Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The average annual incidence of intracranial meningiomas (MNs) is five to six new cases per 100,000 person/years, with the age-related risk drastically increasing from the pediatric population to a peak during the sixth and seventh decades. , In adults (age 24 to 84 years), they occur in 2.4 per 100,000 person/years. The female/male ratio is approximately 2:1 to 3:1, , and this prevalence is presumably related to an as yet poorly known progesterone- and estrogen-receptor–mediated cytoactivation. , ,
MNs arise from the arachnoid cap cells, or “meningothelial” cells, and are the most frequently reported neuro-oncologic challenge, accounting for 12% to 30% of all primary intracranial tumors. , The first case of intracranial MN treated with stereotactic radiosurgery (SRS) was reported by Backlund E-O in 1971. Afterward, the wide diffusion of modern imaging technologies (high-definition computed tomography—CT—and magnetic resonance—MR—imaging) and their combination with SRS apparatuses/systems allowed for SRS treatment of MNs to become increasingly common worldwide. Indeed, since the early 1990s, the role of SRS within the spectrum of therapeutic options for intracranial MNs has been increasingly emphasized even as a primary treatment alternative, especially in the elderly and in tumors in critical locations. The significant impact this has had on surgical morbidity, mortality, and postoperative recurrences, and thus on patients’ and caregivers’ quality of life, has triggered a very dramatic change in therapeutic paradigm. Skull base lesions are classic examples of this phenomenon. Today the majority of these tumors are treated with SRS procedures, limiting surgical approaches to the debulking of larger tumors. In terms of localization, MNs of the cranial base—and specifically of the cavernous sinus and of the posterior cranial fossa—are the most represented.
Up to December 2017, more than 167,000 MNs have been treated worldwide with Gamma Knife (GK) SRS alone, to which thousands of other cases treated with different devices (LINAC, Cyberknife, Proton therapy) have to be added. The increasing number of patients who underwent SRS for MN may be explained by several factors. First, the advantages of the radiosurgical procedure itself, since it represents an uncomplicated treatment plan, with easy and rapid fields shaping with multiple isocenters and with a noninvasive technique involving in most cases a one day-surgery procedure. In particular, for GK SRS, the following technical characteristics can be listed: isocentric irradiation; no moving parts once the patient, helmet, and source assembly are locked in place, and thus no need for treatment verification films and no loss of focusing precision due to mechanical tolerances of moving parts in the radiation source or the patient support; body exposure to radiation up to 100 times less than with other devices; extremely high mechanical and radiation focus precision and coincidence (±0.3 mm); highly conformal treatment plans and extremely high accuracy; high gradient dose (prescribed doses: 12 to 16 Gy at 50% prescription isodose). Second, the biological characteristics showed by most MNs. Most MNs are slow-growing tumors: WHO gastrointestinal (GI) with 0.8 mm/year growing rate. This means that MN belongs at low ∂/β ratio and in this kind of tumor a prescription dose between 10 Gy and 16 Gy is considered long-lasting effective on TLC and extremely well tolerated by surrounding normal brain tissue, with very low risk of radiotoxic permanent complications. This slow-growing tumor (low α/β ratio) characteristic allows adequate time for cytotoxic and obliterative vascular effects to develop following radiation. Third, the neuroimaging characteristics that make MNs an ideal target for SRS. Usually, they present as a dural base, extra-axial location with clear demarcation from normal brain tissue. Therefore, an accurate localization by stereo-MR imaging is achievable. Furthermore, uniform contrast enhancement following the contrast medium injection is usually attainable, with clearly and well-defined borders, good definition of the lesion dural tail, and sometimes including intratumoral calcification. The MRI sequences selected to achieve an accurate localization for SRS treatment usually include: contrast-enhanced volumetric and T1-fat-saturated (to obtain a clear demarcation of MN volume, morphology, and borders) and contrast-enhanced steady-state sequences (for clear definition of cranial nerves and other critical brain structures). Fourth, excellent results in terms of effectiveness and safety with reported 5-year actuarial progression-free survival (PFS) and local tumor control (LTC) rates of 86.2% to 97.9%, stable or improved neurological conditions in the vast majority of cases, very low morbidity rates with severe neurologic worsening extremely rare, and no mortality, if appropriate indications are warranted, particularly regarding tumor volume (TV) and cytologic grading.
Recently, the indications of intracranial MNs suitable for SRS have been expanded. In the last decade, larger MNs and MNs which are closer to critical brain structures such as the anterior optic pathways (AOPs), brain stem, etc., have undergone SRS more and more often thanks to the introduction of innovative “volume staging” and “hypofractionated” irradiation techniques and modalities. Similarly, the number of scientific publications on SRS treatment of intracranial MNs has increased, with growing numbers of treated patients worldwide. Up to August 2018, the overall number of publications on this topic was over than 1000.
In addition to the introduction of new treatment strategies such as “volume staging” and “hypofractionation” mentioned above, the last 6 years have seen important changes in the management of MNs due to the publication and diffusion of guidelines for their diagnosis and treatment, the new WHO classification of tumors of the CNS, including MNs, results on long-term follow-up series of patients with a mandatory minimum period of observation of 10 years to assess treatment effect for usually slow-growth-rate tumors, as well as upgrades of irradiation techniques and treatment plan software. Therefore, both a complete revision of the 6th Edition chapter structure and the inclusion and discussion of some new topics are needed in this updated version to reach an overall view and a comprehensive understanding of the radiosurgical approach to the treatment of intracranial MNs.
During the last decade, the pathologic criteria for the definition of “atypical” as well as of “anaplastic” MNs have been gradually expanded to include not only nuclear pleomorphism, mitoses, atypia, necrosis, and so on but also other relevant landmarks of parenchymal invasion such as the formation of small cell infiltrates, arachnoidal disruption, sheeting, macronucleoli focal malignant macrodifferentiation (e.g., glial fibrillary acidic protein—GFAP—production, melanocytic foci, etc.), and extra-axial diffusion. In fact, the very peculiar metastatic potential of these tumors seems to confirm the cytobiological discrepancy just mentioned, since histologically benign MNs have sometimes shown biological aggressiveness, in terms of not only craniospinal seedings but also of extraneural colonization (lung, liver, bone, lymph nodes). ,
Indeed, the 2000, 2007, and 2016 revisions to the WHO classification and grading scheme of MNs described the characteristics regarding the definitions of atypical (WHO grade II) and anaplastic (WHO grade III) MN very well. While WHO grade I MN remains defined by the well-known characteristics (low mitotic rate, less than 4 per 10 high-power fields (HPFs) and absence of brain invasion, 9 histological subtypes: meningothelial, fibrous or fibroblastic, transitional or mixed, psammomatous, angiomatous, microcystic, secretory, lymphoplasmacyte-rich, and metaplastic), WHO grade II MNs are defined as MNs with 1 or more of the following: (1) 4 to 19 mitoses per 10 hpf; (2) at least 3 of the following 5 atypical features: spontaneous necrosis, macronucleoli, loss of architecture (sheeting), hyper-cellularity, and small cell change; (3) brain invasion; or (4) predominant (>50% of the TV) clear-cell or chordoid morphology. WHO grade III MNs are defined as meningiomas with either 20 or more mitoses per 10 hpf, or predominant rhabdoid or papillary morphology. Interestingly, the 2016 classification introduced brain invasion as a criterion for the diagnosis of atypical MN (WHO grade II). While it has long been recognized that the presence of brain invasion in a grade I MN is associated with recurrence and mortality rates similar to those of a grade II MN in general, prior WHO classifications had considered invasion a staging feature rather than a grading feature and opted to discuss brain invasion under a separate heading. In the 2016 classification, brain invasion joins a mitotic count of 4 or more as a histological criterion that can suffice alone for an atypical MN diagnosis, WHO grade II. , According to these new definition parameters and criteria, the incidence of grade II tumors, which recur earlier and in a more diffuse manner than benign MNs, has dramatically increased its frequency and now accounts for 20% to 35% of newly diagnosed MNs, instead of the 3% to 4% which it represented before the introduction of these new parameters.
Furthermore, the discovery and definition of new biological markers and their correlation with prognosis, further helps in defining the nature and behavior of such tumors. Molecular alterations associated with less favorable clinical courses are expected to be valuable adjuncts to tumor grading when identifying patients at high risk for MN recurrence or progression.
Compared with grade I MNs, the most prominent alteration in grade II and III MNs is a significant increase in chromosomal gains and losses, or copy number alterations, which may have behavioral implications. Monosomy of chromosome 22 , mutations of the NF2 gene on 22q12 in neurofibromatosis type 2 (NF2)—associated MNs, , as well as amplification on 17q and loss on 9p (CDKN2 gene) , and their correlation with WHO grading (more aggressive behavior) of MNs was already known. NF2-positive WHO grade I and II MNs are at higher risk of recurrence and patients should be subjected to postsurgical imaging more frequently. Monosomy of chromosome 7 is frequently reported in radiation-induced meningiomas (RIM).
More recently, new biological markers’ expression of mutated genes has been described and correlated with MN outcome. First of all, protein telomerase reverse transcriptase (TERT) promoter mutations, irrespective of WHO grade, are an indicator for more aggressive growth in MN. Furthermore, the so-called non-NF2 mutations, which are frequently identified in WHO grade I MNs but the presence of a few non-NF2 mutation variants may increase the risk of recurrence. In particular, tumor necrosis factor receptor-associated factor 7 (TRAF7), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α (PIK 3 CA), oncogene homolog 1 (AKT1), Kruppel-like factor 4 (KLF4), smoothened, frizzled class receptor (SMO), and RNA polymerase II subunit A (POLR 2 A) mutations are always associated to a benign behavior (WHO GI MNs), while the detection of v-Akt murine thymoma viral oncogene homolog 1 (AKT1) mutations are usually expression of an intermediate MN aggressiveness. The promoter region of the TERT gene that encodes for TERT is mutated in several cancers. TERT mutations are present in approximately 6% of MN. MNs harboring TERT promoter mutations have variable location and are present in both skull base and cerebral convexities. TERT promoter mutations are more frequently observed in progressive and high-grade MNs. When present in WHO grade I tumors, TERT C228T and C250T mutations are linked to high rates of recurrence and transformation to a higher grade. MNs with TERT promoter mutations should be recognized as aggressive and should undergo frequent imaging. Indeed, progression-free survival is reduced from 179 to just 10 months in patients with TERT promoter mutations compared with patients with TERT wild types. TERT promoter mutations have been correlated with radiotherapy resistance in glioma and it will be important to determine if this is also the case for MN. Mutations in TRAF7 are the most frequently observed gene aberrations in non-NF2 tumors, and they are present in approximately 25% of all MNs. TRAF7 mutations are most common in grade I tumors but are also observed in grade II and III. TRAF7 tumors are typically associated with meningothelial subtypes of the anterior cranial base but location and prognosis are influenced by whether AKT1, KLF4, or PIK3CA mutations are also present. PIK3CA mutations are present in approximately 7% of non-NF2 tumors, with approximately 60% of this group also having a TRAF7 mutation. PIK3CA mutations are generally found in meningothelial and transitional grade I tumors of the skull base. Mutations in KLF4 almost always co-occur with TRAF7 mutations. KLF4 mutations occur in about 12% of WHO grade I MN. Virtually all secretory MNs harbor KLF4 and TRAF7 co-mutations. By contrast, SMO mutations, found in about 5.5% of WHO grade I MN, are mutually exclusive of TRAF7. MNs with an SMO mutation are mostly localized near the midline and at the medial anterior skull base. Mutations in POLR2A are found in 6% of MNs. POLR2A-positive tumors are WHO grade I and have low risk of recurrence. AKT1 is mutated in 7% to 12% of WHO grade I tumors, is rare in WHO grade II, and mostly absent in WHO grade III MNs. Approximately 60% of AKT1 tumors also carry a mutation in TRAF7. AKT1 MNs are frequently located in the anterior cranial base. MN of the skull base may have a reduced time to recurrence if carrying an AKT1 mutation. ,
In light of these recent data, we recommend that, in case of neuroradiological patterns suggesting an aggressive MN, when possible and safe, a more aggressive approach be taken to reach a clear and definitive biological and histopathological definition. From the radiosurgical point of view, in cases of “primary” treatment for MN with tumor location not amenable to surgery and in cases with very high surgical risk MN with imaging characteristics similar to an aggressive lesion, higher prescription doses than those used for WHO grade I MNs (range 10 to 16 Gy) should be delivered. In such cases, several authors suggest to administer prescription doses ranging between 15 Gy and 20 Gy, if allowed by anatomical conditions and radiobiological parameters. , Furthermore, we suggest that patients affected with MN expressing biological markers for mutated genes that are correlated with a more aggressive biological behavior should receive more frequent postsurgical clinical and imaging follow-ups.
The levels of evidence for the diagnosis and treatment recommendations for patients with MNs are low, allowing only recommendations of level B, C, and good practice points, despite the high frequency of such tumors. Nevertheless, the different studies published over the last 6 years show interesting suggestions that deserve to be mentioned. Indeed, since 2012, several guidelines and reviews with level of evidence and grade of recommendations regarding the management of intracranial MNs have been published. In June 2012, the Alberta Health Services published the Clinical Practice Guideline CNS-005 version 2 on intracranial MNs. Evidence was selected and reviewed by a working group comprised of members from the Alberta Provincial CNS Tumour Team and a Knowledge Management Specialist from the Guideline Utilization Resource Unit. For WHO grade I MN, the Alberta Provincial CNS Tumor Team recommends that small, asymptomatic, benign-appearing MNs that are found during a radiographic evaluation are followed without therapy, using CT and/or MRI imaging at regular intervals. Indeed, though most asymptomatic or incidentally detected MNs can be managed by observation with serial surveillance neuroimaging, there is no class I or II evidence to support this approach. The Alberta Provincial CNS Tumour Team recommends that microsurgery be the primary treatment for patients who are not candidates for management by a watch-and-wait approach (recommendation #1). Surgical intervention is recommended when the tumor begins to cause symptoms, or when it displays significant growth on consecutive CT or MRI images. Gross tumor removal (GTR) should always be the goal of surgery and offers the best possibility of a cure for low-grade MNs. However, some meningeal tumors, particularly those involving the cavernous sinus, petroclival region, posterior aspect of the superior sagittal sinus, or optic nerve sheath (ONS), cannot be completely removed due to their relationship to vital neural or vascular structures. For incompletely resected or recurrent symptomatic grade I MNs, the Alberta Provincial CNS Tumour Team recommends the use of radiation therapy (RT) (recommendation #2). Thus, radiation treatments should be considered in cases in which tumor location precludes surgery, where the tumor is unresectable (as primary treatment), in symptomatic residual disease (as an adjuvant treatment), or where there is tumor recurrence (as a salvage treatment). Radiological diagnosis may be sufficient in these cases. Regarding the WHO grades II and III MNs, because of their high rates of postsurgical recurrence, the authors recommend that the standard treatment for such patients is microsurgery followed by postoperative RT (recommendation #3). Patients with selected tumors may be eligible for SRS (recommendation #4). This technique is most often used for patients who have small- to medium-sized MNs, have tumors that are surgically inaccessible, are not candidates for surgery, or have residual or recurrent tumors following surgery with a recommended marginal tumor dose ranging between 9 Gy and 20 Gy (median, 15 Gy). In cases where the tumor is unresectable or all other therapies have failed to prevent a recurrence, the Alberta Provincial CNS Tumour Team suggests that systemic treatment may be considered on a clinical trial basis (recommendation #5) although there are only limited data available on the use of systemic agents in the treatment of MNs.
The National Health System (NHS) in England published in September 2013 the Clinical Commissioning Policy referring to SRS/Stereotactic Radiotherapy (SRT) for MN (NHS England D05/P/e), prepared by the NHS England Clinical Reference Group for SRS. They also stated that the three management options for patients with MN are: (1) surgical removal; (2) RT: SRS (single fraction), SRT (2 to 5 fractions), multi-fraction treatment (>5 fractions) with or without stereotactic localization techniques; and (3) no intervention/radiological surveillance. All treatment options must be considered by a multidisciplinary team (MDT) for all patients with intracranial MNs. These must be discussed with the patient, as patient choice is considered of fundamental importance. Microsurgery is the preferred treatment option in the management of patients with MNs where the operative risks are low or minimal. This enables histological diagnosis to be confirmed, provides an opportunity for complete excision, and permits cytoreduction, even if the tumor origin is not amenable to resection. However, evidence has grown over the past 20 years supporting the use of SRS as a primary treatment for MNs that are in anatomically unfavorable locations where microsurgery is deemed to have an unacceptably high risk of neurological deficit. These locations include, but are not confined to, the skull base, the posterior fossa, parasagittal, parafalcine, and intraventricular sites. They recommend that it is appropriate for clinicians to consider SRS for a small subset of patients with MN that are in a difficult and unacceptable high anatomical risk situation, where there is evidence of effectiveness for SRS, and where conventional surgery is contraindicated or the risk of functional disability would be increased with surgery. This recommendation is supported by the fact that evidence from large numbers of patients indicates that long-term tumor control is achieved in a high proportion of cases treated with SRS. Furthermore, when compared to microsurgery, SRS provides shorter hospitalization and a less detrimental impact on quality of life. Procedural mortality is avoided and there is a lower incidence of treatment-related complications. Finally, there is evidence from a comparative study between microsurgical costs and SRS costs that the overall costs for microsurgery were more than double the costs of SRS. For patients undergoing SRS rather than microsurgery, the cost savings to the NHS can be substantial. SRS is usually delivered as a day-case treatment under local anesthetic. Microsurgical resection is performed under general anesthetic. Operative times usually extend from 2 to 10 hours or more, depending on complexity. Microsurgery patients are managed for 12 to 24 hours minimum in an intensive care/high-dependency care environment. Hospital stay is usually in the range of 4 to 10 days but may extend to several months if postoperative deficits and complications (such as cerebrospinal fluid leak) occur. This can put significant strain on neurological-rehabilitation resources. In addition, SRS limits disease progression in most patients with low retreatment rates. Regarding the evaluation of response to SRS, post-treatment volumetric assessment of the tumor has been suggested at 6 months, annually for 5 years, then every 2 years. From a costing perspective, postoperative patients also undergo regular surveillance scans at similar timepoints. The NHS England Clinical Reference Group for SRS focused their attention on the management of asymptomatic or incidentally detected MN as well. The absence of symptoms, a heavily calcified tumor, or a very small tumor may appropriately lead to conservative management with a radiological surveillance program. This is particularly appropriate in patients with more advanced age in whom symptomatic tumor progression is unlikely to occur. However, in many locations, further growth of the tumor after initial diagnostic imaging will make treatment of the tumor more difficult, leading to universal agreement that intervention should be considered appropriate. Furthermore, studies on the growth rates of MNs are characterized by an inability to reliably predict which tumors will grow and how quickly. Hence, since in many locations (e.g. skull base, parasagittal) any degree of tumor enlargement can lead to compression of adjacent neurological structures with associated permanent neurological deficits, there is universal agreement that intervention is appropriate for many patients with critically placed MNs. In these cases, the NHS England Clinical Reference Group for SRS recommended that a MDT of experienced SRS neurosurgeons, neuro-oncologists, and neuro-radiologists must be involved in determining the operative risks and the likelihood of involvement of adjacent structures for appropriate case selection, treatment planning, and delivery. Accordingly, the NHS England Clinical Reference Group for SRS concluded that because blinded trials cannot be conducted comparing the treatment of MNs with SRS and/or microsurgery and/or a conservative approach and given the fact that many MNs occur in critical areas of the brain, outcomes of treatment are potentially compromised by adopting a watch, wait, and re-scan policy. At the end of their clinical commissioning policy study, the authors stated that indications for SRS/SRT include newly diagnosed MNs (primary SRS/SRT), residual MN after microsurgical resection (adjuvant SRS/SRT), and recurrent MNs (salvage SRS/SRT).
In 2015, Sun et al. reported a comprehensive review of the literature on management of grade II and III MNs based on WHO 2000/2007 criteria and attempted to define optimal treatment strategies for these tumors given the most current evidence. For WHO grade II MNs, recent retrospective studies corroborate the importance of GTR (Simpson grade I–III) resection because maximal extent of resection has been associated with improved long-term outcomes. GTR shows a significant benefit over STR (Simpson grade IV) for grade II MNs (EBM level 3, grade 1C recommendation). However, “maximal safe resection” may be a more appropriate strategy than GTR, given the surgical morbidity associated with resection in certain locations. Hence, while GTR is the goal, STR may be considered in selected patients through traditional open surgery or minimally invasive techniques. Regarding adjuvant RT after GTR, the authors reported that the current literature overall does not favor the addition of adjuvant RT for grade II MNs after GTR. Furthermore, complications of adjuvant RT should also be considered in clinical decision making. Radiation necrosis may occur in 4.2% to 10.2% of patients. Without definitive evidence that RT reduces the risk of tumor recurrence and subsequent retreatment for all 2000/2007 WHO grade II MNs after GTR, the risk of toxicity related to upfront RT needs to be carefully considered. Results from studies relying solely on 2000/2007 WHO guidelines support active surveillance after GTR of grade II MNs (EBM level 3, grade 1C recommendation). MDT management is recommended for those grade II MNs with particularly aggressive histological features. In contrast to gross totally resected WHO grade II MNs, it has been demonstrated that adjuvant RT after STR is associated with improved PFS. Overall, adjuvant RT is reasonable after STR (EBM level 3, grade 1C recommendation), but the results of STR and RT remain suboptimal, with a 5-year PFS of 43% to 91%. Therefore, dose escalation of RT or novel approaches using radiosensitization should be investigated, especially for grade II MNs with necrosis. The authors reported that a review of modern literature on SRS suggests that this modality can be effective for biopsy-proven WHO grade II MNs. In particular, adjuvant SRS following STR resulted in equivalent rates of long-term LTC as adjuvant RT (EBM level 3, grade 2C recommendation), although SRS tended to be used for smaller residual tumors in a smaller resection bed. SRS is also used as a salvage measure for grade II MNs for which surgery and RT have failed. In summary, the authors concluded that RT and SRS are complementary strategies for management of WHO grade II MNs after STR or recurrence, with the volume of the residual or recurrent disease representing the discriminating factor: SRS for smaller residual tumors and RT for larger ones. For recurrent WHO grade II MNs, no studies of WHO 2000/2007 grade II MNs compare outcomes of salvage resection against salvage RT or SRS; for these tumors, the authors recommend tailoring treatment to the specific clinical scenario: salvage resection should be considered if the recurrent grade II MN is accessible, salvage SRS if the recurrent grade II MN is relatively small and localized, and salvage RT if the recurrent grade II MN is radiation-naïve and of a relatively larger volume such that SRS is not feasible. In WHO grade III MNs, maximal but cautious resection strategy and consideration of repeat surgery for recurrence is recommended (EBM level 3, grade 1C recommendation). Authors stated that RT was associated with increased PFS and overall survival in grade III MNs and they conclude that evidence supports the use of RT for such tumors (EBM level 3, grade 1C recommendation). The role of SRS in WHO grade III MNs is limited to recurrent relatively small and localized tumors, nonsurgically accessible, with microsurgery and RT failure. Finally, the role of chemotherapy in WHO grade II and III MNs is still debated. The separate evaluation of the chemotherapy effect in grade II and III MNs is not available because most studies pooled both tumors together. Anyhow, chemotherapy for WHO grade II and III MNs has had limited success. Select trials have suggested that angiogenesis inhibitors (agents against VEGFR and VEGF, including RTK inhibitors and bevacizumab) may play a role in salvage therapy for recurrent or refractory grade II and III MNs (EBM level 2, grade 2B recommendation). At the end of their study, the authors suggested the following EBM classified recommendations for WHO grade II and III MNs: (A) EBM level 3, grade 1C recommendations: (1) maximal safe resection of grade II and III MNs; (2) active surveillance after GTR of grade II MNs; (3) adjuvant RT after subtotal resection of grade II MNs; (4) adjuvant RT after resection of grade III MNs. (B) EBM level 3, grade 2C recommendations: (1) selective RT for grade II MNs based on the absence of histopathological necrosis; (2) adjuvant SRS for small residual grade II MNs after STR.
The MN task force of the European Association of Neuro-Oncology (EANO) assessed the scientific literature and composed a framework of the best possible evidence-based recommendations for health professionals. The EANO recommendations are very similar to those of other recent reported studies. Asymptomatic patients, especially if elderly, can be managed by observation. No class I or II evidence exists to support guidelines for observational management of MNs, but many retrospective series and several reviews validate this approach (evidence level III, recommendation level C). The suggestion to treat with surgery or SRS rather than to manage by observation alone should be made on the basis of a strong indication. If treatment is indicated in MN of any WHO grade, surgery is the first option and complete removal (Simpson grade I) is the primary goal of surgery: evidence level II, recommendation level B for WHO grade I MNs; evidence level III, recommendation level B for WHO grade II MNs; evidence level III, recommendation level C for WHO grade III MNs. Assessment of the extent of resection should be confirmed by MRI performed within 48 hours after surgery, or after 3 months, to avoid artifacts. SRS might be the first option in small WHO grade I MNs in specific locations. Patients with WHO grade I MN who cannot undergo surgery can be treated by fractionated RT or SRS: evidence level III, recommendation level B. Combining subtotal resection and SRS or fractionated RT should be considered to allow comprehensive tumor treatment while reducing the risk of adverse effects from treatment in WHO grade I MNs (evidence level IV, recommendation level C). Patients with incompletely resected WHO grade II MNs should receive fractionated RT or SRS if the tumors are small or if they are tumor remnants (evidence level III, recommendation level C). Pharmacotherapy is experimental in any grade of MN and should only be considered if no further surgical or RT options exist. Regarding the period of observation, the following recommendations are based more on expert consensus opinion than on evidence (recommendation level: good practice point). Follow-up of WHO grade I MNs should be done annually, then every 2 years after 5 years; follow-up of WHO grade II MNs should be done every 6 months, then annually after 5 years; follow-up of WHO grade III MNs should be done every 3 to 6 months indefinitely. In summary, the authors recommend what follows. In WHO grade I MNs with GTR, there is no need for further irradiation, either SRS or fractionated RT. After STR, for elderly patients (older than 65 years), or for tumors not safely accessible by surgery, SRS can be offered for small tumors or fractionated, RT if the TV cannot be treated by a single fraction. In WHO grade II MNs with GTR, active surveillance is recommended without any routine adjuvant RT. For WHO grade II MNs with STR, progression or recurrence lesions, adjuvant/salvage RT or SRS for small tumors or tumor remnants should be considered. In WHO grade III MNs, surgical resection should be as radical as possible and always needs to be followed by fractionated RT.
More recently, the Princess Margaret Cancer Centre has published its Clinical Practice Guidelines. The MDT put forth the following recommendations for each WHO grade MN. In WHO grade I MNs, complete surgical resection is the treatment of choice for most non–skull base tumors. Vertex parasagittal tumor locations present difficulty to achieve a Simpson’s grade I resection due to attachment of the tumor to the superior sagittal sinus. For newly diagnosed symptomatic MN for which surgery is not done (i.e., cavernous sinus, optic nerve, other skull base locations), upfront RT is usually recommended. For MNs where a partial resection has been done with residual disease, upfront RT or delayed RT are possible options. For recurrent MNs, upfront RT is usually recommended. In WHO grade II MNs, GTR is recommended wherever possible; where a GTR has been done, then observation and RT for recurrence is (usually) recommended, as the 10-year relapse rate is approximately 50%. For STR or recurrent tumor, RT is (usually) recommended. In WHO grade III: GTR wherever possible; postoperative RT is recommended in all cases regardless of degree of resection. With reference to SRS, the authors recommended the following indications: maximal tumor diameter should not exceed 3 cm; skull base MNs at initial presentation; residual/recurrent grade I MNs; recurrent grade II/III MNs after prior fractionated RT. The SRS-advised doses range between 12 and 14 Gy, based on location and aggressiveness of tumor.
In summary, evidence-based and guideline studies published in recent years with reference to SRS for the treatment of intracranial MNs are in agreement on several points. First of all, there is an overall agreement about the effectiveness and safety of SRS in selected cases. Indeed, SRS is a shared recommendation as a primary or adjuvant/salvage treatment for WHO grade I MNs, particularly for those in anatomically unfavorable locations where microsurgery is deemed to have an unacceptably high risk of neurological deficit or when MNs cannot be completely removed due to their relationship to vital neural or vascular structures. These locations include, but are not limited to, the skull base and the posterior fossa (particularly, cavernous sinus and petroclival locations), the posterior aspect of the superior sagittal sinus, parasagittal, parafalcine, intraventricular, and ONS sites. Furthermore, SRS provides, when compared to microsurgery: shorter hospitalization; a less detrimental impact on quality of life; avoidance of procedural mortality and lower incidence of treatment-related complications; much lower overall costs. Briefly, for elderly patients (older than 65 years), or for tumors not safely accessible by surgery, or after incomplete surgical resection, SRS can be carried out for small tumors. In WHO grade II MNs, SRS is most often used for patients who have small- to medium-sized MNs, have tumors that are surgically inaccessible and are not candidates for surgery (primary treatment), or have residual or recurrent tumors following surgery (adjuvant/salvage treatment). Results of SRS are similar to those of fractionated RT for small tumors or tumor remnants. Finally, SRS is deemed to be indicated in recurrent WHO grade III MNs that have undergone prior fractionated RT.
The development of SRS has closely followed the impressive evolution of neuroradiologic techniques. The best example is perhaps the exponential increase of radiosurgical indications after the introduction of computerized imaging, particularly magnetic resonance imaging (MRI). Indeed, for routine SRS procedures, stereo-MRI remains a mainstay in target localization. Coregistered multimodality acquisitions (MRI, computed tomography [CT], angiography, positron emission tomography-magnetic resonance imaging [PET-MRI] [ Fig. 95.1 ], PET-CT PET scan) have led on one side to the development of fusion algorithms based on contour definition, signal intensity, and voxel matching that allow detailed, interpolated 3D and 4D pictures, speeding up all the preoperative procedures. On the other, advances in computerized integration of stereotactic and nonstereotactic imaging, including functional MRI and diffusion tensor imaging (DTI), are opening unexpected frontiers to SRS treatments. Improving safety and efficacy of radiosurgical planning is now possible via fusing the conventional sequences of a stereotactic MRI with the three Tesla pictures of corticospinal, visual, or arcuate DTI, with the aim of preserving the specific tract from undue damage ( Fig. 95.2 ) CT and MRI scans sometimes may not adequately identify the regions of functional interest surrounding the planned target. Co-registration of functional MRI becomes mandatory, albeit sometimes still inadequate. Molecular imaging may be the appropriate integration. Using stereo-PET PET-CT or PET-MRI scan metabolic mapping, with FDG or a spectrum of amino acids radiotracer, particularly fluorodopa and 11C-methionine, (68) Ga-DOTATOC , scan be merged with conventional picture, thereby helping to identify the borders of the lesion as well as the metabolically active sites of the tumor or necrotic/radionecrotic foci on the basis of the differential uptake. , Moreover, PET techniques allow us to differentiate the usually octreotide-rich surfaces of MNs from other skull base tumors that generally lack these receptors. ,
Radiosurgery, in the absolute majority of cases, differs from conventional ab externo radiotherapy in various well-known aspects:
A single fractionated high dose is used, with very few exceptions, as opposed to multiple small daily fractions.
The use of stereotactic technology entails considerable accuracy of the treatment (with a standard mechanical error of 0.3 mm).
The dose gradient is extremely steep; thus the resulting lesions are very sharply circumscribed. As a consequence, exposure outside of the target volume is minimized.
Radiotherapy relies on the difference in susceptibility between tumor tissue and normal brain tissue, whereas radiosurgery delivers an ablative dose to the target margin with a higher dose delivered centrally.
Currently SRS techniques may utilize different types of penetrating energy, including particles such as protons or heavy-charged particles, and photon devices such as linear accelerators (LINACs) or the GK. The details and functioning modalities referring to these devices when applied to SRS have been extensively presented and discussed in the 6th Edition chapter. The recent innovations introduced in the last 6 years can be summarized as follows ( Fig. 95.3 ).
Hadron therapy is a form of external beam radiotherapy using beams of energetic protons, neutrons, or positive ions. , The tissue depth at which the Bragg ionization peak is maximal can be adjusted by cross-firing systems according to the target position. The most common type of particle therapy as of 2012 is proton therapy. The drawbacks of this technique include the great expense of building and the use of these charged particles has developed rapidly in recent decades.
Linear accelerator-based radiosurgery uses x-ray beams. Multiple non-coplanar arcs converge at a single isocenter, where a nearly sphere-shaped dose distribution is created. This technique has proved to be an effective, alternative radiosurgical option, particularly in cases of larger target volumes, or whenever fractionated radiosurgical procedures are required. , , Comparative analyses of the literature concerning LINAC versus GK results in MNs, however, still show similar tumor control indexes on one side, and much higher complication rates with LINAC on the other, with comparable or shorter follow-up periods. , With the increasing use of SRS, the growing interest for highly sophisticated, photon linear accelerators has led to micro-multileaf (MML) technology and a flattening filter-free (FFF) linear accelerator, potentially more competitive with GK and proton beam devices, and characterized by an easy patient setup and greater possibilities of reaching any irradiation position. Basic parameters that allow complex field shaping with the LINAC include the volumetric modulated arc therapy (VMAT), patient couch angle, isocenter position, and collimator size. The Magnetic Resonance Linear Accelerator (MR-LINAC) is a technology that enables simultaneous radiation delivery and fast acquisition of high-quality MR images. MRI yields superb soft-tissue visualization (see Fig. 95.3A and B ).
The CyberKnife (Accuray, Sunnyvale, CA) has clearly shown basic pros and cons of the technique. , , Regarding the latter, the absence of a stereotactic frame, as well as the mobility of the radiation source, should somehow entail a slightly superior error margin if compared to the GK. The CyberKnife (see Fig. 95.3C ) combines a lightweight 6-MeV LINAC designed for radiosurgery and mounted onto a highly maneuverable robotic arm that can position and point the LINAC. Internally controlled real-time image guidance eliminates the need of skeletal fixation for either positioning or rigid immobilization of the target. Complex radiosurgical treatments may be performed, in which beams originate at arbitrary points in the workspace to target arbitrary points within the lesion. Even nonisocentric beams can be focused anywhere within a volume around the center.
Helical tomotherapy unit is a new modality for radiation treatments, the first dedicated to intensity-modulated irradiation using a fully integrated image-guided radiotherapy machine with on-board megavoltage CT capability. The tomotherapy system (see Fig. 95.3D ) uses a 6-megavoltage accelerator and a binary. Radiation is delivered in a helical way, obtained by concurrent gantry rotation and couch/patient movements. Tomotherapy has been used for the treatment of benign brain tumors (MNs and neurinomas), resulting in good target coverage with high-dose homogeneity and respecting organs-at-risk constraints. In addition, this technique allows treatment of larger brain lesions than GKS. Once again, the most important difference seems to be that the latter still maintains a better conformity index, and non-negligible advantages in terms of the integral dose to the brain.
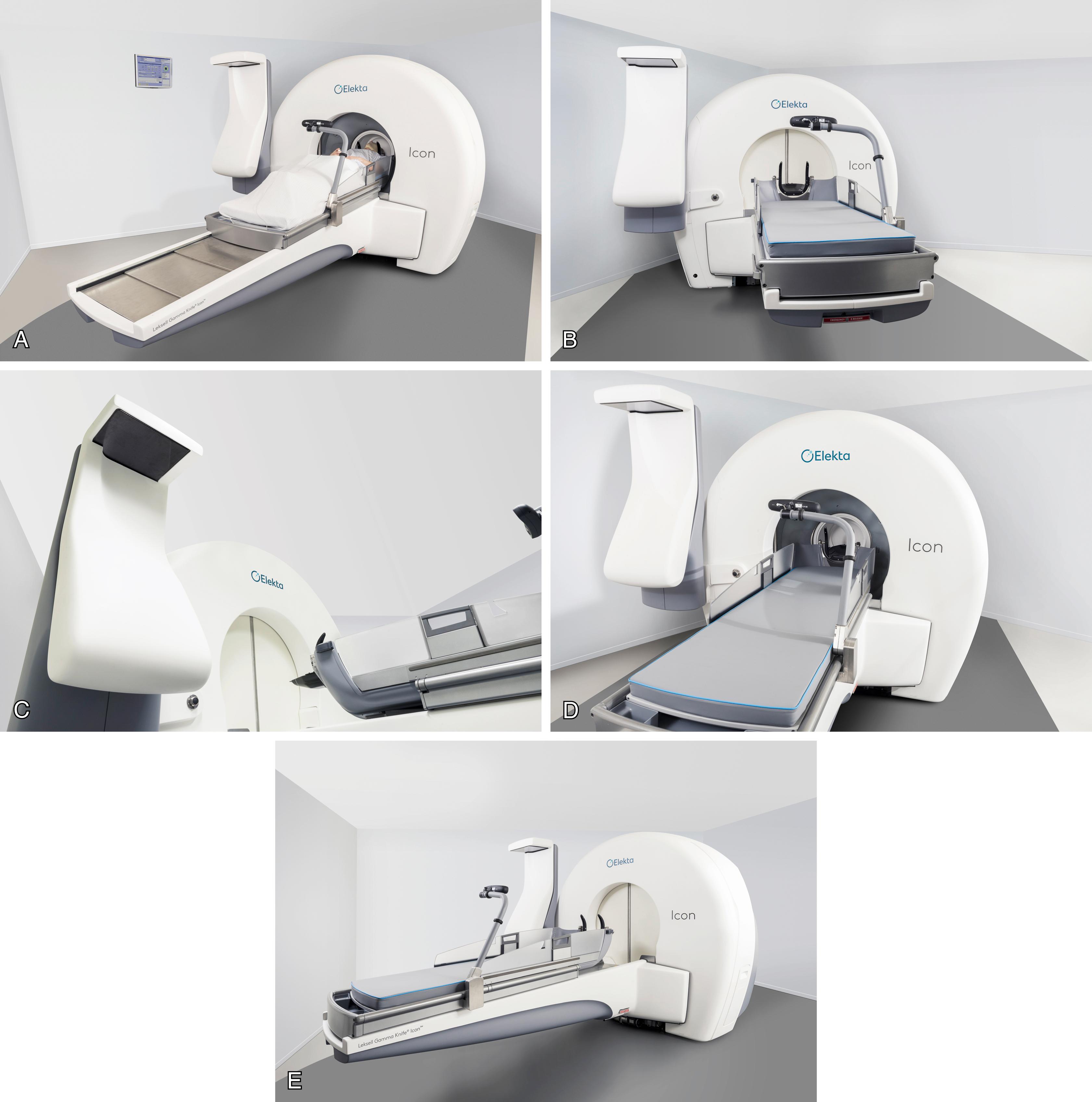
The GK (Elekta, Sweden) contains an array of 192 (model Perfexion and Icon) (see Fig. 95.3E ) individual Co sources aligned with a collimation system that directs each of the radiation beams to a very precise focal point. The Icon model integrated stereotactic Cone Beam CT for frameless treatment and gives clinicians the option to perform single or fractionated frame-based or frameless treatments, allowing for more individualized delivery, without sacrificing precision and accuracy. GK treatments are therefore quite heterogeneous in terms of dose distribution inside and outside of the target; tumor control and tissue sparing is achieved via the steep dose gradient at the periphery rather than exploiting the radiobiological differential between normal and pathologic tissue as in fractionated radiotherapy. With the multiple isocenters routinely used, GK plans tend to be more conformal but less homogeneous than LINAC-based plans. Due to the typically steep dose gradients of radiosurgery, an accurate alignment of the plan’s isocenter with the physical isocenter of the machine is a challenging issue. Moreover, the surgeon can modify the shooting collimator size using the sector drive motors that move the sources along their bushing to the correct position; the localization of each sector is monitored by linear and rotational encoders characterized by a positioning repeatability of less than 0.01 mm. ,
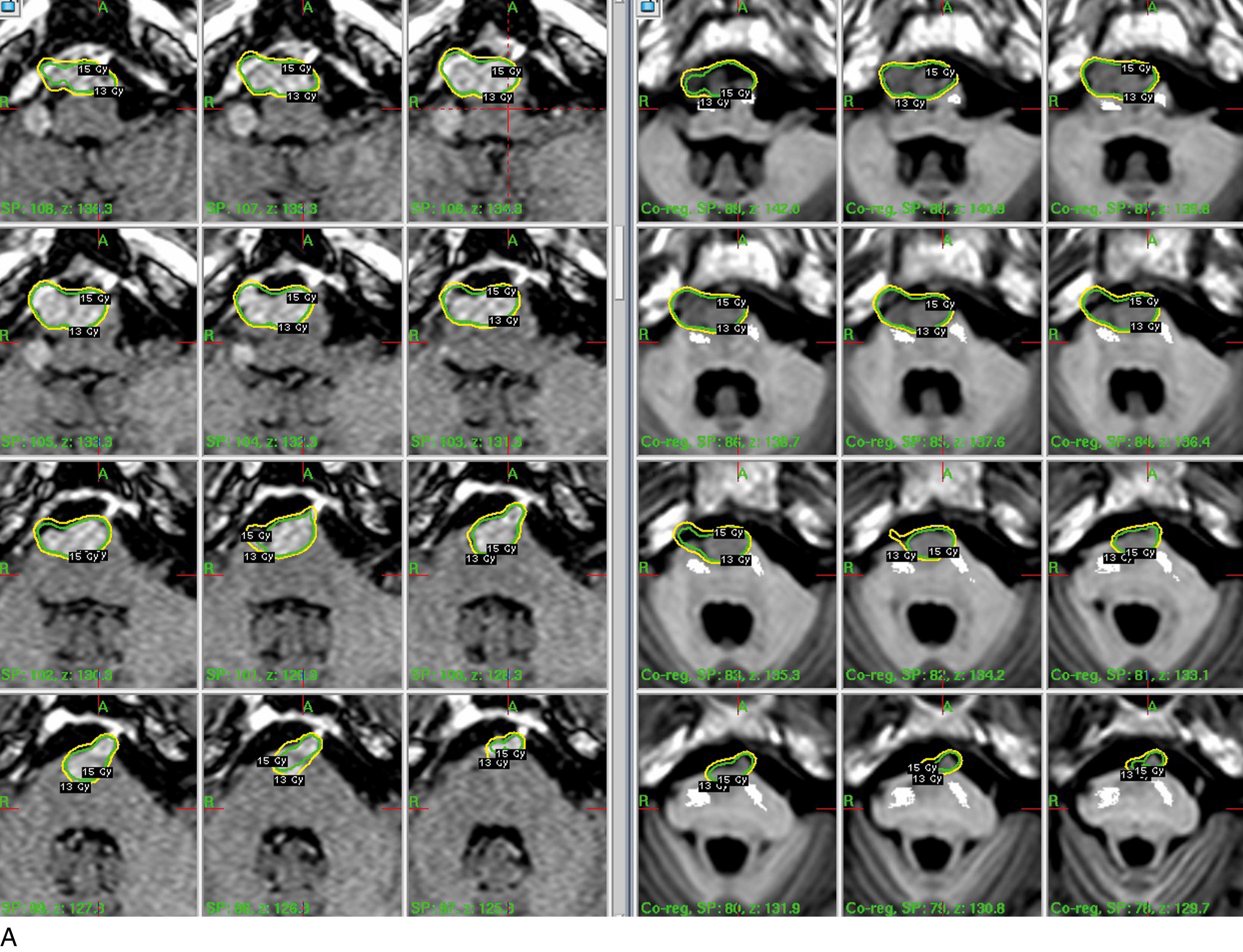
The goal of dose planning is to create an extremely conformal and selective isodose configuration, with complete covering of the tumor, and minimal exposure of the surrounding tissues. Using a combination of multiple isocenters of different sizes, differential weighting of the crucial shots, selective blocking of the collimated beams, and typical of the GK using hybrid shots, it is possible to produce dose plans that closely conform not only to the main shape of the MN but also to all the MRI-documented dural attachments, while sparing the neighboring neural structures. These newer approaches in treatment planning have consistently improved Paddick’s conformity index, while reducing treatment times for GKR , , and for MML LINAC. , To date, the recommended surface (SD), edge (ED), or peripheral (PD) doses for MNs range from 12 to 15 Gy at the 50% isodose; the higher levels are currently reserved for more aggressive histotypes. , T1 contrast-enhancing image plus a supposedly infiltrated margin of a few millimeters, , to the controversial inclusion of the “dural tail” that—according to studies with extremely refined doses—should be essentially composed of hypervascular dura with none of the expected tumor colonies. , Another deceptive variable in the definition of the target volume is represented by hyperostosis, , , particularly after the studies of Pieper et al. showing that (in a series of 26 consecutively operated patients) hyperostotic bone was almost always present (25/26 cases), even where there was no evidence from imaging. In these cases, ablative radiosurgery on the hyperostotic bone might have the same meaning as Simpson’s grade 1 in surgical approaches. As mentioned previously, the novel and impressive advances in radiosurgical treatment strategies, particularly coregistration and fusion algorithms dose-planning software, and automatic positioning systems are helping to overcome some of the major constraints of SRS, and specifically GKR. We can reasonably target unusually larger volumes, even in crucial sites, by using lower dosages or by performing “staged” SRS procedures, via means of dose and/or volume fractionation. ,
Published results are interesting, and there is no evidence of increased adverse radiation effects (AREs). , , In fact, the well-known relationship between the dose-volume integral and the risk of AREs does not seem to apply at lower-dose regimens. , , , Nonetheless, in MN treatment, relevant limits, pitfalls, and risks remain to be tackled. An instructive example is represented by dosimetry planning for cavernous sinus MNs. The dose heterogeneity of these treatments usually requires an extremely careful evaluation of dose-distribution algorithms, because of the occasional reported cases of radio-induced vascular injury to the carotid arteries, and because of the morbidity that is still observed with this technique on sensory nerves, and also because of the sometimes disappointing results in atypical and anaplastic lesions. , Finally, a controversial issue in treatment planning is the so-called “radiation-induced meningiomas.” , These tumors usually appear at variable intervals from radiation exposure, depending on the dose and timing. They are often deep seated, multifocal in 4.6% to 18.7% of the cases. Surgical resection is considered to be the best therapeutic option. However, when dealing with frail patients or with particularly crucial locations, radical surgery may be too risky, opening the question on the SRS alternative. The published literature is rather scanty. , Eligibility criteria (limited volume, well-defined imaging, etc.) adopted in these cases do not differ from standard protocols. Clinical-radiologic results—particularly the LTC (75% to 78%) and the 5-year PFS (similar)—are satisfactory, and only slightly inferior to the published data regarding regular MNs. Morbidity remains low (5%) with no reported cases of newcarcinogenesis. ,
Radiosurgery, like most radiation treatments, results in the formation of free radicals as electrons are freed from their atoms. Their main biological effect occurs at the DNA level: The transfer of energy results in breaks in the DNA strands (direct effect). Additional radiation damage to the DNA is mediated through reactive species of water (indirect effect). Whenever single-strand breaks occur, the aberrations are of little eventual consequence, because the breaks are easily repaired. To the contrary, double-strand breaks or chromosome breaks with the “sticky ends” rearranging and rejoining in grossly distorted, nonviable formations can lead to lethal aberrations (mutagenesis) and/or cell death. The number of lethal aberrations and subsequent killed cells is closely related to several conditioning factors: the specific oncotype and a complex series of cellular parameters (“alpha-beta ratio,” superoxide-enzyme characterization, etc.) defining the specific radiosensitivity, the radiation dose, the TV, and the microscopic model of energy deposition. On the basis of these features, MNs mostly belong to relatively radiosensitive, late-responding tissues.
Effective dosages are in the lower range, not far from normal-cell thresholds, while the time interval for the effect is close to the maximum in vivo doubling time. Moreover, all kinds of ionizing radiations are currently identified, not only according to dose level but also to characteristic pattern of energy transfer and consequent relative biological effectiveness (RBE). Linear energy transfer (LET) is defined as the average energy that is locally delivered by a particle in an absorbing medium divided by unit length of the crossed distance. Larger particles are correlated with higher LET values, with increasing ionization density and greater RBE. Some of the main reactive molecules produced by radiation beams (i.e., ion pairs and free radicals) are superoxide compounds with unpaired valence electrons, which break DNA-protein chemical bonds. Therefore, in most cases the permanent effect of this free radical-mediated injury is dependent on the presence of oxygen.
When tumor cells rapidly divide, they outgrow their vascular supply and become chronically hypoxic. With increased LET beams, this close dependence on oxygenation for biological effect decreases. By contrast, low-LET sources such as x-rays and gamma-rays rely significantly on local oxygen levels for their biological effect. Hence, the importance sometimes associated with the radiosurgically typical dose inhomogeneity that may compensate for this disadvantage is the hot spot in the “core” of a tumor, which may be desirable for several reasons. First, it offsets the relative protection offered by the poor oxygenation of the tumor core; second, it may increase the cell kill around the hot spot due to the “penumbra effect.” Fractionated radiation results in the distribution of lethal and sublethal effects within a wider targeted field. By contrast, the typically small targeted size and sharp dose fall-off of radiosurgery allows the delivery of high radiation levels to a limited area.
In SRS, the use of a single dose results in a higher incidence of lethal damage to cells, enhancing the biological efficacy of the beam. Additional factors may explain the spectrum of responses: (1) rate of cell proliferation, resulting in increased sensitivity of endothelial, glial, and subependymal plate cells; (2) vascular obliteration also seems to play a role in the death of tumor cells; and (3) due to the steep dose gradient at the margin of the target volume, normal tissue is exposed to lower levels than the target periphery, and this explains why SLD is of little concern.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here