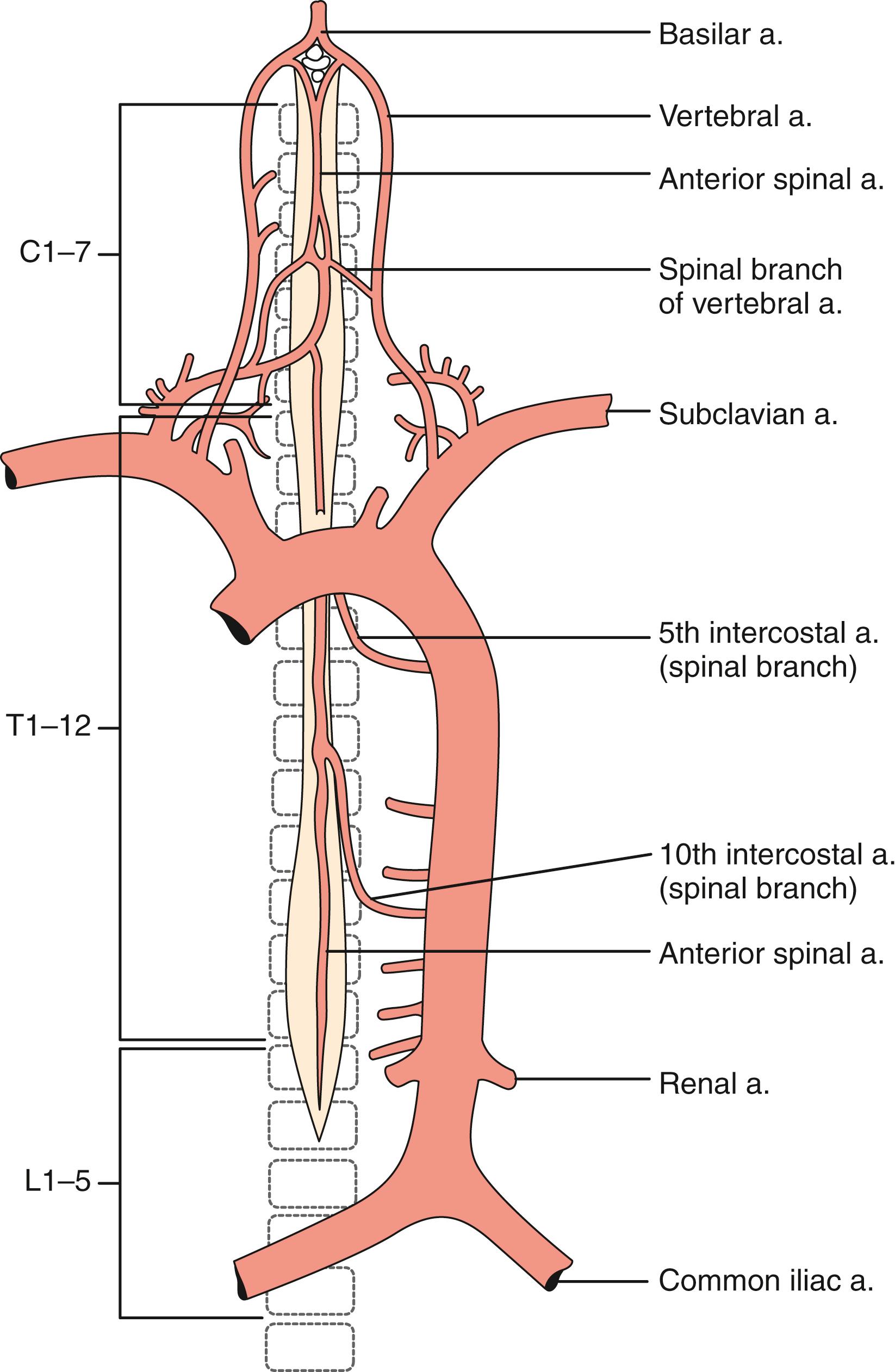
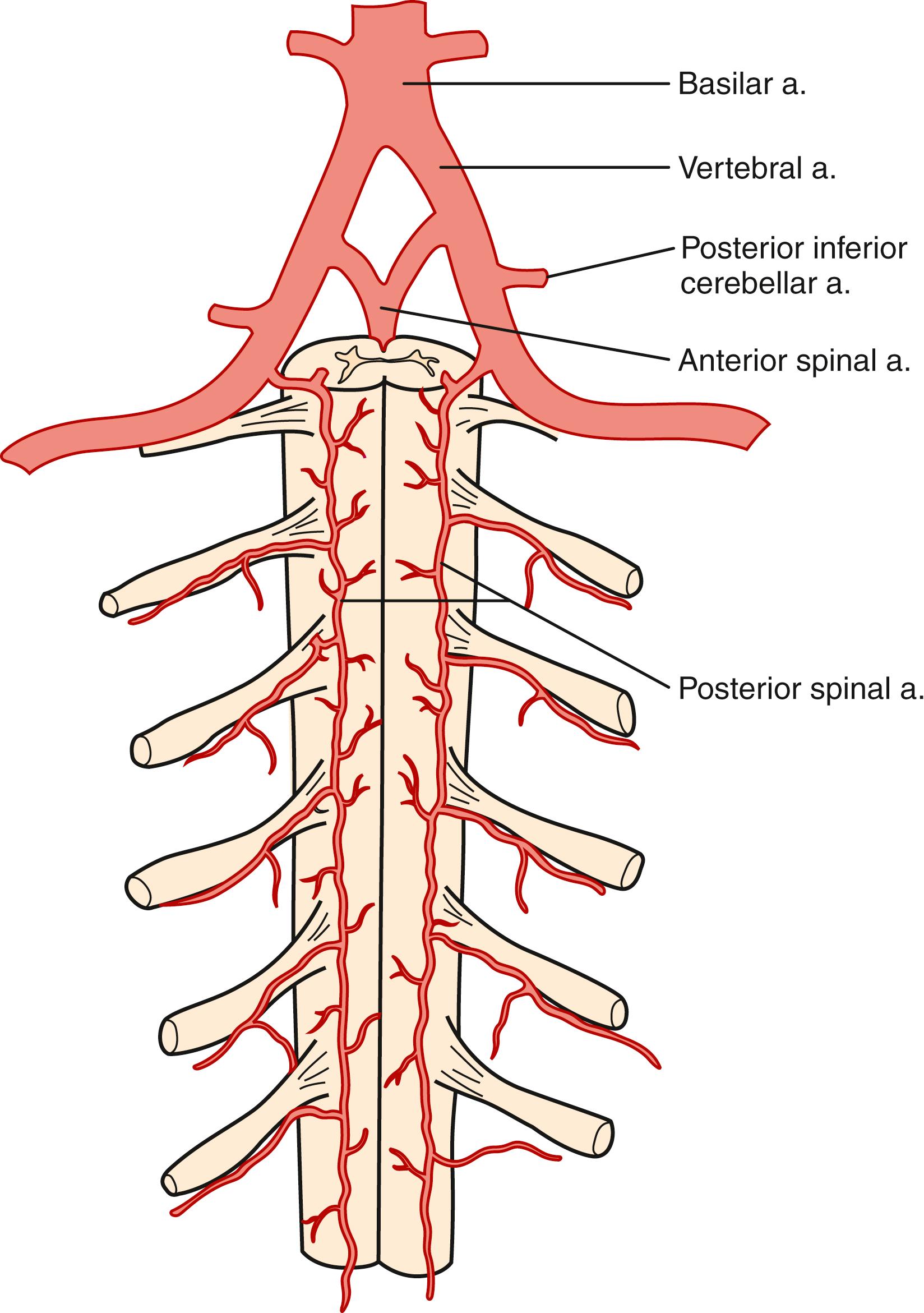
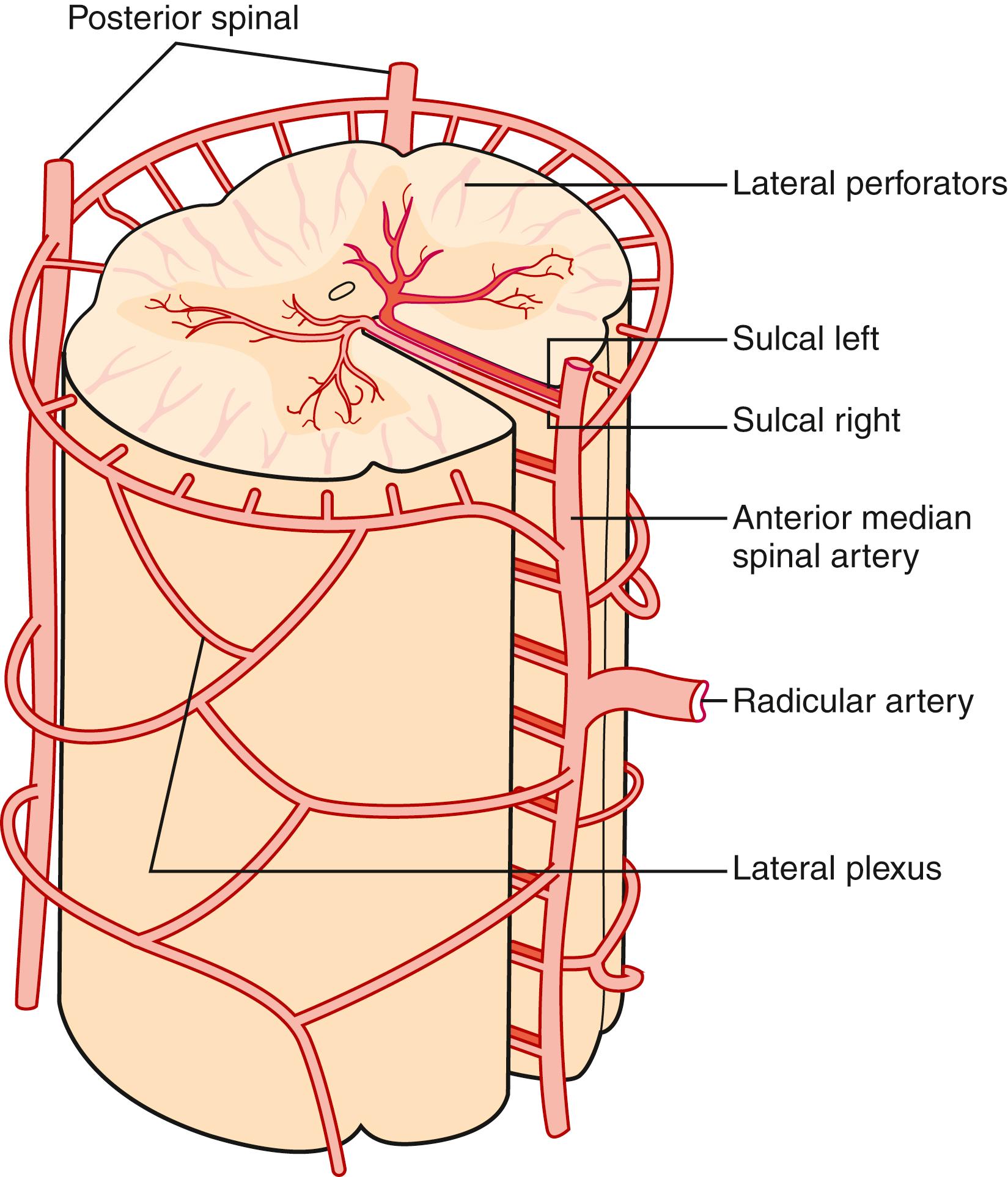
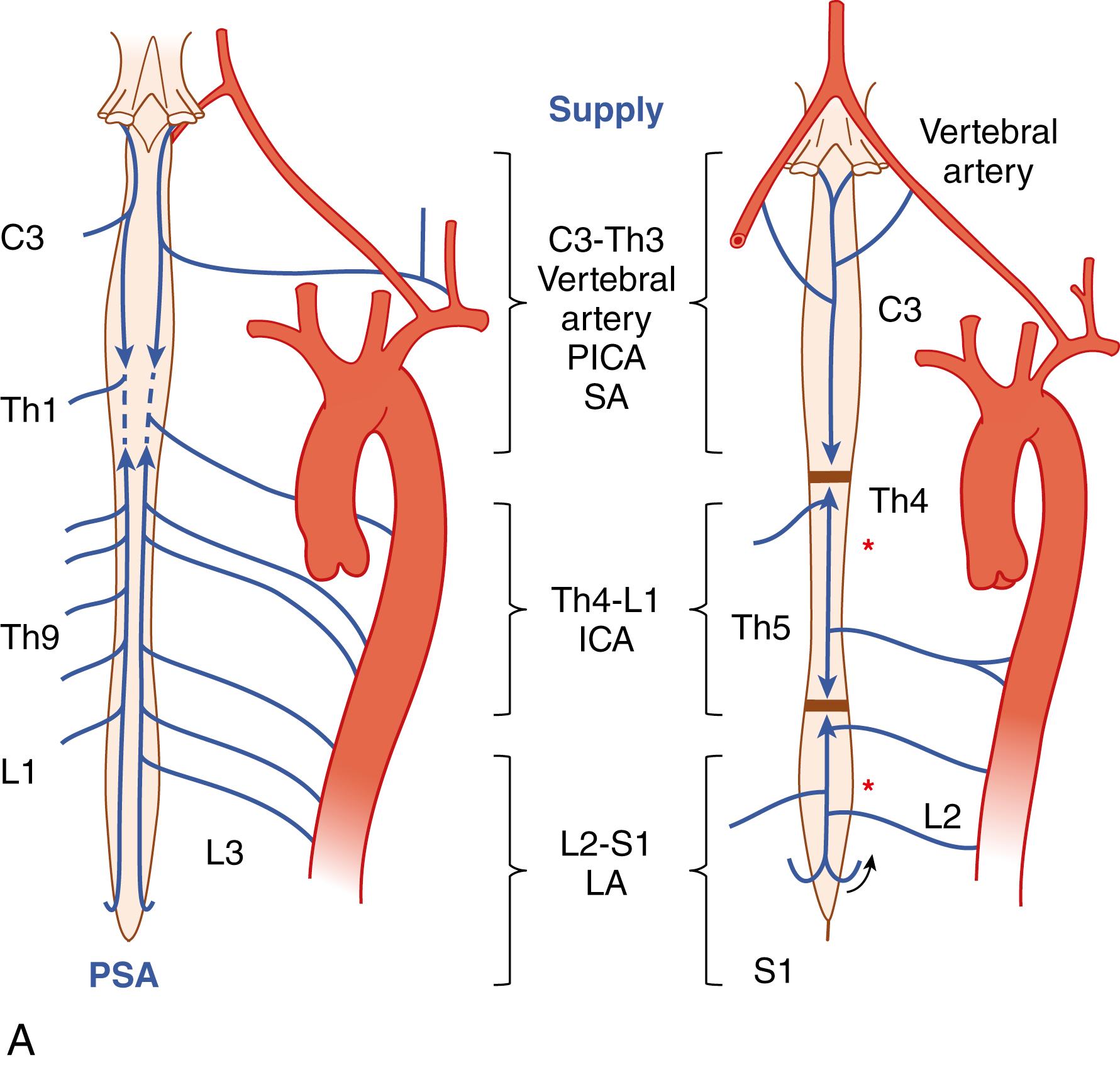
The pattern of arterial blood supply to the spinal cord consists of three families of arteries with a specific anatomic location: longitudinal vessels that extend in a cranial–caudal direction from the upper cervical cord to the conus medullaris, radicular tributary vessels feeding into longitudinal vessels across multiple spinal segments, and numerous feeder (intrinsic) arteries that enter the parenchyma ( Figs. 31.1–31.3 ). Anastomoses between the longitudinal and radicular vessels lead to the formation of a rich vascular plexus from which medullary vessels penetrate both white and gray matter. This network has been identified with the “collateral network concept” to explain resilience in the spinal cord to occlusions in spinal arteries ( Fig. 31.4 ). These vessels are end arteries and do not anastomose further.
The venous drainage from the spinal cord exhibits greater variability but can be subdivided into the extrinsic and intrinsic systems.
There are three longitudinal arteries, the anterior spinal artery and the two posterior spinal arteries. The single anterior spinal artery forms rostrally from the union of the two anterior spinal branches of each vertebral artery at the level of the foramen magnum. From here it descends up to the tip of the conus medullaris. The caliber of the artery is largest in the region of the lower thoracic and upper lumbar enlargement and smallest in the midthoracic region. The anterior spinal artery is reinforced by successive feeder arterial branches, which enter the artery in a caudal direction and supply the spinal cord below the point of entry. At the conus medullaris and along the filum terminale, the anterior spinal artery communicates through anastomotic branches with the posterior spinal arteries.
The two posterior spinal arteries originate directly from the vertebral arteries (see Figs. 31.1–31.3 ). Each vessel descends on the posterior surface of the spinal cord along the posterolateral sulcus. Throughout its course, each posterior spinal artery gives off branches that penetrate the cord to supply the posterior columns, dorsal gray matter, and superficial dorsal aspect of the lateral columns.
Thirty-one pairs of radicular arteries penetrate the spinal canal through the intervertebral foramina. Usually the 62 radicular branches contribute to the vascularization of the spinal cord and define three major spinal arterial territories—cervicothoracic, midthoracic, and thoracolumbar. These radicular tributaries may be further subdivided according to their origin. The first group consists of those derived from the subclavian artery; the second group is supplied directly from the aorta. At the level of the second thoracic spinal cord segment the arterial supply changes from the subclavian artery to supply directly from the aorta.
The cervicothoracic territory consists of the cervical spinal cord, its brachial plexus enlargement, and the first two or three thoracic segments. This territory is richly supplied by the anterior spinal artery arising from the intracranial vertebral arteries, the midcervical radicular branches of the vertebral artery, and the branches of the costocervical trunk. In the midthoracic territory, the radicular arteries supplying the middle and lower thoracic cord are less prominent. This territory is usually supplied by a radicular branch arising at about the T7 level; it comprises the fourth to eighth segments of the thoracic cord.
The lower thoracic and upper lumbar segments, which contain the lumbar enlargement and lumbosacral plexus, receives its blood supply from the artery of Adamkiewicz (also called the arteria radicularis magna), which variably arises from T8 to T12 and exhibits notable differences in size between individuals. , The artery of Adamkiewicz may be fed by collateral segmental arteries in the setting of chronic occlusive disease, which may have implications for the development for spinal cord infarction in the setting of aortic surgery. During aortic surgery reimplanting the artery of Adamkiewicz is commonly performed to optimize spinal cord perfusion with variable results. Occlusion of the artery of Adamkiewicz during surgery has been associated with a higher risk of spinal cord ischemia. On the other hand the rich collateral network is likely equally important to spinal cord perfusion, even compromise of the artery of Adamkiewicz, and is likely to include flow in a caudal to cephalad direction via collaterals from the internal iliac arteries. These collaterals, as well as the artery of Adamkiewicz, may be further visualized by computed tomography or magnetic resonance angiography.
When the radicular arteries reach the surface of the spinal cord, they form two distinct systems of intrinsic blood supply (see Fig. 31.3 ). The first is the posterolateral and peripheral plexus formed by the two posterior spinal arteries, which are interconnected by anastomotic channels. This plexus, a centripetal vascular territory, is formed by radial arteries directed inward as branches from the coronal arterial plexus surrounding the spinal cord. It supplies from one-third to one-half of the outer rim of the cord, including the lateral and ventral spinothalamic tracts. These radial arteries are longer in the posterior white columns than in the anterior and lateral columns. The second arterial system to the spinal cord is a centrifugal system formed by the sulcal arteries, which arise from the anterior spinal artery and pass backward in the anterior medial sulcus. These arteries enter the gray commissure and, turning left or right, supply the gray matter and adjacent white matter. The corticospinal tract is nourished by both arterial systems.
Both arterial systems are interconnected by a capillary anastomosis in the spinal cord. The number of sulcal arteries supplying each segment of the spinal cord varies with the region of the cord. They are most numerous in the thoracolumbar segment and least numerous in the upper thoracic segment.
The intrinsic venous system can be classified into two groups based on the pattern of drainage in the anterior and posterior directions. The anterior median group (central veins) collects blood from both halves of the medial aspects of the anterior horns, anterior gray commissure, and white matter of the anterior funiculus. The central veins also drain adjacent levels above and below through intersegmental anastomoses. They commonly anastomose with other veins within the fissure. Finally, the central veins empty into the anterior median spinal vein. The second group consists of radial veins that arise from capillaries near the periphery of the gray matter or from the white matter. They are radially oriented and directed outward toward the surface of the spinal cord, where they join the superficial plexus of veins surrounding the cord and form a venous vasa corona or corona plexus. These veins are more numerous in the white matter of the posterior and lateral funiculi, but they are also found in the anterior funiculus. The radial veins are more prominent at certain cervical and thoracic levels; they drain laterally from the gray matter of the lateral horns as well as posteriorly from the dorsal nucleus of Clark.
The extrinsic venous system is conspicuous on the posterior aspect of the spinal cord and is especially prominent in the lumbosacral region. There is a rich anastomosis between the large venous trunks. The median posterior spinal vein descends in the region of the posterior median septum. This vessel drains blood from the posterior white columns and the end of the posterior horns.
The anterior spinal vein accompanies the anterior spinal artery and receives the sulcal veins. Both the anterior spinal veins and the median posterior spinal vein empty into the radicular veins, which accompany the anterior or posterior spinal roots. These radicular veins drain into the paravertebral and intervertebral plexuses, and then into the azygous and pelvic venous systems.
The regulation of spinal cord blood flow is similar to that of brain blood flow. The spinal cord blood vessels are affected by changes in pO 2 and pCO 2 , with hypercapnia increasing spinal cord blood flow in a similar manner as in cerebral blood flow. Autoregulation keeps the regional as well as the total spinal cord blood flow constant. As in the brain, the blood flow requirement and metabolic rate are high in the gray matter. The total cerebral blood flow of the human brain is 50 mL/min/100 g. Spinal cord flow varies, depending on the species and the area of cord studied. In monkeys, total flow in cervical, upper trunk, and lumbar areas is 15, 10, and 20 mL/min/100 g, respectively. The intrinsic blood supply of the cord is directly proportional to the area of gray matter, this being most abundant in the thoracolumbar and cervical segments where the cord enlarges. Consequently, these segments may be more vulnerable to emboli or thrombotic occlusions; in case series of spinal cord infarction these locations are the most commonly involved, with the cervical cord being the second most commonly affected location in up to 25% of patients. The mid-thoracic cord, notably close to the T4 segment, has been commonly described as the most vulnerable to ischemia from hypoperfusion, leading to the description as the “watershed territory,” though the exact location has been called into question by some radiologic studies. ,
Another important consideration is that the cord is contained within the spinal canal, which has fixed dimensions. Akin to the changes observed with elevated intracranial pressure, a rise in intraspinal canal pressure can cause a lowering of spinal cord blood flow and subsequent ischemia. The parallel of this process is the potential therapeutic benefit of lowering spinal pressure with cerebrospinal fluid (CSF) drainage and inducing hypertension to increase spinal perfusion pressure as an adjunct to prevent perioperative spinal cord stroke in aortic surgery. The blood flow of the spinal cord has a different pattern in the anterior and posterior systems. Flow in the anterior spinal artery is mainly caudal and unidirectional. Infarcts are most likely to be located in the anterior spinal artery distribution. Flow in the posterior spinal arteries is bidirectional, caudal in the cervical and thoracic regions, and rostral in the lumbosacral region.
Occlusion of any vessels supplying the spinal cord stemming from a wide range of etiologies may lead to infarction in the cord, while systemic or local hypoperfusion may lead to ischemia in the spinal cord “watershed” territory. The final pathologic processes appear to be similar regardless of the etiology ( Fig. 31.5 ).
The cellular events in spinal cord infarction are similar to those seen in cerebral infarction. Depending on the intensity of tissue damage, the infarct can be complete or incomplete. In complete infarction, all cellular elements die rapidly. In incomplete infarction blood vessels and, to a lesser extent, astrocytes are unaffected, while neurons undergo delayed cell death that is likely mediated through apoptosis. An initial ischemic and inflammatory cascade is triggered, including N -methyl- D -aspartate-mediated excitotoxic neuronal injury. Microglial cell activation through activation of toll-like receptor 4 mechanisms has been noted in animal models of spinal cord ischemia similar to cerebral ischemic injury models. Subsequent cell death, delayed up to 2 days after the initial ischemic injury, occurs due to apoptotic cell death via activation of caspases; this delay after the initial injury may in turn be mediated after the failure of initial endogenous neuroprotective mechanisms. Post-ischemia activation of inflammatory and apoptosis pathways may further worsen injury to the cord and could be an explanation of delayed-onset paraplegia that can be observed after surgery on the aorta. , The presence of heat-shock proteins, which can be observed in a range of ischemic injuries, has been noted in CSF samples of patients undergoing aortic surgery with subsequent spinal cord stroke. The presence of edema association with spinal cord infarction is mediated in part by varying expression of aquaphorin-4. The pathologic changes observed at autopsy in the spinal cord mirror those seen with cerebral infarction.
Infarction of the spinal cord involving the territory supplied by the anterior spinal artery may result from interruption of arterial flow in any vessel from the aorta to the intramedullary vasculature from either local occlusive disease or embolic phenomena; systemic hypoperfusion presents another possible etiology. Several conditions have been identified as leading to spinal cord infarction, with most descriptions originating from single case reports or small series ( Box 31.1 ). In broad terms the etiology can be divided into spontaneous and iatrogenic.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here