Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In Western countries neuromodulation is increasingly used for peripheral neuropathic pain (PNP). To know the indications for neuromodulation therapies and understand how to diagnose PNP disorders, one must be familiar not only with the principles of the techniques, but also with (the basics of) the nervous system and its diseases. Detailed information about the relevant anatomy of the nervous system can be found in Chapter 3 . This chapter can be considered as guidance for the clinician who intends to implement neuromodulation therapies in patients with PNP.
According to the definition of the International Association for the Study of Pain, neuropathic pain is pain that arises as a direct consequence of a lesion or disease affecting the somatosensory system. The prevalence of neuropathic pain is estimated to be between 3.3% and 8.2% ( ). Neuropathic pain is associated with a high social and economic burden and therefore should be a priority in research ( ). Clinical assessment of neuropathic pain is very important for selecting the most optimal treatment, but can be difficult. The assessment should contain a careful history and neurological examination, and, if possible, be based on objective diagnostic tests ( ).
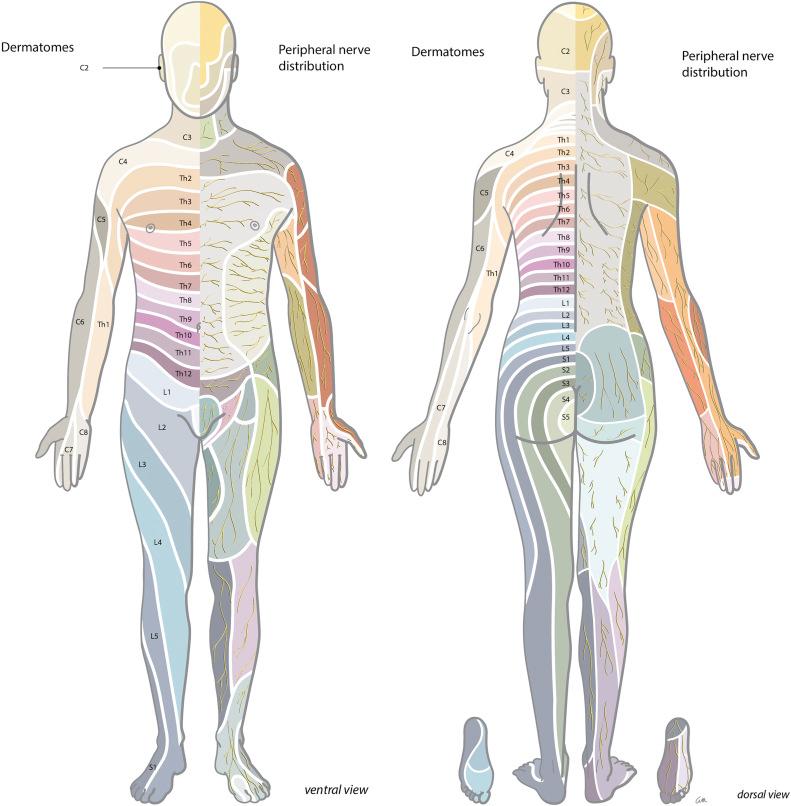
In diagnosing a neurological disorder of either central or peripheral origin it is helpful to distinguish the symptoms that belong to a default/defect in the central nervous system (CNS) (brain, spinal cord) from those of the peripheral nervous system (PNS) (spinal roots, plexus, peripheral nerves); see Fig. 49.1 . A lesion in the PNS can lead to weakness, atrophy, diminished reflexes, hypotonia, sensory loss (pain, touch, thermal sense), and/or hypersensitivity and pain, and usually has a specific neuro-anatomical distribution (see Fig. 49.2 ).
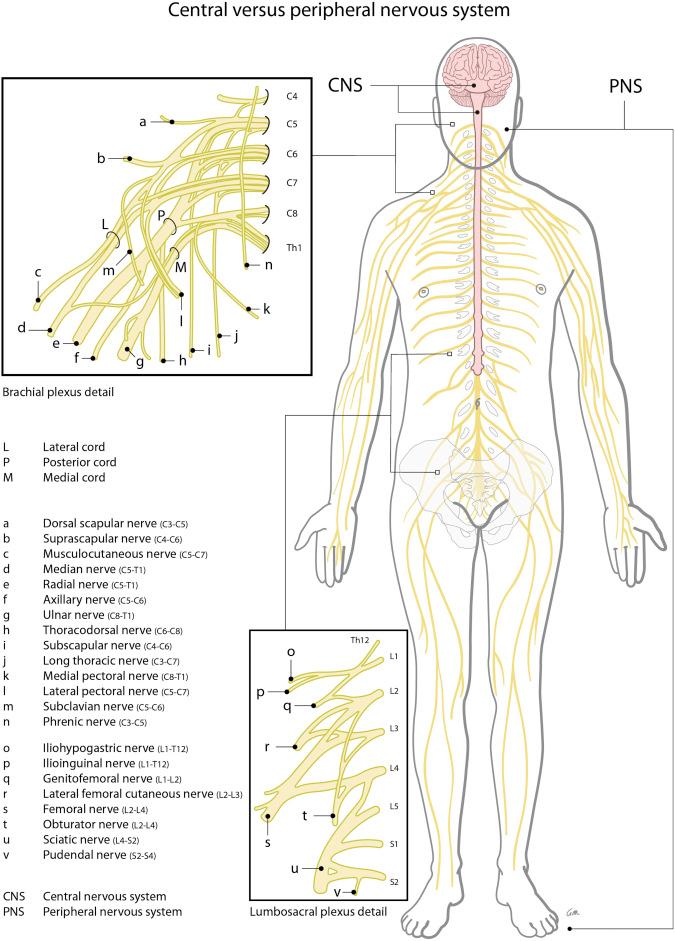
Until now, the first steps in treatment of PNP have been based on noninvasive pharmacological interventions. Pharmacological intervention used for treatment of neuropathic pain usually consists of antidepressant, antiepileptic, and opioid medications ( Table 49.1 ). These medications, however, may have unsatisfactory results and often unacceptable side-effects ( ). If pharmacological intervention does not result in adequate pain reduction, neuromodulation via SCS of the dorsal columns may be a second-line treatment option. The history and mechanisms of SCS of the dorsal columns, the proposed techniques, and current indications are discussed in this chapter.
| First line | SNRI | Duloxetine Venlafaxine |
| TCA | Amitriptyline Nortriptyline | |
| Α2δ calcium antagonist | Pregabalin Gabapentine |
|
| Second line | Weak opioid | Tramadol |
| Capsaicin 8% patches | If pain localized to limited area | |
| Lidocaine patches 5% | If pain localized to limited area | |
| Third line | Strong opioids (slow release) | |
| Botulin toxin A |
The mechanisms of action (MOAs) of SCS in humans are difficult to study, so various animal models have been used. Based on a translational, experimental animal model of chronic PNP, were the first to study the pain-relieving effects of SCS at the cellular and biochemical levels. Furthermore, with this experimental animal model, conventional SCS settings (approximately 50 Hz, 0.2 ms, 1.4 mA) of dorsal column stimulation results in a comparable pain-relieving effect and percentage of responders as observed in patients ( ).
In these chronic PNP animal models SCS significantly decreases the tactile hypersensitivity (responders to SCS), and this behavioral effect is correlated with a decreased intracellular GABA level and an increased extracellular GABA level in the DH ( ). Moreover, SCS of the dorsal columns induces a decrease in release of glutamate, the predominant neurotransmitter of nociceptive afferent neurons ( ). Interestingly, in nonresponders to SCS the effect on GABA release in the DH is less prominent, further substantiating the pivotal effect of SCS on GABA interneurons in the DH network ( ).
In SCS responders, intrathecal (IT) application of a selective GABA-B antagonist can prevent the SCS-induced glutamate decrease. This phenomenon is in line with the gate-control theory, where GABAergic interneurons presynaptically modulate the glutamate release of nociceptive afferents in the DH ).
Likewise, the IT application of a subeffective dose of baclofen could potentiate the attenuation of neuropathic pain by conventional SCS ( ). The importance of glutamate and its receptor was further substantiated by the fact that nonresponders to conventional SCS could be turned into responders when a subeffective dose of ketamine, a N -methyl- d -aspartate (NMDA) glutamate receptor antagonist, was additionally applied ( ). The latter is notable, as the NMDA receptor is very important for the increased sensitivity of glutamate transmission or central sensitization ( ).
The question still remains whether SCS is mainly acting via a segmental (antidromic) or a suprasegmental (orthodromic) mechanism (see Fig. 49.3 ).
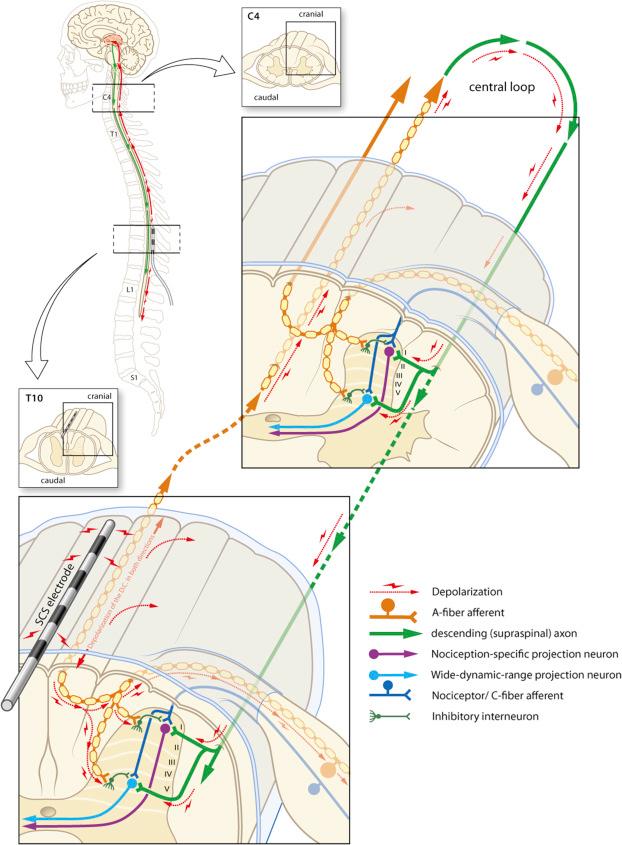
Electrophysiological evidence showed that in rats with neuropathic pain, SCS induces long-lasting depression of hyperexcitable wide dynamic range neurons by attenuating both spontaneous and evoked after-discharges, and that SCS of the dorsal columns at the same time reduces the C-fiber evoked central sensitization ( ). These results indicate a spinal segmental modulation of excitatory and inhibitory molecules to be pivotal in the MOA underlying the pain-relieving effects of SCS. Although dorsal nociception is now known to be much more complicated than the gate-control network originally presented by Melzack and Wall in , the balance between many inhibitory and excitatory cells forms the essence of the concept.
This so-called DH gate-control network ( ) is a network triggered by conventional SCS and the antidromic stimulation of AB-fibers in the dorsal columns. In an elegant series of experiments where the leads were positioned either very close to the level where activated nociceptive afferents enter the spinal cord or much more rostrally, the pain-relieving effect of SCS was shown to be predominantly segmental ( ).
Although the primary effect of conventional SCS has been attributed to the antidromic activation of ascending dorsal column fibers inducing segmental neurochemical changes in the DH, there is evidence that orthodromic activation also involves supraspinal mechanisms via a loop in which the descending dorsolateral funiculus plays a role in the attenuation of neuropathic pain ( ).
For all indications, the following basic rules are recommended regarding the use of conventional SCS.
Conventional SCS should only be used after proper assessment of the patient. That means clear somatic neuropathic pain diagnoses such as failed back surgery syndrome (FBSS), postherpetic neuralgia (PHN), plexopathy, or painful diabetic polyneuropathy (PDP) have to be confirmed.
Only use conventional SCS when a patient suffers from chronic neuropathic pain with no sufficient response to conservative treatment.
Preimplantation psychiatric screening is advised.
Be careful with patients with blood-clotting disorders and immune deficiency.
Be careful with patients with chronic opioid use. We tend to advise minimizing the use of opioids to an equivalent of 30 mg morphine a day or less.
Successful treatment of neuropathic pain requires consideration of the neuroanatomy and its clinical context. We systematically discuss several indications for conventional SCS on the basis of the anatomical distribution of the lesion. Typical clinical features are presented regarding patient history, physical examination, and ancillary tests.
FBSS, sometimes called postlaminectomy pain syndrome, is chronic pain that occurs after spinal surgery and is estimated to occur in about 40% of these patients ( ). The cause of this syndrome can be multifactorial. Somatic contributing factors include (persistent) pressure on the spinal nerve by residual or recurrent disc herniation or epidural fibrosis and/or traumatization of a nerve root. Psychological factors may also play an important role ( ) .
History : FBSS patients complain about persistent low-back pain, but can also have persistent leg pain in the projection territory of the affected nerve root.
Neurological exam : Neurological examination can be normal, but changes can be found in strength, sensibility, and reflexes in the distribution of the affected nerve root (see Fig. 49.2 and Table 49.2 ).
Diagnostic testing : Electromyography can be helpful, but is usually not necessary. Magnetic resonance imaging (MRI) can show either a compressive lesion such as epidural fibrosis and disc herniation or no actual lesion at all.
Spinal cord stimulation : Conventional SCS is recommended for the use in patients with pronounced leg pain and insufficient pain relief with conservative treatment, and is superior to reoperation ( ). In 2005 North et al. published a randomized controlled trial (RCT) in 50 patients with FBSS. Conventional SCS proved to be more effective than reoperation. Furthermore, a success rate of almost 50% (>50% pain relief) in the conventional SCS-treated FBSS patient group as compared to a success rate of about 10% in the nonSCS group was reported in another multicenter RCT ( ).
Remarkably, conventional SCS is presently underutilized and only used in 2.4% of the cases of FBSS, and a longer delay to implantation has been shown to result in higher healthcare resource utilization ( ). For more information about SCS for FBSS and low-back pain we refer readers to Chapter 50 .
| Nerve Root | Motor Weakness | Reflexes |
|---|---|---|
| Arm | ||
| C5 | Infraspinatus (external rotation of the arm) | Biceps reflex |
| Deltoid (arm abduction or shoulder flexion) | ||
| Biceps brachii (elbow flexion with forearm in supination) | ||
| C6 | Extensor carpi radialis brevis and longus (wrist extension) | Biceps reflex |
| Deltoid (arm abduction or shoulder flexion) | ||
| Biceps brachii (elbow flexion with forearm in supination) | ||
| C7 | Extensor digitorum communis (extension of digits 2–5) | Triceps reflex |
| Flexor carpi radialis (wrist flexion) | ||
| C8 | Flexor carpi ulnaris (wrist flexion with ulnar deviation) | Triceps reflex |
| Abductor digiti quinti (abduction of digit 5) | ||
| Leg | ||
| L2 | M iliopsoas (hip flexion) | – |
| M quadriceps, rectus femoris (knee extension) | ||
| L3 | M iliopsoas (hip flexion) | – |
| M quadriceps, rectus femoris (knee extension) | ||
| L4 | M quadriceps, rectus femoris (knee extension) | Knee jerk |
| L5 |
|
– |
| S1 |
|
Ankle jerk |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here