Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Stereotactic ablative radiotherapy (SABR) is recommended in treatment guidelines as the nonoperative therapy of choice for early-stage nonsmall cell lung cancer (NSCLC).
SABR can be adequately performed using either traditional linear accelerators equipped with suitable image-guidance technology or linear accelerators specifically adapted for SABR and using dedicated delivery systems.
Clinical assessment, staging of disease, and multidisciplinary discussion should be based on published guidelines for early-stage NSCLC.
Guideline-specified nodal staging should be performed before SABR, as nodal regions are not radiated.
SABR dose constraints, which are based on the constraints used in the RTOG 0618, 0813, and 0915 SABR trials, are summarized in the current National Comprehensive Cancer Network guidelines.
Results of SABR have been consistent, with both the high local control rates and low toxicity found in prospective clinical trials also being reported in large single-institution series and pooled multi-institutional analyses.
A widely used working definition for so-called central lung tumors is tumors located either adjacent to the proximal bronchial tree or located 1 cm or less from the heart or mediastinum.
Three distinct cohorts of patients with oligometastases can be identified: patients with oligometastatic disease at the time of diagnosis, patients with oligoprogressive disease after cytoreductive therapy, and patients with oligorecurrent disease after curative local–regional therapy.
Results appear generalizable across centers when current SABR guidelines are followed.
Changes in the epidemiology of lung cancer are particularly relevant for the field of radiation oncology. Globally, lung cancer represents the leading cause of cancer death in men and is the second leading cause in women. A key challenge is that older patients are the fastest growing population—nearly 25% of patients are 75 years of age or older. Approximately 20% of all patients diagnosed with nonsmall cell lung cancer (NSCLC) have stage I disease, and surgery is currently the guideline-specified treatment for fit patients who are willing to accept the procedure-related risks. However, a population study from the Netherlands showed that, among patients with stage I disease, resection was done in 49% of patients 75 years of age or older compared with 91% of patients who were 60 years of age or younger. Similarly, in an analysis of data in the Surveillance, Epidemiology and End Results (SEER)-Medicare database for the period 1998 to 2007, the percentage of patients who had a surgical procedure decreased over time (75.2% in 1998 vs. 67.3% in 2007) and the percentage of patients who did not receive any local treatment increased (14.6% in 1998 vs. 18.3% in 2007). These findings were explained by the increase in the proportion of patients 85 years of age or older (from 4.5% to 9%), as well as an increase in patients with three or more comorbidities (from 15% to 30%) during the study period. The reluctance to operate on older patients is mainly due to their frailty, as comorbidities are more common in the older population. Although severe comorbidity has the greatest impact on outcomes during the first month following surgery, the increased death rate associated with impaired performance status persists with longer follow-up.
The apparent reluctance of clinicians to refer older patients for conventional radiotherapy was partly due to the 30 or more once-daily treatments that were typically required, which is cumbersome for frail older patients. In the era before stereotactic ablative radiotherapy (SABR), outcomes of radiotherapy in early-stage NSCLC were poor despite treatment with doses ranging from 60 to 66 Gy. Local tumor recurrences occurred in approximately 40% of patients, with an overall survival rate at 3 years of approximately 30%. Furthermore, a modest 6-month increase in median survival was reported in an analysis of 2010 SEER data when older radiotherapy techniques were applied.
In the mid-1990s, the principles of cranial stereotactic radiotherapy (or radiosurgery) were transferred to extracranial sites by work pioneered at the Karolinska Hospital in Sweden. Stereotactic body radiation therapy (SBRT) and SABR are equivalent terms for this technique in the body. This stereotactic approach was further developed by centers in Japan and Germany. In subsequent years, encouraging results from both prospective and retrospective studies resulted in rapid adoption of SABR for early-stage NSCLC. A national survey in the United States found that 57% of all responding physicians used SABR for the treatment of lung cancer in 2010, whereas a similar survey in Italy found that 41% of responding radiotherapy centers used SABR in 2009. At present, SABR is recommended in treatment guidelines as the nonoperative therapy of choice for early-stage NSCLC.
Guidelines for SABR have been released by several professional groups: the American Association of Physics in Medicine Task Group 101, the American Society for Therapeutic Radiology and Oncology and the American College of Radiology, the Canadian Association of Radiation Oncology-Stereotactic Body Radiotherapy, the National Radiotherapy Implementation Group of the UK, and the working group Stereotactic Radiotherapy of the Germany Society of Radiation Oncology. Current definitions of SABR adhere to the following criteria: a high degree of accuracy, use of high doses of radiation, and delivery of radiation in one or a few treatment fractions to an extracranial target.
The rationale of SABR for early-stage NSCLC is that higher radiation doses are more effective for local control of the tumor, which translates into longer overall survival. SABR differs from conventional radiotherapy in that SABR involves delivery of very high radiation doses only to the visible tumor, with planning and delivery of treatment optimized to ensure safety margins of a few millimeters. In addition, radiation doses to surrounding normal organs are often lower than with conventional techniques. As a result, local tumor control rates of 90% and higher can be achieved and rates of severe toxicity are typically below 10%.
SABR for lung cancer is a multidisciplinary endeavor, involving all disciplines related to the diagnosis and treatment of the disease, but particularly specialists working on a radiotherapy team. Accuracy of delivery is achieved using an optimized workflow and appropriate quality assurance procedures, including development of written protocols, which is an essential component of the process.
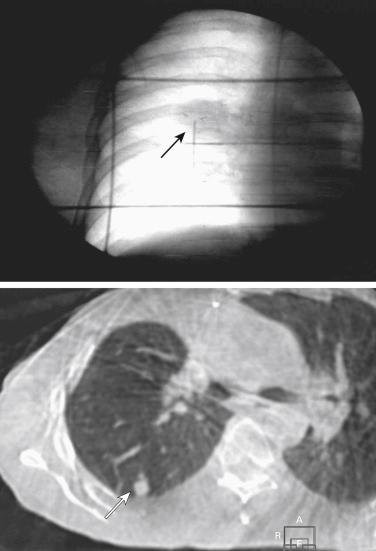
SABR can be adequately performed using either traditional linear accelerators equipped with suitable image-guidance technology or linear accelerators specifically adapted for SABR and using dedicated delivery systems. The SABR procedure was initially defined by the use of frame-based patient set-up, the goal of which was stable and reproducible patient positioning. However, frame-based stereotactic patient set-up has been replaced by image guidance, which makes the term stereotactic somewhat misleading. With non–frame-based patient set-up, external stereotactic coordinates are replaced by visualization of a patient’s anatomy using images acquired on-table and subsequently compared with pretreatment planning images. Soft-tissue images of the tumor itself, or of an implanted fiducial marker, can be used for setting up the target ( Fig. 37.1 ).
A dedicated SABR team should consist of radiation oncologists, medical physicists, and technicians (e.g., radiographers, radiation therapists), all of whom should have attended appropriate training courses organized by professional bodies and/or industry in accordance with the above-mentioned guidelines. Written treatment protocols that are consistent with national regulations, institution-specific equipment, and training and education of the individual radiotherapy team members should be available. SABR requires additional and more frequent physical quality assurance: verification and quality assurance of the entire SABR treatment chain is mandatory, and end-to-end tests for overall uncertainty estimation are recommended. It is paramount to verify that the radiation isocenter coincides with the mechanical isocenter, including couch rotation, room lasers, and, especially, the imaging isocenter.
Clinical assessment, staging of disease, and multidisciplinary discussion should be based on published guidelines for early-stage NSCLC. Unlike the toxicity found following surgery in older individuals, no increased toxicity or treatment-related mortality has been noted when SABR has been extensively applied to patients between 75 and 80 years old. The poorer overall survival rates reported in this older population after SABR are related to comorbidities, and the number of comorbidities predicts overall survival after both SABR and surgery. SABR-related toxicity is also not increased in patients with very poor pretreatment pulmonary function, and the available data suggest that SABR should be offered to all patients regardless of age and preexisting pulmonary comorbidities, unless their predicted survival time is short.
Diagnosis and confirmation of NSCLC based on tissue biopsy is recommended before starting any local treatment for early-stage NSCLC. However, obtaining a histologic diagnosis may not be possible for peripheral lung lesions or may pose a high risk of toxicity in a patient group with considerable medical and/or pulmonary comorbidities. In the latter case, radiographic criteria of malignancy are used to establish a diagnosis. Models that predict the probability of malignancy in solitary pulmonary nodules based on both clinical and radiographic characteristics have been described and validated. It is important to note that these criteria may not hold true in geographic regions with a high incidence of infectious and/or granulomatous lung diseases. Therefore, current guidelines state that any treatment for a possible early-stage NSCLC without a pathologic diagnosis should proceed only after assessment by an experienced multidisciplinary tumor board. If the clinical and radiographic findings are inconclusive, repeated imaging to evaluate the growth pattern is an option for some patients, but careful follow-up is required because patients with malignancy are at risk for early disease progression.
Guideline-specified nodal staging should be performed before SABR, as nodal regions are not radiated. Staging with 18 F-2-deoxy- d -glucose-positron emission tomography (FDG-PET) is essential because of its higher diagnostic accuracy for the detection of node metastases (negative predictive value, 90%) and also because unsuspected distant metastases and second primary tumors can be excluded. In the event of pathologic FDG uptake in regional lymph nodes, further evaluation by endobronchial ultrasound or endoscopic ultrasound is recommended and, if the findings are inconclusive, a mediastinoscopy may be necessary. Staging with PET–computed tomography (CT) should ideally be performed within 6 to 8 weeks before SABR is given because of the risk of disease progression in the interim.
All imaging should be acquired in the treatment position, with standard practice dictating that planning CT images encompass the entire lung volume, and a slice thickness of 2 mm to 3 mm is used. Using intravenous contrast medium may improve the delineation of centrally located primary tumors. As conventional three-dimensional (3-D) CT risk introduces artifacts and systematic errors, four-dimensional (4-D) CT, also known as respiration-correlated CT, is the recommended technique for SABR planning. Although a single 4-D CT planning image provides only a snapshot of a patient’s breathing pattern, several studies have demonstrated that the motion pattern and amplitude are stable over time, making routine repeated 4-D CT imaging unnecessary. The use of FDG-PET alone is not appropriate for a reliable assessment of target motion in SABR planning.
Gross tumor volume is determined on the basis of CT findings in the lung and soft-tissue window. Current guidelines do not recommend the use of clinical target volume margins when SABR is delivered, as the high radiation doses combined with rather flat dose profiles in pulmonary tissue of low electron density result in sufficient coverage of potential microscopic disease extension. Integration of breathing-induced target motion into the target volume concept will ensure patient-tailored tumor targeting, with clinical implementation dependent on the chosen motion management strategy. Several different approaches are used in routine clinical practice. Continuous radiation in free breathing is performed using the internal target volume concept, the mean target position concept, or real-time tumor tracking. Tumor tracking is possible using dedicated robotic delivery machines, by dynamic multileaf tracking, a gimbaled multileaf collimator, or a dynamic treatment couch. Noncontinuous radiation of the tumor in a reproducible position is performed using gated beam delivery in predefined phases of the breathing cycle, in voluntary breath-hold, or in breath-hold using the active breathing coordinator.
It is important to emphasize that active motion management strategies, such as gating and tracking, require continuous intrafractional monitoring; however, continuous intrafractional monitoring is less critical for passive strategies, such as the internal target volume or mean tumor position concept. Although patient-specific motion management is strongly recommended, data from the available prospective trials did not involve the use of advanced motion management strategies. Of the individualized 4-D motion management strategies used, resulting field sizes are largest for the internal target volume concept; however, this motion management strategy is straightforward to implement and ensures adequate target coverage. Even if all uncertainties in treatment planning and delivery are minimized by currently available technologies, residual errors still remain and require minimum planning target volume (PTV) margins of about 5 mm.
Dose prescription and reporting should comply with the International Committee on Radiation Units and Measurements Report on Prescribing, Recording, and Reporting Intensity-Modulated Photon-Beam Therapy as closely as possible, but historical practice and experiences need to be considered as well. Most prospective and retrospective studies used inhomogeneous dose distributions within the PTV, with maximum doses ranging between 105% and 150% of the prescribed dose. Inhomogeneous dose distributions offer the opportunity to deliver an extra dose to the center of the PTV, where the (potentially hypoxic) macroscopic tumor is located, without increased doses to the peripheral normal tissue.
As a result of large differences in single-fraction and total doses between SABR studies, a comparison of physical doses is less meaningful. The linear quadratic model has been widely used for modeling of SABR outcomes data, but it has not been validated for very high single doses. Despite uncertainty surrounding the linear quadratic model, several groups have independently demonstrated a clear dose-effect relationship for local tumor control using biologic effective doses (BEDs), with a minimum PTV dose of more than 100 Gy BED (α/β ratio, 10 Gy) required for local tumor control rates of higher than 90%. The current recommended tumor dose for SABR of lung tumors is a minimum of 100 Gy BED, prescribed to the target volume encompassing isodose. A meta-analysis demonstrated a potential detrimental effect of SABR doses exceeding more than 146 Gy BED.
Total doses are typically delivered in one to eight fractions, but insurance reimbursement rules have resulted in a widespread use of five or fewer fractions in the United States. However, use of very high single-fraction doses and total doses (e.g., delivery of three fractions of 20 Gy) can damage normal tissues in or adjacent to the target volume. Consequently, treatment of tumors in proximity to critical normal organs has led to the use of so-called risk-adapted fractionation schemes that deliver the required dose of 100 Gy BED in a larger number of treatment fractions with lower single-fraction doses. Fractionation appears to spare some critical normal organs while ensuring sufficiently high doses to achieve local tumor control.
Fractionation appears to be especially valuable in SABR for centrally located tumors, as it allows for radiobiologic sparing of critical organs such as large bronchi, vessels, heart, and esophagus. High-quality prospective data on the safety and efficacy of SABR for centrally located lesions are limited, but a systematic review of the literature demonstrated local control rates of 85% or greater when the prescribed BED to the tumor was 100 Gy or higher. The overall treatment-related mortality was 2.7%, and when the BED to normal tissue was 210 Gy or lower (α/β ratio, 3 Gy), the rate was 1.0%. Until mature prospective multicenter data become available, a recommended fractionation scheme for experienced centers is 8 × 7.5 Gy, with D max (PTV) of 125%.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here