Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Few hematologic malignancies arise primarily in the spleen; most conditions occurring at this site represent secondary involvement by diseases originating elsewhere in the body. The role of the pathologist in most cases is to confirm the known or suspected diagnosis and to exclude unsuspected pathology. Careful gross evaluation of the organ and optimal tissue fixation are essential for the successful interpretation of splenic pathology. Because of the amount of blood in the spleen, thin sections are particularly important. In addition, care must be exercised in isolating lymph nodes of the splenic hilum. Their examination can provide valuable additional information, particularly in the diagnosis of low-grade B-cell lymphomas. Obtaining adequate clinical information is often critical for the diagnostic characterization of disorders that involve the spleen; this need cannot be overemphasized.
In this chapter, we present a comprehensive account of those aspects of splenic pathology likely to be encountered by diagnostic hematopathologists. We outline principles for a systematic histopathologic analysis that can be applied to achieve a specific diagnosis after the recognition of broad categories of abnormalities affecting individual splenic compartments. To avoid repetition of material covered elsewhere in this text, specific splenic disease entities, such as hepatosplenic T-cell lymphoma and splenic marginal-zone lymphoma, are only mentioned briefly. The reader is directed to the specific chapters covering these entities as well as chapters covering other hematopoietic tumors that may secondarily involve the spleen, such as Hodgkin's lymphoma and the various non-Hodgkin's lymphomas.
The characterization of disorders that involve the spleen can best be understood in light of the structure and function of that organ. The spleen is composed of two anatomically and functionally distinct regions ( Table 60-1 ). The lymphoid tissue of the spleen, called the white pulp, appears grossly as uniformly distributed white nodules. The white pulp is intimately associated with the splenic arterial circulation. The central arteries, which arise from trabecular arteries within the fibrous trabeculae, are surrounded by cylindrical cuffs of lymphocytes called periarteriolar lymphoid sheaths. The periarteriolar lymphoid sheaths contain an admixture of B cells and T cells, with a predominance of CD4-positive T lymphocytes. Periodically, splenic lymphoid follicles (malpighian corpuscles) occur as outgrowths of the periarteriolar lymphoid sheaths. The morphology of the splenic white pulp varies with age and with its functional activity (e.g., presence of antigenic stimulation). Inactive or hypoplastic white pulp, in which no germinal centers are seen, is characteristic of infancy, senescence, and the immunologically unstimulated adult spleen. In the immunologically activated state, the splenic lymphoid follicle shows three distinct zones. The germinal center, structurally similar to germinal centers in other lymphoid organs, is surrounded by a mantle zone. The mantle zone is encased by the outer marginal zone, a cellular layer at the interface between the white and red pulp. The marginal zone, composed of both B cells and T cells, is the site of initial antigen trapping and processing ( Fig. 60-1 ).
| Compartment | Elements | Description |
|---|---|---|
| White pulp | Follicles | |
| Primary | Composed of small nodules of mantle-type B lymphocytes (see below) | |
| Secondary | Composed of a mixture of small, irregular B lymphocytes and large transformed cells, with intermixed dendritic cells and macrophages | |
| Mantle zone | Surrounds the germinal center; composed predominantly of small B lymphocytes with round to irregular nuclei, condensed chromatin, and scant cytoplasm | |
| Periarteriolar lymphoid sheaths | Sheaths of predominantly small T lymphocytes that surround arterioles and arteries; other cells include larger transformed lymphocytes, NK cells, plasma cells, and B cells | |
| Red pulp | Sinusoids | Lined by specialized endothelial cells with macrophage capacity; lack a continuous basal membrane |
| Cords | Lie between the sinusoids; composed of extracellular space and cordal macrophages | |
| Supporting stroma | Capsule and trabecular septa | Paucicellular dense fibrous tissue; thickened in reactive or chronic conditions |
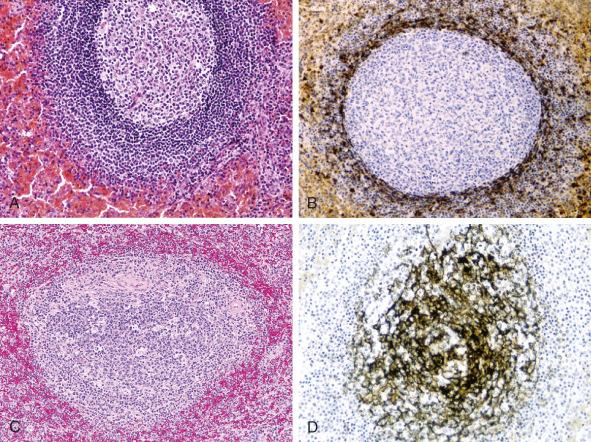
The red pulp of the spleen is composed of splenic vascular sinuses and the cords of Billroth, which are made up of splenic macrophages, scattered cord capillaries, venules, and stromal cells. All these cellular elements linked together, along with a relatively scanty amount of extracellular matrix, are responsible for the peculiar architecture of the red pulp. The splenic vascular sinuses provide the mechanism for filtration of the peripheral blood, one of the important functions of the spleen. The sinus-lining cells, also known as littoral cells, have long cytoplasmic processes that overlap and are closely apposed. However, because no tight junctions are present, circulating blood cells are able to squeeze through the interendothelial spaces and percolate through the cords of Billroth before entering the splenic sinuses and the venous system, thus returning to the systemic circulation. The ability of circulating blood cells to enter the splenic sinuses and subsequently percolate through the cords depends on their deformability. Cells without the ability to deform cannot enter the sinuses and are destroyed in the acidotic, hypoxic environment of the cords of Billroth.
The T cells found in the red pulp are predominantly CD8-positive small lymphocytes, which are rarely found in the periarteriolar lymphoid sheaths and are virtually absent in the germinal centers. Gamma-delta T cells also reside normally in the red pulp. The distribution of immunoglobulin-containing B cells is comparable to that seen in the lymph nodes. The mantle-zone B cells bear surface immunoglobulin, with co-expression of immunoglobulin (Ig) M and IgD. The marginal-zone B cells express predominantly IgM, with only a small minority expressing IgD. IgG expression is lacking in these areas and is limited to scattered cells in the red pulp, where rare IgA-containing cells are also found. The red pulp contains numerous cells of monocyte-macrophage lineage, only a few of which are found in the white pulp. Natural killer (NK) cells are found scattered throughout the red pulp. The red pulp also contains granulocytes, monocytes, and lymphocytes that pass transiently through the red pulp circulation.
The initial evaluation of the spleen should consist of a gross examination of the organ. Three major patterns are recognized, based on involvement of the white pulp, red pulp, or more focal lesions ( Table 60-2 ).
| Pattern | Predominantly Red Pulp Based | Predominantly White Pulp Based | ||
|---|---|---|---|---|
| Neoplastic | Non-neoplastic | Neoplastic | Non-neoplastic | |
| Diffuse | HCL and HCL variant Splenic diffuse red pulp small B-cell lymphoma Hepatosplenic T-cell lymphoma LGLL Acute leukemias MPN, other myeloid neoplasms CLL/SLL (rare) LPL (rare) |
Hemolytic anemias Non-specific congestion Extramedullary hematopoiesis Storage diseases Cytokine effects HPS |
Small B-cell lymphomas (CLL/SLL, LPL, SMZL, MCL) PTCL |
Hyperplasia |
| Focal * or variable | Hodgkin's lymphoma DLBCL T-PLL EBV-DCT Other dendritic cell tumors Mast cell disease Vascular tumors † Metastases |
— | — | Inflammatory pseudotumor Hamartoma Cyst Peliosis |
* Focal lesions may have considerable overlap, with both red pulp and white pulp involvement. At times, this division is arbitrary.
† Diffuse involvement is seen in systemic angiomatosis as well as in some cases of littoral cell angioma.
Most proliferative disorders of the splenic lymphoid tissue produce a micronodular pattern owing to the abnormal expansion of preexisting splenic lymphoid structures (follicles and periarteriolar lymphoid sheaths). Grossly, multiple small, whitish nodules are noticeable on the cut surface, an appearance that is occasionally referred to as a miliary pattern. This pattern is most often seen in small B-cell lymphoid neoplasms, other than hairy cell leukemia, involving the spleen. The nodules occasionally become confluent or present as larger, dominant masses. Lymphoid malignancies that affect the white pulp are largely the same as those that affect lymph nodes. These disorders include both classical and nodular lymphocyte-predominant Hodgkin's lymphoma and non-Hodgkin's lymphomas, primarily of B-cell lineage.
Red pulp involvement has a different gross appearance. Typically, expansion of the red pulp gives the spleen a more homogeneous red or “beefy” appearance. The normal nodularity of the white pulp is typically diminished or not seen. Microscopically, the white pulp is often atrophic or compressed by the expanded red pulp. Neoplastic proliferations that involve the red pulp include myeloid and lymphoid leukemias, myeloproliferative neoplasms, and a variety of non-hematopoietic tumors. In general, disorders with a large component of circulating cells (e.g., chronic lymphocytic leukemia, large granular lymphocytic leukemia, hairy cell leukemia, acute leukemia) have significant red pulp involvement. However, some lymphomas (e.g., hepatosplenic T-cell lymphoma, intravascular large B-cell lymphoma, as well as other less well-defined lymphoid malignancies) also involve the red pulp.
Some benign and malignant proliferations produce focal lesions rather than more diffuse involvement of the red or white pulp. These include lesions that involve vascular, stromal, and hematolymphoid elements.
Pathologic rupture of the spleen can be seen in a variety of hematologic disorders, both benign and malignant. Spontaneous rupture of the spleen should always prompt a pathologic evaluation of the splenic tissue because various infectious causes (particularly infectious mononucleosis) have pathologic findings that are distinctive enough to make a presumptive diagnosis or suggest additional serologic studies. Other causes, such as storage diseases, present with characteristic findings as well. Splenic rupture as a primary presentation of hematologic malignancy is rare, but it has been reported with both low-grade and high-grade lymphoid malignancies. Acute and chronic myeloproliferative disorders and, rarely, acute lymphoblastic leukemia can present as splenic rupture. Nonhematopoietic lesions associated with splenic rupture include cysts, infarctions, vascular lesions or neoplasms, and metastatic malignancies.
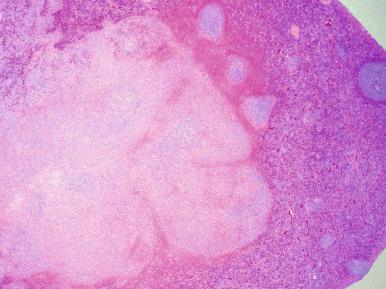
Various reactive conditions that affect the splenic white or red pulp can simulate hematopoietic malignancies ( Box 60-1 ). Reactive follicular hyperplasia, with the formation of germinal centers, is usually easily recognized as benign (see Fig. 60-1 ). However, follicular hyperplasia must occasionally be distinguished from follicular lymphoma. The finding of tingible body macrophages and a polymorphic lymphoid cell population within polarized splenic follicles points to the diagnosis of a reactive hyperplasia. A rare entity that may grossly simulate lymphoma is localized (nodular) reactive lymphoid hyperplasia. The area of nodular hyperplasia appears quite distinct from adjacent normal spleen and may raise the suspicion of lymphoma ( Fig. 60-2 ). Histologically, this area is composed of a focal aggregation of hyperplastic follicles that have typical, benign features.
Immune reactions
Florid follicular hyperplasia
Marginal-zone hyperplasia
Congenital immunodeficiencies
Autoimmune conditions
Disorders of the reticuloendothelial system
Storage diseases
Hemophagocytic syndrome
Langerhans cell histiocytosis
Castleman's disease
Reactive myeloid proliferations due to cytokine treatment
Non-hematopoietic lesions
Cyst
Hamartoma
Inflammatory pseudotumor
The marginal zones may become widely expanded, a phenomenon referred to as splenic marginal-zone hyperplasia. This usually occurs in association with follicular hyperplasia, but such expansions may also occur with B-cell lymphomas other than splenic marginal-zone lymphoma, particularly follicular lymphoma. It may be impossible to distinguish these reactive changes from cases of early marginal-zone lymphoma on morphologic grounds alone, although reactive marginal-zone hyperplasia is usually not associated with an increase in red pulp B cells, which is common in true splenic marginal-zone lymphoma. Some autoimmune disorders can result in splenic marginal-zone hyperplasia, including systemic lupus erythematosus or idiopathic thrombocytopenic purpura.
Reactive lymphoid hyperplasia without germinal center formation, which is characteristic of infectious mononucleosis as well as herpes simplex and other viral infections, can simulate both Hodgkin's and non-Hodgkin's lymphoma. The white pulp in these conditions lacks expanded follicles and, on low-power examination, resembles the immunologically unstimulated spleen. This is the splenic equivalent of paracortical hyperplasia and is primarily a reaction of T cells. High-power examination reveals morphologic evidence of antigenic stimulation, characterized by the presence of lymphocytes in varying stages of transformation, including small and large lymphocytes, often with plasmacytoid features, and immunoblasts. Transformed lymphocytes and immunoblasts also proliferate around splenic arterioles and may infiltrate the subendothelial zones of the trabecular veins and the connective tissue framework, resulting in splenic rupture in extreme cases. This pattern of lymphoid hyperplasia can be seen in immunocompromised individuals, such as patients treated with steroids or other immunosuppressive therapies for conditions such as immune thrombocytopenic purpura or autoimmune hemolytic anemia. Some peripheral T-cell lymphomas may produce a similar pattern of white pulp expansion. Nodular T-cell hyperplasia, simulating a peripheral T-cell lymphoma, can rarely be observed in patients with hypersensitivity reactions to phenytoin. Abnormalities of the white pulp that may be worrisome for lymphoma can also be seen in patients with congenital conditions characterized by immunodeficiency or by abnormalities causing deregulated lymphoid production (e.g., autoimmune lymphoproliferative syndrome).
Occasional cases of Castleman's disease of both the unicentric hyaline-vascular type and the multicentric type associated with Kaposi's sarcoma–associated herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) reportedly occur in the spleen. Multicentric Castleman's disease represents the majority of cases reported in more recent years. Splenic involvement is rare in the unicentric form, and most such reports are from the older literature, before cases were evaluated for HHV-8/KSHV; thus, the nature of these proliferations is not clearly established. The white pulp is expanded, with hypervascular germinal centers; the red pulp shows marked plasmacytosis. As seen in lymph nodes, immunoblastic cells expressing IgMλ are distributed in the perifollicular areas of the white pulp. Multicentric Castleman's disease is generally negative for Epstein-Barr virus (EBV), but rare cases resembling germinotropic lymphoma have been described. These tumors are coinfected with EBV and HHV-8/KSHV.
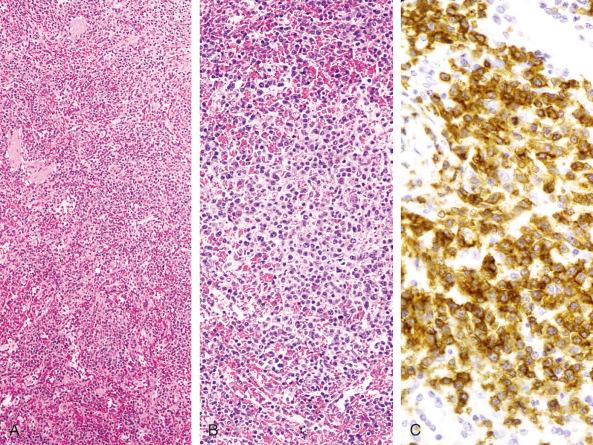
Autoimmune lymphoproliferative syndrome is a rare disorder that can mimic lymphoma in the spleen. It is a hereditary disorder, usually due to mutations of the CD95 (FAS) gene, that presents in early childhood (younger than 2 years). Autoimmune lymphoproliferative syndrome is characterized by lymphoid hyperplasia, autoimmunity, and splenomegaly; the spleen frequently enlarges to more than 10 times its age-normal size. Histologically, the white pulp shows variable degrees of follicular hyperplasia, often with enlarged marginal zones. The periarteriolar lymphoid sheaths and red pulp are also expanded, owing to a markedly increased number of T cells ( Fig. 60-3 ). These cells consist of a mixture of small lymphocytes and immunoblasts. As in lymph nodes in this disorder, many of these T cells are negative for both CD4 and CD8. The pathologic picture of the spleen is complicated by the frequent association with immune cytopenias affecting red blood cells, granulocytes, and platelets, contributing to splenomegaly. Patients with this disorder have an increased risk of having both Hodgkin's and non-Hodgkin's lymphomas.
Although the spleen is the most common extranodal organ involved by Hodgkin's lymphoma, primary Hodgkin's lymphoma of the spleen is extremely rare. The documentation of splenic involvement has therapeutic and prognostic implications, although these implications now appear to be less critical in light of the high rates of remission and cure obtained with current regimens of combination chemotherapy. Involvement of the liver and bone marrow is rarely found in the absence of splenic involvement. All histologic subtypes of Hodgkin's lymphoma can involve the spleen; nodular sclerosis and mixed cellularity are the most common, and involvement by nodular lymphocyte-predominant Hodgkin's lymphoma is less common. Lymphocyte-depleted Hodgkin's lymphoma characteristically presents with subdiaphragmatic disease and splenic involvement.
Hodgkin's lymphoma produces either small miliary nodules or, more frequently, solitary or multiple tumor masses in the spleen ( Fig. 60-4 ). Splenic involvement is generally detectable grossly but may be subtle ( Fig. 60-5 ). Foci of involvement may be only a few millimeters in size. For this reason, the gross examination of the spleen must be meticulous in patients with Hodgkin's lymphoma so that small foci of involvement are not missed. The early lesions of Hodgkin's lymphoma in the spleen are found microscopically in the periarteriolar lymphoid sheaths or in the marginal zones. As the disease progresses, the nodules expand to efface the lymphoid follicles and may involve the red pulp.
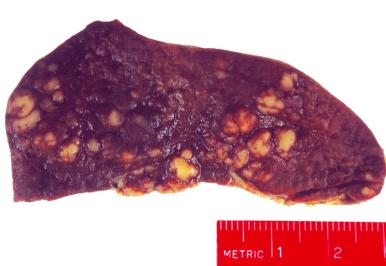
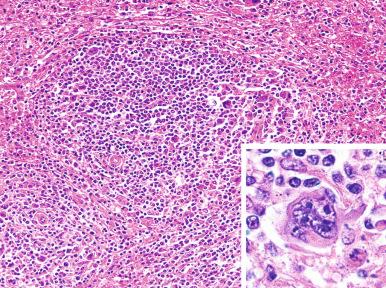
Sarcoid granulomas may be found in the spleens of patients with Hodgkin's lymphoma, in addition to various other disorders associated with abnormal T-cell function. The granulomas are not related to prior lymphangiography, and their origin is unknown. Several studies have suggested that granulomas occur more frequently in spleens uninvolved by Hodgkin's lymphoma than in those involved by the disease. Grossly, the granulomas may be so large as to mimic involvement by Hodgkin's lymphoma. Microscopically, the granulomas are composed of clusters of epithelioid histiocytes that occur in the white pulp in close association with the arterial circulation. It has been suggested that patients with splenic sarcoid granulomas have a better prognosis.
The criteria for the diagnosis of Hodgkin's lymphoma in the spleen are the same as those for other non-nodal sites (see Chapters 27 and 28 ). The subclassification of Hodgkin's lymphoma in the spleen is sometimes difficult and is unnecessary in cases with a previous nodal diagnosis. However, the unique morphologic and immunophenotypic characteristics of nodular lymphocyte-predominant Hodgkin's lymphoma allow its distinction from the classical Hodgkin's lymphoma subtypes (see Chapter 27 ).
Non-Hodgkin's lymphomas may involve the spleen in three clinical settings. In the first and rarest setting, termed true primary splenic lymphoma, the tumor is confined to the spleen or splenic hilar lymph nodes, without evidence of involvement of other sites. In the second and most common setting, the organ is involved as part of generalized, systemic lymphomatous spread. In the third setting, the lymphomatous process is characterized by prominent or predominant splenomegaly and often distinctive clinicopathologic features.
Primary splenic lymphoma is rare, accounting for less than 1% of all lymphomas. Excluding lymphomas thought to arise in the spleen, such as splenic marginal-zone lymphoma, most of these cases were described in the older literature and are not well defined. Two cases occurred in HIV-positive patients, and rare cases have been associated with hepatitis C infection. Several studies of primary splenic lymphomas fulfilling the most stringent diagnostic criteria (i.e., tumor confined to the spleen and splenic hilar lymph nodes) include almost entirely adult patients with a slight male preponderance. The most common presenting symptoms include left-sided abdominal pain and systemic symptoms such as fever, malaise, and weight loss. The gross findings and the histologic characteristics were similar to those observed in spleens secondarily involved by malignant lymphoma. Most reported cases are of B-cell lineage. Large B-cell lymphoma, some showing CD5 expression, appears to be the most common subtype, with the remainder being mostly low-grade B-cell malignancies. A report of 32 patients presenting with follicular lymphoma first diagnosed in the spleen identified two variants: one with the t(14;18), high BCL2, and CD10 expression, similar to nodal follicular lymphoma, and a second subset that lacked t(14;18) and was of a higher histologic grade. The majority of patients relapsed with systemic disease.
Clinical assessment of the likelihood of splenic involvement by malignant lymphomas may be difficult. The weights of involved spleens vary widely. Although tumor involvement usually results in palpable splenomegaly, Goffinet and colleagues found that approximately one third of non-palpable spleens were involved by lymphoma at staging laparotomy. Staging laparotomy has been replaced by imaging studies; positron emission tomography, in particular, provides an accurate determination.
Non-Hodgkin's lymphomas of different types involve the spleen with variable frequency. Splenic involvement is particularly frequent in low-grade B-cell lymphomas. As mentioned earlier, evaluation of splenic hilar lymph nodes is very important. Histologic findings of lymphoma that are ambiguous or incompletely diagnostic in splenic sections may be more distinctive in splenic hilar lymph nodes. Liver involvement by lymphoma is rare in the absence of splenic disease.
Although enlargement of the spleen often occurs during the course of either B-lymphoblastic or T-lymphoblastic malignancies, it rarely approaches clinical significance. The histopathologic features are similar to those of other leukemic disorders, with diffuse infiltration of the red pulp by blast cells.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here