Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Staphylococci are well adapted as commensals and as pathogens. Coagulase-negative species constitute a significant proportion of the normal human cutaneous microbiome. Staphylococcus aureus , a coagulase-positive species, is a nasopharyngeal colonizer in a third of individuals, most of whom will not become infected. As a pathogen, S. aureus infections range in severity from relatively trivial skin infections to lethal invasive disease. Coagulase-negative species, which are intrinsically less virulent and less invasive than S. aureus , nevertheless are responsible for one in four health care–associated infections, particularly those involving indwelling medical devices. The increasing prevalence of antibiotic-resistant strains of staphylococci has a profound impact on therapy.
The genus Staphylococcus consists of more than 30 distinct species. These organisms have coevolved as normal flora of mammals and birds. These gram-positive spherical cells (i.e., cocci) are 0.5 to 1.5 µm in diameter and divide in multiple orthogonal planes to form clusters resembling grapes when viewed under the microscope. Staphylococci are nonmotile, nonsporulating, hardy organisms that are resistant to desiccation, extremes of pH, and high salt concentrations. Staphylococci can grow under both aerobic and anaerobic conditions. The ability of staphylococci to produce catalase, which is an enzyme that degrades hydrogen peroxide into water and oxygen, definitively distinguishes them biochemically from streptococci and enterococci. The coagulase test is the basis for differentiating S. aureus from the numerous other nonpathogenic, coagulase-negative species. Coagulase is a secreted protein that, in the presence of a prothrombin-like plasma protein, converts fibrinogen to fibrin, thereby forming a clot. S. aureus produces a variety of other surface proteins (e.g., protein A) that differentiate it from other species.
The staphylococcal chromosome is circular. Approximately 75% of the genes in a given strain constitute a core genome. The remaining 25% contain species-defining elements and mobile genetic elements that have been acquired by horizontal gene transfer and that shape diversity within the species. The S. aureus genome is abundant in genes encoding toxins, superantigens, and adhesins, whereas coagulase-negative species contain few adhesin and no toxin or superantigen genes. Genetically, S. aureus is sufficiently uniform to classify it as a single species; greater diversity among coagulase-negative species merits their classification as distinct species.
S. aureus is maintained in the human population primarily by asymptomatic colonization of the anterior nares, oropharynx, and other moist areas of the body, including the groin, perineum, and perianal area. Infants become colonized by strains from their mothers within weeks of birth. Carriage rates are higher in children than in adults. Higher-than-average S. aureus carriage rates are associated with atopic dermatitis, eczema, chronic skin ulcers, and other acute and chronic skin conditions; insulin-dependent diabetes; dialysis; human immunodeficiency virus infection; and injection drug use. Carriers have a several-fold higher risk for developing an S. aureus infection compared with noncarriers. The principal mode of transmission of S. aureus is direct contact with an infected individual or an asymptomatic carrier, probably through transient hand carriage. S. aureus may also contaminate environmental surfaces, where it can persist for days. The role of environmental contamination in transmission is not well defined, but it may be important if heavily contaminated surfaces or materials are contacted. Droplet and aerosol transmission of S. aureus plays little, if any, role.
S. aureus is responsible for millions of infections in the United States each year, most of which are community-acquired skin infections. Approximately 5 to 10% of S. aureus infections are invasive, three fourths of which are associated with bacteremia. S. aureus also causes hundreds of thousands of health care–associated , ( Chapter 261 ) infections each year, about half of which are caused by methicillin-resistant S. aureus (MRSA) in the United States. MRSA strains are now prevalent in the community, but the frequency of and mortality of MRSA have been declining. Although MRSA is now disseminated globally, there is marked geographic variation in MRSA burden that is due mainly to differences in local infection control practices and to pathogen-specific characteristics of the indigenously circulating clones. Community-associated strains circulate in both the community and the health care environments, whereas health care–associated strains are largely restricted to the health care environment. The strains are distinct from each other in several ways ( E-Table 267-1 ). For example, Panton-Valentine leukocidin, which is frequently present in community-associated strains, causes distinct clinical syndromes, such as primary skin abscesses and necrotizing community-acquired pneumonia.
| PROPERTY | COMMUNITY-MRSA | HOSPITAL-MRSA |
|---|---|---|
| Methicillin-resistance gene cassette | SCCmecIV ∗ | SCCmecI, II, III |
| Common genotypes † | USA300 (ST8) ST59, ST80 |
USA100 (ST5) USA500 (ST8) USA800 (ST5) |
| Panton-Valentine leukocidin | Present in ≥75% | Present in <5% |
| Antimicrobial resistances | β-Lactams, macrolides, fluoroquinolones | β-Lactams, macrolides, clindamycin, tetracycline, fluoroquinolones, aminoglycosides |
| Doubling time ‡ | 30 minutes | 45-60 minutes |
| agr mutants § | Uncommon | Common |
∗ Staphylococcal chromosomal cassette type; this element contains the methicillin resistance gene mecA; numerous other variants occur as well.
† Pulsed-field gel electrophoresis type with the multilocus sequence type (ST) in parentheses.
S. aureus produces well above 50 virulence factors, including adhesins, toxins, enzymes, surface-bound proteins, and capsule polysaccharides ( E-Table 267-2 ). Genes encoding virulence factors may be located on the chromosome as part of the core genome, within mobile genetic elements (or their remnants, including bacteriophages, pathogenicity islands, and cassettes), or on plasmids. α-Toxin, Panton-Valentine leukocidin, and phenol-soluble modulins, all of which provoke potentially deleterious host inflammatory responses and cause host cells to lyse, appear to be important virulence factors that mediate the severity of disease, especially in community-associated MRSA strains. Protein A, which is a B-cell superantigen that promiscuously triggers B-cell proliferation as well as supraclonal expansion and apoptosis, interferes with the host’s antibody-mediated adaptive immunity.
Virulence factors promote binding to host tissues; allow the organism to evade, circumvent, or disrupt host immune responses; and facilitate cell injury and tissue invasion. Variability in both the presence of virulence determinants and their expression among strains allows extreme diversity among clinical isolates, remarkable adaptability and versatility of S. aureus as a pathogen, and a wide spectrum of clinical syndromes. An extensive network of two component response systems, DNA-binding proteins and regulatory RNAs, controls the expression of virulence (and other) factors in response to environmental conditions. Principal among these factors is the accessory gene regulator agr , which is a two-component quorum sensing and global gene regulator that coordinately controls the expression of numerous surface and secreted proteins. Mutations in agr are associated with loss of virulence.
Biofilm formation, a feature of coagulase-negative infections in particular, occurs in the presence of foreign material, such as vascular catheters or implanted devices. Biofilms are a complex network of extracellular polysaccharides, DNA, and protein in which bacterial cells become embedded, thereby rendering them inaccessible to clearance by host defense mechanisms. Organisms within biofilms tend to be metabolically inactive and show tolerance with reduced susceptibility to the lethal actions of antimicrobial agents.
| ACTIVITY | GENES | PROTEIN OR MOLECULE |
|---|---|---|
| Inhibition of antimicrobial peptides | icaA, icaD, icaB, icaC, icaR | Polysaccharide intercellular adhesin (PIA) |
| isdA, isdB | Iron-regulated surface determinants of S. aureus (IsdA and IsdB) | |
| mprF | Multiple peptide resistance factor (MprF) | |
| Sak | Staphylokinase | |
| Inhibition of chemotaxis | chp | Chemotaxis inhibitory protein of S. aureus (CHIPS) |
| ecb | Extracellular complement-binding protein (Ecb) | |
| efb | Extracellular fibrinogen-binding protein (Efb; inhibitor of C5a generation) | |
| scn | Staphylococcal inhibitor of complement (SCIN) | |
| Inhibition of oxygen-mediated bacterial killing | crtM, crtN | Carotenoid pigment, staphyloxanthin ( S. aureus golden pigment; resistance to reactive oxygen species) |
| spn | Staphylococcal peroxidase inhibitor (resistance to reactive oxygen species) | |
| isdA, isdB | Iron-regulated surface determinants of S. aureus , IsdA, and IsdB (promote resistance to neutrophil reactive oxygen species) | |
| sodA, sodM | Superoxide dismutase, SodA, SodM (promotes resistance to neutrophil reactive oxygen species) | |
| Inhibition of neutrophil function | cap5 or cap8 | Capsular polysaccharide (inhibits phagocytosis) |
| clfA | Clumping factor A (ClfA; inhibits phagocytosis) | |
| eap | Extracellular adherence protein (Eap; inhibits leukocyte adhesion) | |
| hlgA, hlgB, hlgC | Gamma hemolysin subunits A, B, and C; HlgA, HlgB, HlgC (causes cell lysis) | |
| lukAB, lukED lukSF-PV ( Panton-Valentine) | Leukocidins (cause cell lysis) | |
| psm | Phenol-soluble modulin-like peptides (PSMs; causes cell lysis) | |
| Perturbation of T-cell function | sea, seb, sec n , sed, see, seg, seh, sei, sej, sek, sel, sep | Staphylococcal enterotoxins: SEA, SEB, SEC n , SED, SEE, SEG, SEH, SEI, SEJ, SEK, SEL, SEP (superantigens) |
| tst | Toxic shock syndrome toxin 1 (TSST-1; superantigen) | |
| Perturbation of B-cell function | spa | Protein A (B-cell superantigen) |
| Adhesion | cna | Collagen-binding protein |
| fnA, fnB | Fibronectin-binding proteins A and B | |
| clfA, clfB | Clumping factors A and B (binds platelets and fibrinogen) |
The pathogenesis of invasive S. aureus disease usually proceeds from local tissue invasion to systemic infection. This process is dependent on the local and systemic effects of many virulence factors acting in combination. The hallmark lesion in tissues is the abscess, which is a focal collection of pus (liquefied and necrotic host tissue, blood, inflammatory cells, DNA, and cellular debris) and bacterial cells surrounded by an ill-defined layer of edematous and inflamed tissue infiltrated by acute and chronic inflammatory cells.
Host defenses against S. aureus tissue infection primarily consist of an intact, normal skin barrier and the innate immune system. Conditions in which these defenses are breached or impaired are associated with increased risk of S. aureus infection. Among these conditions are injection drug use ( Chapter 365 ), presence of vascular access devices, burns ( Chapter 97 ), chronic skin diseases, use of systemic steroids, traumatic wounds, minor skin abrasions or trauma, surgical procedures, insulin-dependent and non–insulin-dependent diabetes ( Chapter 210 ), peritoneal dialysis ( Chapter 117 ), hemodialysis ( Chapter 117 ), subcutaneous and intramuscular injections, acupuncture, prosthetic implants, congenital or acquired disorders of neutrophils (e.g., chronic granulomatous disease [ Chapter 153 ]), and advanced age. If the cutaneous barrier is breached, the next line of defense is the innate immune system ( Chapter 35 ). Neutrophils recruited to the site of infection ingest and kill staphylococci.
Staphylococci elaborate numerous virulence factors that specifically thwart each step of the host response. If large numbers of organisms are present, the host response is overwhelmed, infection is not contained, and dissemination occurs. Endothelial cell injury and invasion can also occur. Intracellular organisms and small colony variants within phagocytes and endothelial cells may play a role in relapse and persistent bacteremia by acting as a protected sanctuary against the innate immune host response and antimicrobial therapy. High tissue burdens of organisms and bacteremia are usually, but not always, accompanied by fever, tachycardia, and other signs of the systemic inflammatory response syndrome, including frank septic shock ( Chapter 94 ).
Toxinoses are a separate category of disease for which a toxin is both necessary and sufficient. The three toxin-mediated syndromes, which can occur in the absence of invasive disease, are staphylococcal food poisoning, staphylococcal toxic shock syndrome, and staphylococcal scalded skin syndrome. Staphylococcal food poisoning is caused by the ingestion of a preformed heat-stable enterotoxin. The emetogenic activity of enterotoxin is mediated by the intestinal release of 5-hydroxytryptamine and the stimulation of receptors present on afferent vagal neurons. Toxic shock syndrome is caused by a specific toxin, TSST-1, or other staphylococcal enterotoxins acting as superantigens that bind to major histocompatibility complex class II molecules of antigen-presenting cells and T-cell receptors, thereby stimulating the massive release of cytokines from T cells and resulting in shock and death. Staphylococcal scalded skin syndrome and bullous impetigo are caused by either of two exfoliative toxins, A or B. These toxins are serine proteases that specifically cleave desmoglein 1, which is a desmosomal protein that anchors the overlying superficial epidermis to the stratum granulosum.
Skin and soft tissue infections are by far the most common infections caused by S. aureus; millions of cases occur annually in the United States ( Chapter 408 ). This heterogeneous group of skin diseases includes impetigo ( Fig. 408-1 ), folliculitis, furuncle ( Fig. 408-2 ), abscess, erysipelas ( Fig. 408-4 ), cellulitis ( Fig. 408-3 ), mastitis (cellulitis of the breast), necrotizing fasciitis, and wound infections. Community-acquired MRSA strains encoding Panton-Valentin leukocidin have been associated with an increased rate of primary skin and soft tissue infections in the United States, and recurrent infections occur in about 20% of individuals.
Impetigo, folliculitis, and furuncle ( Chapter 408 ) are superficial infections; fever and other systemic signs of infection are not present. Impetigo, which is a focal infection of the epidermis, occurs most commonly in children (see Fig. 408-1 ). The typical lesion, which may be multiple or in clusters, is about 1 cm in diameter, with erythema surrounding a bulla or bullae (caused by the production of exfoliative toxin) that contains cloudy fluid or has a crusty or scabbed-over appearance. Folliculitis is a superficial infection with tender, erythematous, maculopapular or pustular lesions centered around hair follicles. A furuncle is simply a boil, a painful focal collection of pus with surrounding erythema measuring 1 to 2 cm or more in diameter that extends through the dermis into the subcutaneous tissue (see Fig. 408-2 ). The distinction between a furuncle and an abscess is somewhat arbitrary. Abscesses tend to be larger and deeper and may be associated with systemic signs of infection and bacteremia. Furuncles may extend to fascia or deeper tissues and coalesce into carbuncles.
Erysipelas (see Fig. 408-4 ) and cellulitis, which are similar in appearance, are painful, warm, indurated, erythematous, nonlocalized infections that may be accompanied by lymphangitis. Cellulitis extends into the dermis and subcutaneous fat; erysipelas is more superficial. Cellulitis due to streptococci ( Chapter 269 ) cannot reliably be distinguished from that caused by S. aureus owing to their similar appearance, although associated purulence suggests staphylococcal infection.
Necrotizing fasciitis is an infection of the deep layers of skin and subcutaneous tissues, extending to muscle and along fascial planes. It is associated with systemic toxicity, leukocytosis, and severe pain often out of proportion to the physical findings. The overlying skin may appear to be uninvolved, belying the serious nature of this infection. Necrotizing fasciitis, which is more typically caused by group A streptococci ( Chapter 269 ) or mixed aerobic and anaerobic organisms, has been associated with community MRSA infection.
Pyomyositis (also termed tropical myositis) is a deep abscess or multiple abscesses within skeletal muscle. S. aureus is the most common cause. The patient presents with fever, pain, swelling, and induration that can be felt on deep palpation. The overlying skin and soft tissue may appear normal. There is often a history of trauma to the infected area. Although pyomyositis can occur in otherwise normal persons, acquired immunodeficiency syndrome ( Chapter 358 ) and other immunocompromising conditions are predisposing factors. This infection is thought to occur as a consequence of metastatic seeding from a subclinical bacteremia, although blood cultures may not be positive at the time of diagnosis. Computed tomography or magnetic resonance imaging should be obtained to identify lesions. The diagnosis is established by culture of pus collected by needle aspirate.
Secondary S. aureus infection due to skin injury is unlinked to Panton-Valentin leukocidin and occurs in a variety of situations such as diabetes, underlying skin diseases, and surgery. S. aureus causes 30% of surgical site infections overall in the United States. These infections occur at the site of incision and are associated with significant morbidity, particularly in the setting of a prosthetic device. Signs and symptoms are fever accompanied by erythema, edema, induration, drainage, pain, and tenderness at the surgical site.
Bacteremia is present in approximately 75% of cases of invasive infection. The most common sources of bacteremia are skin and soft tissue infections, central venous catheters and other intravascular devices, bone and joint infections, pneumonia, and endocarditis. Bacteremia can originate from any source, which may not be obvious in 25% of cases. Once organisms have invaded the bloodstream they can disseminate widely throughout the body, thereby establishing multiple metastatic sites of infection and perpetuating the bacteremia. Fever is usually, but not always, present. Patients with S. aureus bacteremia have high rates of organ failure and septic shock ( Chapter 94 ).
S. aureus , which is the leading cause of both native valve and prosthetic valve endocarditis ( Chapter 61 ), accounts for approximately 30% or more of all cases. Endocarditis may be community-acquired or, increasingly, health care–associated. Risk factors include injection drug use ( Chapter 365 ), diabetes mellitus ( Chapter 210 ), hemodialysis ( Chapter 117 ), presence of a prosthetic valve ( Chapter 60 ) or other implantable intracardiac device, and recent hospitalization. Endocarditis may present as an acute febrile illness with high fever that develops over a few days. The patient may appear toxic and septic, but some patients have surprisingly few acute symptoms and complain only of protean symptoms such as shortness of breath, malaise, and weakness. The intracardiac source of infection initially may not be evident because a pathologic murmur may not be heard when the patient first presents. A quarter or more of patients have a coincident infection of bone, joint, or skin and soft tissue. The aortic and mitral valves are most commonly involved in native valve infection, but the tricuspid valve can be involved, especially in persons who inject drugs. Systemic embolization to the brain, kidneys, spleen, gut, or other large vessels is clinically evident in about one third of cases. Peripheral manifestations, including Roth spots ( Fig. 391-26 ), Osler nodes ( Fig. 61-2 ), Janeway lesions, splinter hemorrhages ( Fig. 39-10 ), and petechiae ( Fig. 61-1 ), occur with a similar frequency.
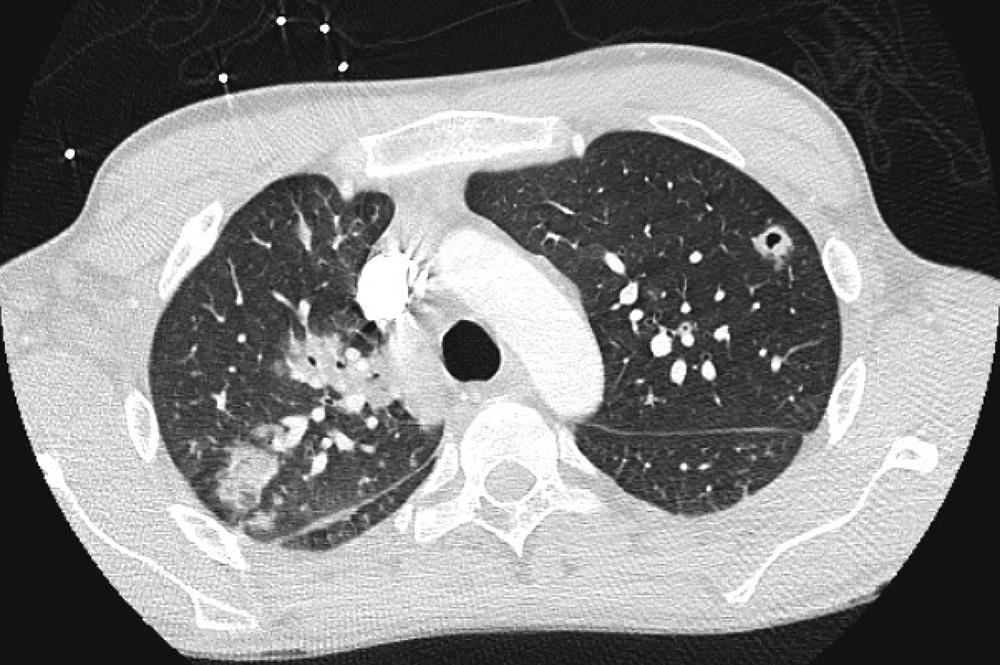
Native valve endocarditis in persons who inject drugs involves the tricuspid valve in approximately three quarters of cases. Patients typically have fever, cough, hemoptysis, and pleuritic chest pain as a consequence of hematogenous seeding of the lung and septic emboli from the valve. The chest radiograph may show pulmonary infiltrates, signs of consolidation, or pleural effusion. Multiple, often peripheral, nodular pulmonary infiltrates with cavitation are hallmark features of septic embolization ( Fig. 267-1 ).
S. aureus is the most common cause of purulent pericarditis ( Chapter 62 ) after cardiac surgery in adults. It may occur by contamination at the time of surgery; by bacteremic seeding from another site of infection; as a complication of endocarditis, paravalvular abscess, or myocardial abscess; or by direct extension of infection from pneumonia, lung abscess, or empyema. The presentation is that of acute pericarditis ( Chapter 62 ), with fever and severe chest pain, tachycardia, and hemodynamic instability. The clinical course may be extremely rapid, terminating in septic shock or cardiac tamponade.
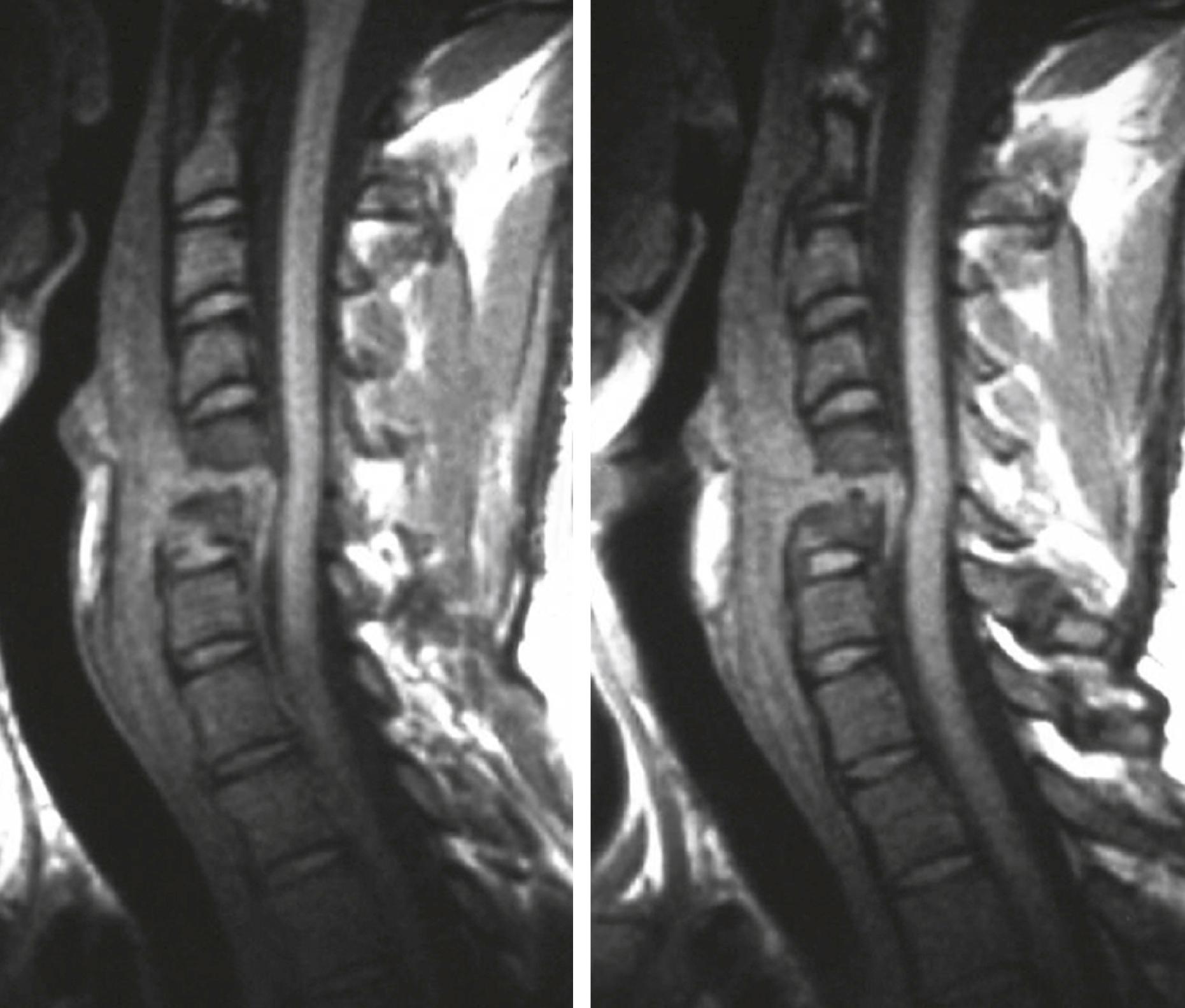
S. aureus is the most common cause of osteomyelitis, both acute and chronic ( Chapter 251 ). Disease arises from hematogenous dissemination or contiguous spread. Hematogenous infection is often monomicrobial. Contiguous infection is often polymicrobial and results from trauma, inoculation during surgery, or soft tissue infection. Acute osteomyelitis—defined as an initial episode with a clinical course of days to weeks, but not months—is manifested with fever and pain at the site of infection. Vertebrae (most commonly lumbar and cervical) are more commonly infected in adults, but adults also can have long bone osteomyelitis, usually from a contiguous focus of infection or at a site of fracture, prior trauma, or orthopedic hardware. Vertebral osteomyelitis is frequently accompanied by paravertebral or epidural abscess ( Fig. 267-2 ; Chapter 382 ). Back pain accompanied by signs of cord compression, such as radicular pain, sensory loss, lower extremity weakness, urinary retention, and bowel or bladder incontinence, is an emergency.
S. aureus is the most common cause of septic arthritis ( Chapter 251 ), usually as a consequence of bacteremic seeding, trauma, or a surgical procedure. Risk factors include diabetes, recent joint surgery or joint prosthesis ( Chapter 255 ), and rheumatoid arthritis ( Chapter 243 ). Cardinal features are joint pain, history of joint swelling, and fever. Hip, knee, ankle, and wrist are most commonly affected. S. aureus also has a predilection for infecting the sternoclavicular, sacroiliac, and symphysis pubis joints. Multiple joints are involved in 5% of cases.
S. aureus is an uncommon cause of community-acquired central nervous system infections, such as meningitis ( Chapter 381 ), primary brain abscess ( Chapter 382 ), or subdural empyema. These infections are often associated with endocarditis or a contiguous focus of infection, such as cavernous sinus thrombosis. S. aureus is an important cause of nosocomial meningitis after head trauma, craniotomy, or implantation of intraventricular or extraventricular catheters.
S. aureus is an uncommon cause of community-acquired pneumonia ( Chapter 85 ), accounting for only 1 to 5% of cases but with geographic and seasonal (i.e., increased during influenza season) variability. S. aureus pneumonia is usually a fulminant necrotizing pneumonia accompanied by evidence of cavitation on chest radiographs.
Hospital-acquired and ventilator-associated pneumonias ( Chapter 91 ) often are caused by S. aureus and community-acquired or hospital-acquired MRSA strains. Patients with traumatic or medical brain injury (e.g., stroke, hemorrhage) are particularly prone to early colonization and subsequent pneumonia by this pathogen.
Lung abscess ( Chapter 78 ) and pleural empyemas ( Chapter 86 ) most commonly are caused by oral anaerobic bacteria but are occasionally caused by S. aureus . The clinical course may be subacute or even indolent. Empyema occurs as a complication of prior chest tube placement, surgery, trauma, staphylococcal pneumonia, or tricuspid valve endocarditis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here