Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
During the last quarter century, the management of many skull base tumors has shifted away from complete surgical resection toward subtotal resection to preserve function, and then control of growth. This is particularly true for vestibular schwannomas (i.e., acoustic neuromas) and is now applicable to glomus jugulare tumors. The principal modality for such treatment is Gamma Knife surgery, although other conformal radiation treatment systems are available as will be discussed later. Gamma Knife surgery is advantageous in that it requires a single session for the treatment of most skull base lesions, which increases its appeal to both surgeons and patients. This chapter is focused primarily on the methods used and important considerations in treating skull base tumors with Gamma Knife surgery.
Gamma Knife surgery, similar to microsurgery, has advantages and disadvantages that must be thoroughly discussed with the patient. , For the patient it is alluring to undergo an outpatient procedure rather than microsurgical management that requires a much longer period of care ( Fig. 59.1 ). Further, Gamma Knife outcomes show excellent tumor control and, with the current methods, low cranial nerve morbidity. Gamma Knife surgery is a viable treatment modality for the appropriate patient as defined by age, medical history, tumor characteristics, and physical findings. As such, many neurotologists now offer Gamma Knife surgery as part of their armamentarium for managing vestibular schwannomas, glomus jugulare tumors, and many other skull base neoplasms. ,
As of September 2019, there were 339 Gamma Knife units in use worldwide. The parent company, Elekta Instrument AB, requires that all centers submit annual treatment statistics to the company. Based upon these data, there are impressive Gamma Knife surgery treatment statistics through 2018 regarding the specific types of tumors and diseases often managed surgically by neurotologists: 116,879, vestibular schwannoma; 182,899, meningioma; 76,427, trigeminal neuralgia; 8091, craniopharyngioma; 7578, trigeminal schwannoma; 4633, glomus jugulare paraganglioma; 3647, chordoma; and 893, jugular foramen schwannoma procedures. Worldwide, several institutions offer training courses for physicians and radiation physicists at centers with the Leksell Stereotactic System or Leksell Gamma Knife. To date, more than 4000 neurotologists, neurosurgeons, physicists, and radiation oncologists have received such training. In addition, Elekta Instrument AB offers basic and advanced training courses and workshops. The courses typically consist of didactic lectures, observation of patient treatment, and practical hands-on training. Further, all new installations of Leksell Gamma Knife are accompanied by 1-week on-site start-up training for the neurotologists, neurosurgeons, radiation oncologists, and physicists comprising the Gamma Knife treatment team.
Opting for Gamma Knife surgery over observation or microsurgical resection is a complex decision. There are the preferences of the informed patient, the comfort and experience of the surgeon, the patient’s medical history and condition, and the characteristics of the tumor. Although there are no definitive measures defining or restricting the use of Gamma Knife surgery, particular guidelines can inform the decision-making process.
Although a tissue diagnosis is not typically acquired before Gamma Knife treatment, radiographic and clinical diagnoses of vestibular schwannoma and glomus jugulare are sufficient to initiate a discussion of Gamma Knife surgery. The other potential neoplasms amenable to Gamma Knife treatment by the neurotologist are cerebellopontine angle meningiomas, posterior fossa and jugular foramen nonvestibular schwannomas, other cranial nerve neoplasms, temporal bone metastatic lesions, and primary vascular neoplasms. An absolute contraindication to Gamma Knife treatment is tumors extending too far inferiorly to enable placement into the centrum of the collimator helmet, which could result in an undeliverable shot or a helmet collision. Special care should be taken to place the frame as low as possible in cases with a low-lying tumor. With such placement it may be possible treat as inferiorly as C3 or even C4 depending on patient anatomy.
Gamma Knife surgery is also contraindicated in large tumors causing life-threatening brainstem and central aqueduct compression. Such large tumors, in the absence of clinically significant problems, provide a relative contraindication to Gamma Knife surgery because the posttreatment swelling may cause obstructive hydrocephalus requiring emergent intervention. Typically, vestibular schwannomas greater than 2.5 cm in the cerebellopontine angle should be cautiously approached if the Gamma Knife proves the best option, given other medical concerns. If treatment is to be delivered to a large lesion, the use of fractionation (2 to 5 doses of radiation) can be considered, as prior studies have shown an improved balance of tumor control and toxicity compared with single-fraction radiosurgery for large lesions. Most surgeons will not treat vestibular schwannomas greater than 3.0 cm in the maximum axial dimension within the cerebellopontine angle because of the risk of posttreatment obstructive hydrocephalus. Likewise, caution should be exercised with cystic vestibular schwannomas because of their unpredictable expansion behavior.
The other guidelines for Gamma Knife surgery require clinical judgment as to the medical condition of the patient, the expected growth and potential morbidity of the tumor, the functional status of the patient, audiometric and vestibular performance, age, and expected lifespan of the patient. Individualized treatment plans depend on a frank and thorough dialogue between physician and patient as to the options available, risks and benefits of each approach, and expected outcomes based upon evidence-based reviews or an analysis of each institution’s outcomes.
Informed consent for Gamma Knife surgery requires the surgeon to discuss alternative options such as observation and microsurgical resection. The risks and benefits of these alternatives should be frankly described and compared with the Gamma Knife treatment. Many patients have received information from the Internet or from physicians with limited experience with the Gamma Knife and may have erroneous information. Common misconceptions include the expectation that Gamma Knife surgery completely removes the tumor and that hearing will improve, or, conversely, that the cranial nerve morbidities are significant. These misconceptions must be addressed with evidence-based reports and information.
One treatment outcome that is particularly alarming to patients considering Gamma Knife surgery is vestibular schwannoma malignant transformation. This topic has been reviewed by Nicolli and Ruckenstein as well as by Maducdoc et al. , To be formally considered a radiation-induced malignancy, the tumor must fulfill the Cahan criteria: (1) the tumor must occur at the irradiated site, (2) the time latency is longer than 5 years, and (3) the tumor is a different pathological type from the previously irradiated tumor. Unfortunately, in the context of vestibular schwannomas, these criteria are difficult to meet, because the diagnosis is based on clinical and radiographic information, and a pretreatment biopsy is rarely obtained.
A 2010 review by Demetriades et al. found 14 cases of malignant vestibular schwannomas reported in the literature. Surprisingly, only 6 of the 14 patients actually had prior radiotherapy, and of those 6, only 3 had a histologically confirmed benign pathology before treatment. Also included in the review were five patients with malignant vestibular schwannomas who did not undergo radiosurgery. These patients highlight the difficulty of definitively establishing a causal relationship between radiosurgery and malignant transformation.
As with any treatment decision, patient selection is critical for stratifying risk. Age at the time of exposure and certain genetic susceptibilities such as neurofibromatosis 2 (NF2) have been identified as increasing the risk of radiation-induced malignant transformation. In addition to these risk factors, a 2011 review by Husseini et al. suggested a possible association with the pretreatment tumor size. Of the 17 patients with vestibular schwannoma who underwent malignant transformation of their tumor, none had a tumor less than 2.5 cm in size: 13 of the 17 tumors were in patients with NF2. The study authors postulated that radiosurgery serves as the “second hit” in a two-hit theory of oncogenesis (the “first hit” being the chromosomal mutation for the merlin protein). This two-hit theory could also explain the increased risk in younger patients. In that scenario, the radiosurgery would serve as the first hit, and because, younger patients have a longer lifespan, they have a longer interval over which to acquire a second hit to the tumor DNA.
As the duration of posttreatment follow-up increases for patients with vestibular schwannoma who undergo stereotactic radiosurgery, more specific criteria regarding high-risk patients will be established. In 2013 Hasegawa et al. reported 440 vestibular schwannoma patients treated with Gamma Knife surgery and followed for a median 12.5 years. In their series only one patient underwent malignant transformation of the tumor; however, they did not meet the Cahan criteria because they did not have a preradiosurgery histological diagnosis of the tumor. Even with the assumption that this lesion was benign pretreatment, the overall malignant transformation rate was calculated to be 0.3%, and the annual incidence of malignant change was 0.02%. This incidence, although not zero, compares favorably with that of perioperative mortality for patients treated with microsurgery, which in one systematic review was calculated as 0.63%.
Pollock et al. also addressed the delayed development of radiation-induced neoplasms in 1998. They reviewed more than 20,000 patients treated with radiosurgery worldwide and found no increased incidence of new neoplasia (i.e., benign or malignant). A retrospective cohort study comparing the Sheffield, England radiosurgery patient database with the national mortality and cancer registries identified a single new astrocytoma among those treated. Based on their national incidence figures, 2.47 cases would have been predicted. The risk of radiosurgery-induced malignancy in patients with NF2 and von Hippel–Lindau disease was similarly studied. Of 118 NF2 and 19 von Hippel–Lindau disease patients, totaling 906 and 62 patient-years of follow-up data, respectively, only two cases of intracranial malignancy were found. Both of these were in NF2 patients. One was thought to have arisen before the radiosurgery; the other was a glioblastoma diagnosed 3 years after radiosurgery. Gliomas may occur in as many as 4% of NF2 patients, and the single case may not represent an increased risk. It was suggested that the late risk of malignancy arising after irradiation must be put in the context of the condition being treated, the treatment options available to these individuals, and their life expectancy.
Maducdoc et al. also reviewed the literature malignant transformation literature after stereotactic radiosurgery and microsurgery. They identified four cases of malignant transformation after microsurgery only. Since malignant transformation of vestibular schwannomas can also occur de novo, they concluded that it is possible that these malignant transformations after stereotactic radiosurgery and microsurgery are spontaneous events.
The Congress of Neurological Surgeons recently published a systematic review of radiosurgery and radiation therapy of vestibular schwannomas. Based upon level 3 evidence, they recommended that patients be informed that there is minimal risk of malignant transformation of vestibular schwannomas after stereotactic radiosurgery. Thus, it is important to counsel patients about the possibility of malignant transformation or induction. A handful of tumors suggestive of radiation-induced malignancy have been reported; however, this must also be considered in the context of 116,879 vestibular schwannomas treated with Gamma Knife surgery as of 2018. Lustig et al. reported the development of a squamous cell carcinoma following the radiation treatment of vestibular schwannoma. Hanabusa et al. reported the malignant transformation of a vestibular schwannoma following Gamma Knife surgery. There was histological evidence of vestibular schwannoma following a retrosigmoid resection. Four years after this resection, a recidivistic tumor was identified, and the patient subsequently underwent Gamma Knife surgery. Six months posttreatment, the tumor had grown, and the patient underwent surgical resection via a combined retrosigmoid-translabyrinthine approach. Abnormal mitotic figures were observed on histological sections, and the diagnosis of malignancy was assigned.
Based upon these data, we do not recommend that young patients and those with NF2 undergo Gamma Knife surgery of their vestibular schwannoma.
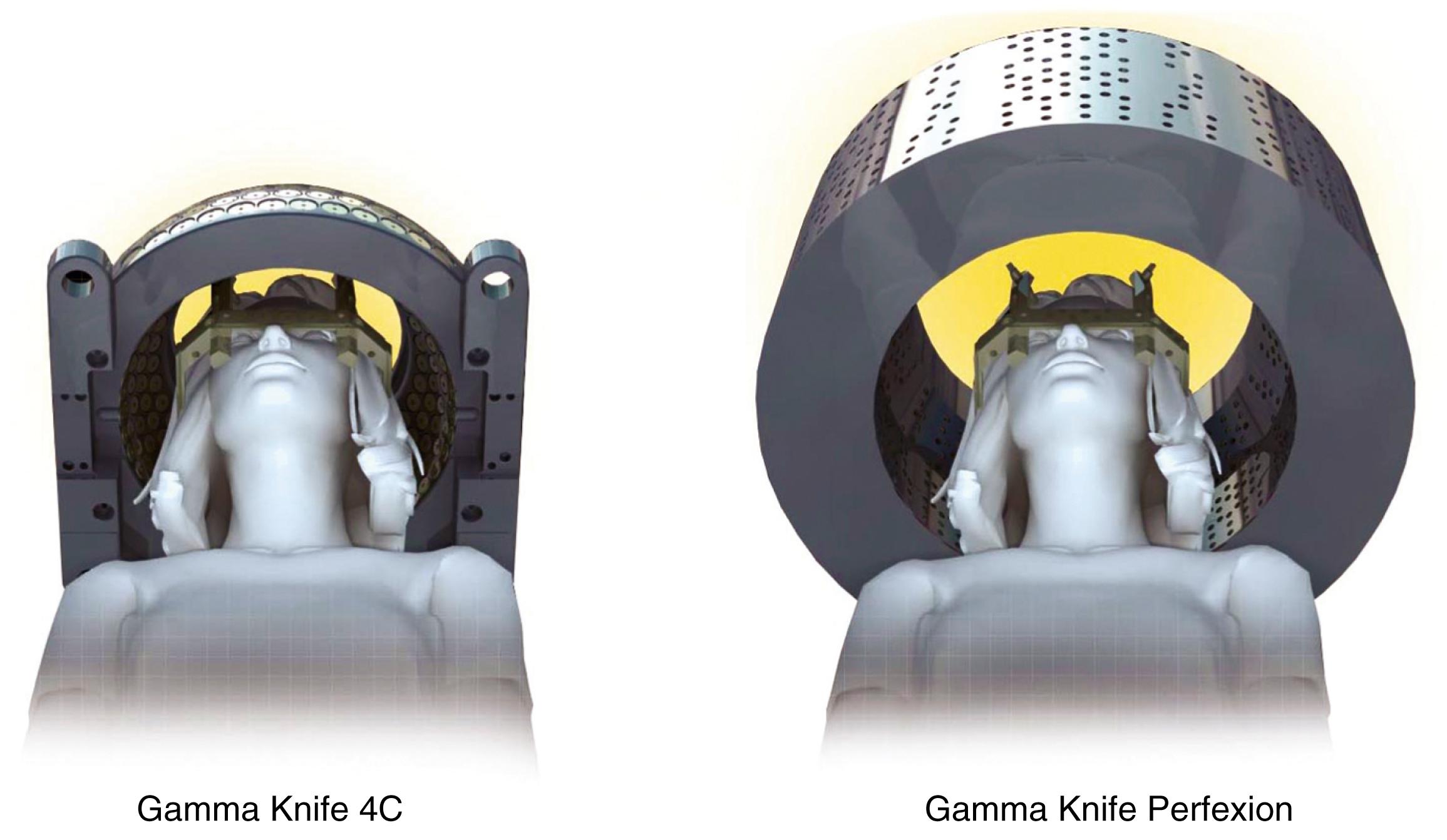
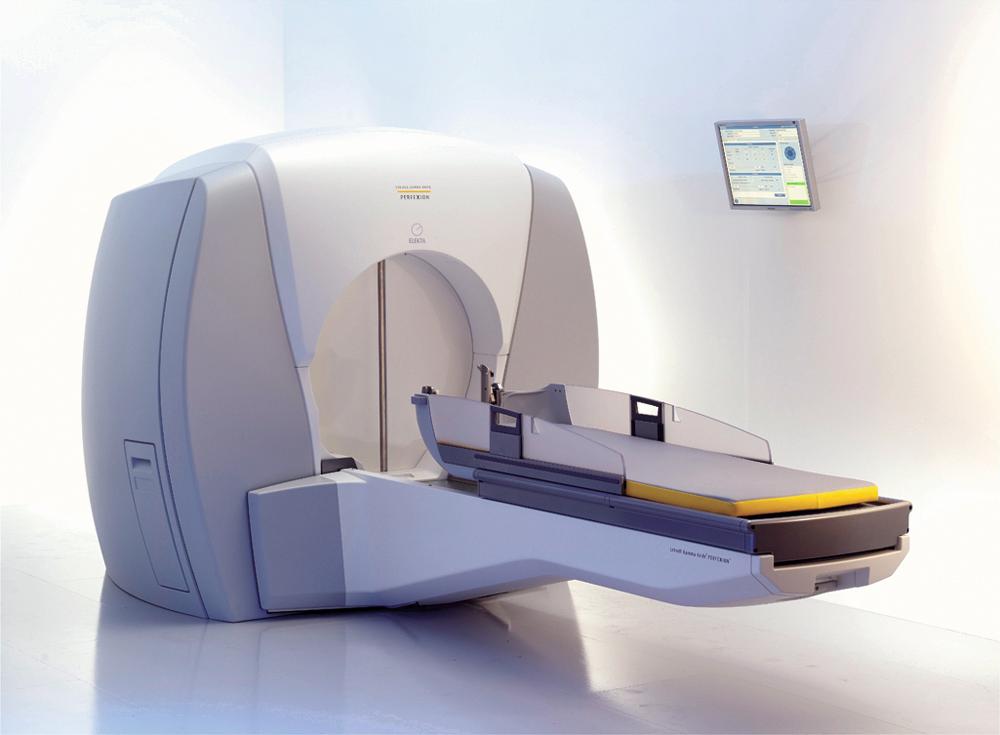
The first Gamma Knife unit (Elekta Instrument AB) was installed in Stockholm, Sweden, in 1968. It was not until 1987 that the first Gamma Knife (model U) was installed in the United States, at the University of Pittsburgh. The Gamma Knife model B (1996) became the workhorse used throughout the United States until the Gamma Knife model 4C was introduced 3 years later. The model 4C had a major upgrade consisting of an automatic positioning system (APS) that obviated the need to change the X, Y, and Z coordinates of the headframe manually. The units were otherwise similar and contained 201 radioactive isotope cobalt 60 ( 60 Co) sources and beam channels. Because of the physical restraints, these units could only treat lesions intracranially or along the skull base. In 2008, a completely redesigned Gamma Knife unit, named Perfexion, was introduced. It uses 192 60 Co sources, has a single collimator helmet with variable diameters, and can be used to treat lesions within the entire head and neck, down to the level of the clavicles ( Fig. 59.2 ).
Building on the principles of Perfexion, the newest generation model, Gamma Knife Icon, has a number of advancements compared to all prior models ( Fig. 59.3 ). This results in increased flexibility with new workflow, immobilization options, and the expansion in treatment capabilities. With Icon, the immobilization can be via standard frame placement, or, for the first time, a mask-based frameless alternative ( Fig. 59.4 ). Due to its less invasive nature, the mask-based system allows for the ability to offer frameless single-fraction and multi-fractionated radiosurgery, similar to LINAC-based radiosurgical systems like CyberKnife.
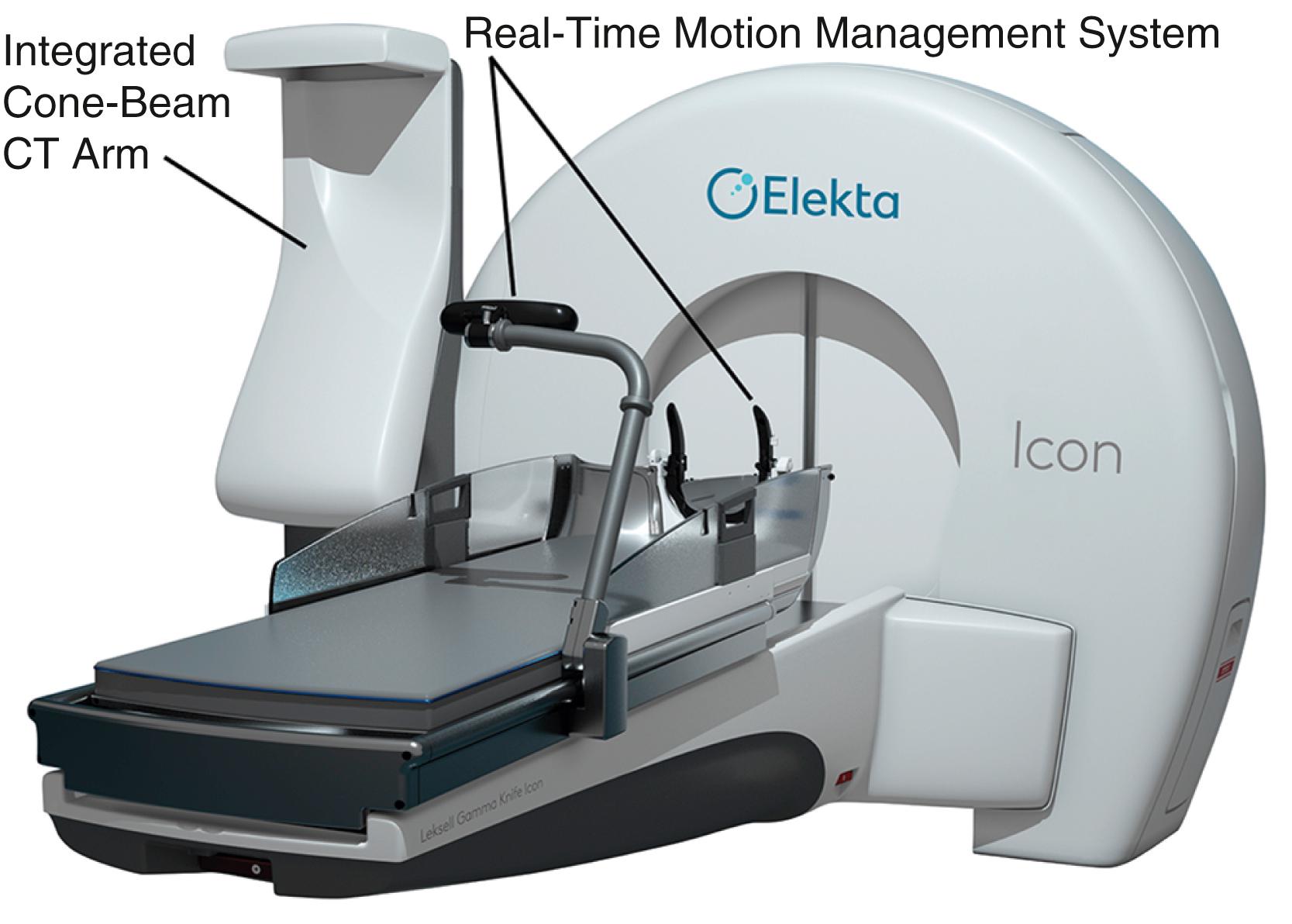
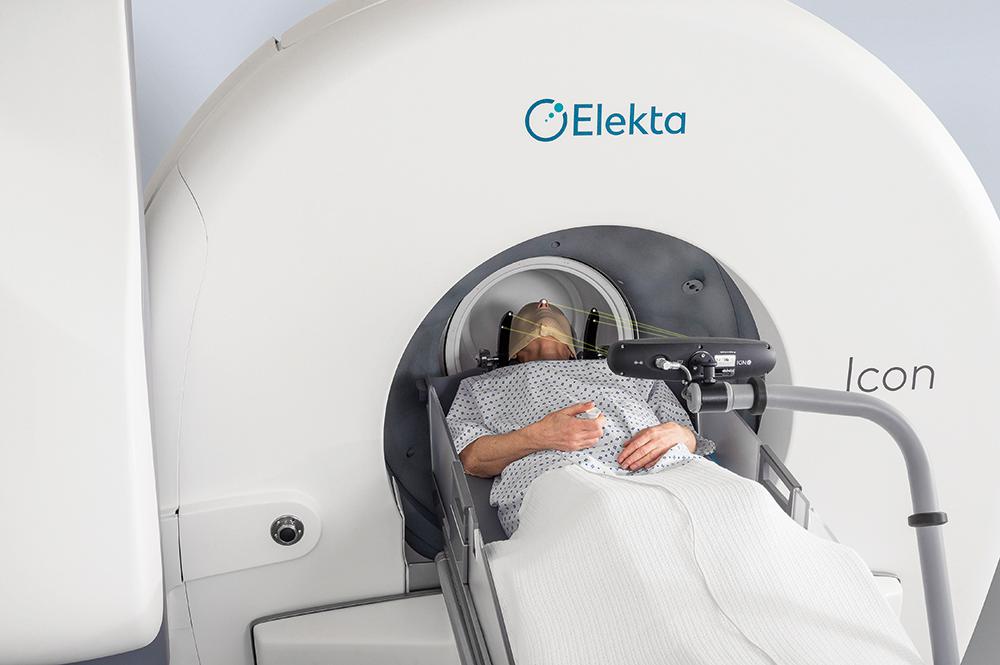
In order to confirm accuracy during masked treatment, the unit employs real-time motion management with the use of an infrared camera with a resolution of 0.15 mm. The users can pre-specify a cut-off tolerance such that if a patient’s movement is beyond this, the unit will automatically stop delivering the dose to allow for the repositioning of the patient. Also new to the Icon is an integrated stereotactic cone-beam CT arm, which can determine treatment coordinates without the use of the classic fiducial-based registration. This results in a great advancement in workflow and treatment efficiency over the older models, as patients no longer need to have the frame placed with same-day MRI before any treatment planning can occur. Therefore, patients can undergo imaging and plan creation prior to the actual treatment day, greatly reducing the duration of their treatment day and their time in the frame or mask.
Finally, this model introduces a feature called Online Adaptive DoseControl which can account for a shift in patient position at the time of the actual dose delivery. Treating physicians now have the ability to make minute changes to some or all of the planned shots, giving unparalleled confidence in dose delivery.
The basic principle of Gamma Knife surgery is to provide focused radiation to the tumor while minimizing radiation delivery to surrounding tissues. As such, a semicircular shield called the collimator helmet is used to generate 192 (Icon and Perfexion) or 201 (model B and 4C) individual gamma radiation “beams.” In the center of the helmet where the beams meet, the radiation delivery is maximal, but along each individual radiation tract, the tissue exposure is relatively low. When the collimator helmet is locked into position, the openings of the collimator helmet coincide with the cobalt sources. There is a shielded chamber within which the 60 Co sources are contained, and stainless steel shielding doors protect the treatment room from the 60 Co sources. There is a treatment couch with an adjustable mattress that slides into the Gamma Knife unit together with the collimator helmet and the patient. Fig. 59.5 schematically shows the orientation of the components of the Leksell Gamma Knife 4C and Fig. 59.6 shows the overall appearance of the Leksell Gamma Knife Perfexion.
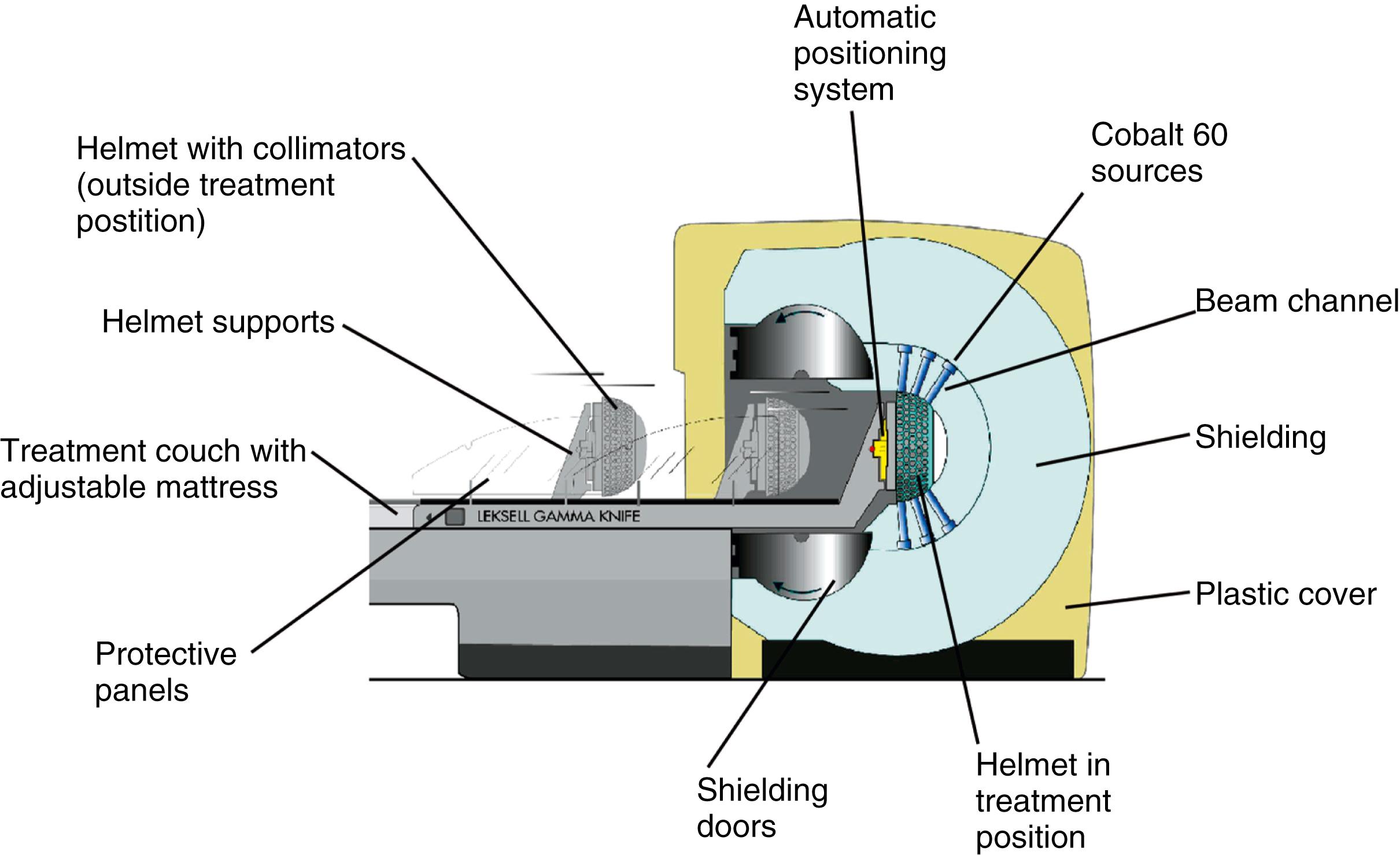
When treatment is initiated, the treatment couch is automatically moved from its idle position into the treatment unit together with patient and helmet. Once the couch is docked in its treatment position, the helmet collimator and corresponding collimators in the unit form a beam channel, allowing the radiation that is continuously emitted by the sources to reach the patient. At the end of each irradiation “shot,” the couch is automatically withdrawn, either to its idle position or to one outside the radiation focus, to reposition the patient for the next irradiation shot. For the model B and 4C, there are four interchangeable helmets, by means of which the size of the collimator (that part of the treatment unit that shapes the beam) can be changed to 4, 8, 14, or 18 mm. The combination of four different-sized collimators and repositioning the patient in the three-dimensional (3D) space defined by the stereotactic headframe are effective to deliver the radiation dose selectively and conformally to radiosurgical targets of any shape. In both the Perfexion and Icon, the helmet-based cones are replaced by a collimator system based on a fixed, single, tapered tungsten cylinder. The system contains three apertures (4, 8, and 16 mm) and a blocked position for each of the 192 60 Co sources. Unlike the previous versions, the sources are not fixed; rather, they move linearly until aligned with the desired collimator size. The sources are grouped into eight sectors, each containing 24 sources. Although all the sources in a given sector must align with the same size of cone, the different sectors can use different collimator sizes, which allows for considerably greater shaping of individual shots than what was possible in previous models. In addition, because the adjustment of collimator size is completely automatic, there is little time penalty associated with using multiple collimator sizes in a single shot. Further, when combined with the automatic movement of the patient and couch, extremely complicated and customized treatments can be delivered much more quickly than was possible in the past.
The stereotactic headframe is used to coordinate the location of the tumor within the collimator helmet. As such, its proper placement is of utmost importance to providing adequate treatment. There are two general principles guiding the headframe placement for Gamma Knife surgery. First, the target should be as close to the center of the frame as possible. This prevents possible collisions of the frame with the sides of the collimator helmet, especially when attempting to align laterally extended tumors in the center of the unit. Second, the frame attachment should be stable, to prevent movement and ensure accuracy and correlation among the pretreatment imaging study, workstation treatment plan, and delivery of focused radiation. These principles should be addressed at the time of frame attachment. In lateral targets, such as vestibular schwannomas or glomus tumors, the frame should be shifted toward the tumor side. In skull base tumors, the frame should also be positioned lower than for the treatment of more superior intracranial lesions. The anterior-posterior alignment should also be accounted for and can be adjusted by varying the lengths of the pins used to secure the frame. To ensure stability, avoid screw fixation in bone flaps, cranioplasty materials, burr holes, or skull defects.
The method of anesthesia used during frame placement is surgeon- and patient-dependent. Our program uses a combination of topical lidocaine 2.5% and prilocaine 2.5% cream (EMLA) combined with the local injection of 0.5% bupivacaine with 1:200,000 epinephrine, and an oral 5 mg oxycodone immediate-release tablet and 1 mg of lorazepam. Other programs use either sedation with versed and fentanyl, or monitored anesthesia with propofol, followed by the injection of local anesthetic at the pin sites. Fig. 59.7A shows the typical array of tools used for the frame attachment. A variety of screw lengths allows the surgeon to choose those ideal for the individual location of the posts and tumor. The placement of the frame should begin with an accurate orientation of the location of the target within the patient’s head. Ideally, the target should be located within the fiducial range and placed centrally within the frame, thereby avoiding later collisions with the collimator helmet and granting sufficient accuracy for the stereotactic target definition.
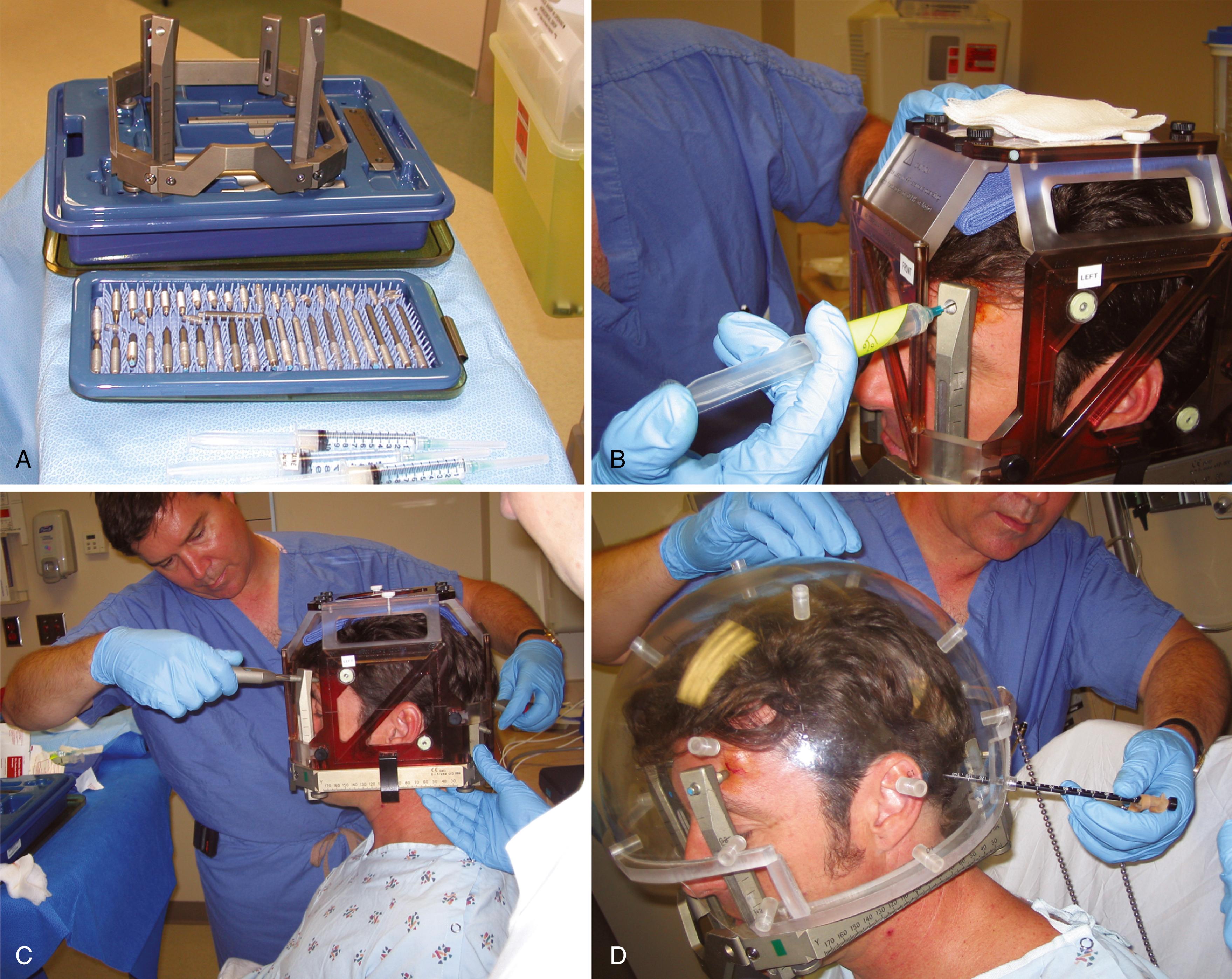
The stereotactic frame is assembled and preliminarily supported by external auditory canal support pins, a Velcro band, or a stereotactic fiducial box. When using a fiducial box to facilitate the frame placement, it is important to use the magnetic resonance imaging (MRI) fiducial box, rather than the CT or angiography fiducial box, because this is the smallest of the three Plexiglas fiducial boxes ( Fig. 59.7B and C ). Asymmetric frame placements are possible and do not impair the accuracy of imaging. The frame can be shifted from side to side or moved as far as possible to the front or back to facilitate centering of the tumor. An assistant stabilizes the frame against the patient and the surgeon should adjust the lengths of the posts to maintain the relative tumor position. A low position of the anterior posts can help avoid anterior collisions with the collimator helmet for skull base posterior fossa tumors. In critical positions, collisions can sometimes be avoided by using the curved posts in the anterior position.
Once the post position is determined, the screws can be inserted. The surgeon should work on diagonally opposing screws to provide the best stability without changing the desired frame position. For asymmetric frame placement, apply the longest screws first, thereby defining the desired distance of the target to the frame. Protrusion of the screws from the posts should be kept to a minimum to avoid collisions. Approximately 8 to 10 mm may be considered acceptable, but at our institution we prefer to limit this projection to less than 3 mm. If a screw extends farther, it should be exchanged for a shorter screw.
Measurements of the frame and placement are then performed to allow the computer to identify any potential collisions after the plan is formulated. These measurements are required for the frame and skull section in Leksell GammaPlan treatment planning software. Measurements include the length of the four posts and the length of the screws that protrude from the posts. In addition, the volume of the head is measured using the plastic collimator bubble, simulating the relationship of the frame to the treatment collimator helmet ( Fig. 59.7D ). This concludes the frame placement, and the patient may proceed to imaging.
If the practitioner determines that the patient would be better suited for treatment with the mask-based immobilization system, the patient will no longer need the invasive frame placement for treatment. The creation of the mask is quick, usually taking under 20 minutes, and relatively straightforward. It can be performed either prior to the day of treatment delivery or on the same day immediately before the dose delivery. Unlike frame placement, most patients will not need IV placement or any medications to tolerate the procedure, besides an occasional oral anxiolytic for the claustrophobic patient.
At the time of mask creation, patients are encouraged to remove all jewelry above the neck and to wear comfortable clothing, without any hood or collar, which could interfere with a proper mold. Once the patient is supine, a custom head cushion is molded from the back of the head. This cushion will conform tightly to the patient’s neck and posterior head, immobilizing the head and docking the patient to the machine’s treatment couch. Once this cushion is made, a rigid thermoplastic sheet is warmed and thus becomes malleable. While still warm, with the patient’s eyes closed and their nose normal to the treatment surface, the thermoplastic sheet is stretched across the forehead, cheeks, and chin of the patient. Multiple connectors are locked and the patient is told to avoid speaking or moving the head for at least 10 minutes to allow for the mask to cure. Strict attention is paid to tight mask conformation around features like the forehead, chin and sides of the nose.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here