Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A theory of pain transmission published in 1965 inspired researchers to develop a reversible, nondestructive pain therapy that relied on equipment adapted from cardiac pacemaker technology to deliver electrical stimulation to the spinal cord. The initial results of this therapy, now known as spinal cord stimulation (SCS), were inconsistent, but many patients benefited dramatically. During the intervening decades, refinements in SCS techniques, stimulation patterns, equipment, and patient selection criteria as well as new targets ( Table 119.1 ) have led to clinical results that continue to improve.
|
|
|
|
|
|
|
Melzack and Wall’s gate control theory provided a theoretical rationale for the use of electrical stimulation in the management of pain. The theory proposes that a neuronal “gate” controls the transmission of pain signals from the dorsal horn of the spinal cord to the brain. An excess of small-fiber afferent input opens the gate, and a dominance of large-fiber afferent activity closes it. The gate concept is, in some aspects, a bit similar to Head and Holmes’ 1911 proposal that parallel “epicritic and protopathic” input systems govern sensory influx. This hypothesis provided the physiologic basis for Gabriel Mazars’ therapeutic trials with sensory thalamic stimulation in Paris beginning in the 1960s, which were the first modern attempts to treat severe neuropathic pain with electric stimulation. ,
Because large fibers are more susceptible than small fibers to electrical depolarization, it seemed reasonable to attempt to close the gate and stop pain transmission with low-amplitude stimulation that would selectively recruit large-fiber activity in a mixed population of nerves. Electrical stimulation of mixed peripheral nerves can achieve this effect, but stimulation of peripheral nerves at an amplitude close to that required for a therapeutic effect can cause unwanted motor effects. In addition, pain generally involves multiple peripheral nerves. Therefore, investigators decided to apply electrical stimulation to the spinal cord, where they could recruit large fiber primary afferents from multiple segments conveniently isolated in the posterior columns. As expected, antidromic activation of these primary afferents, whose collateral processes extend into the dorsal horn, yielded a wide area of paresthesia with, in successful cases, ensuing pain relief. The electrical stimulation techniques that grew out of the gate control theory has been succeeded, but the theory itself remains controversial. One reason is that activation of peripheral large fibers can result in increased pain (hyperalgesia or allodynia) in some pathologic circumstances. Thus, peripheral nerve stimulation or SCS might relieve pain by blocking the conduction of primary afferents at the branch points of dorsal column fibers and their collaterals. The mechanism of action of SCS, however, cannot depend solely on blocking conduction (e.g., by impulse collision) because electrical stimulation does not inhibit all types of pain, and therapeutic SCS does not normally evoke the pain that would occur if SCS also activated small-diameter, high-threshold fibers in the spinothalamic tracts. Dorsal column activation is more successful than ventral stimulation, which is closer to the spinothalamic tracts.
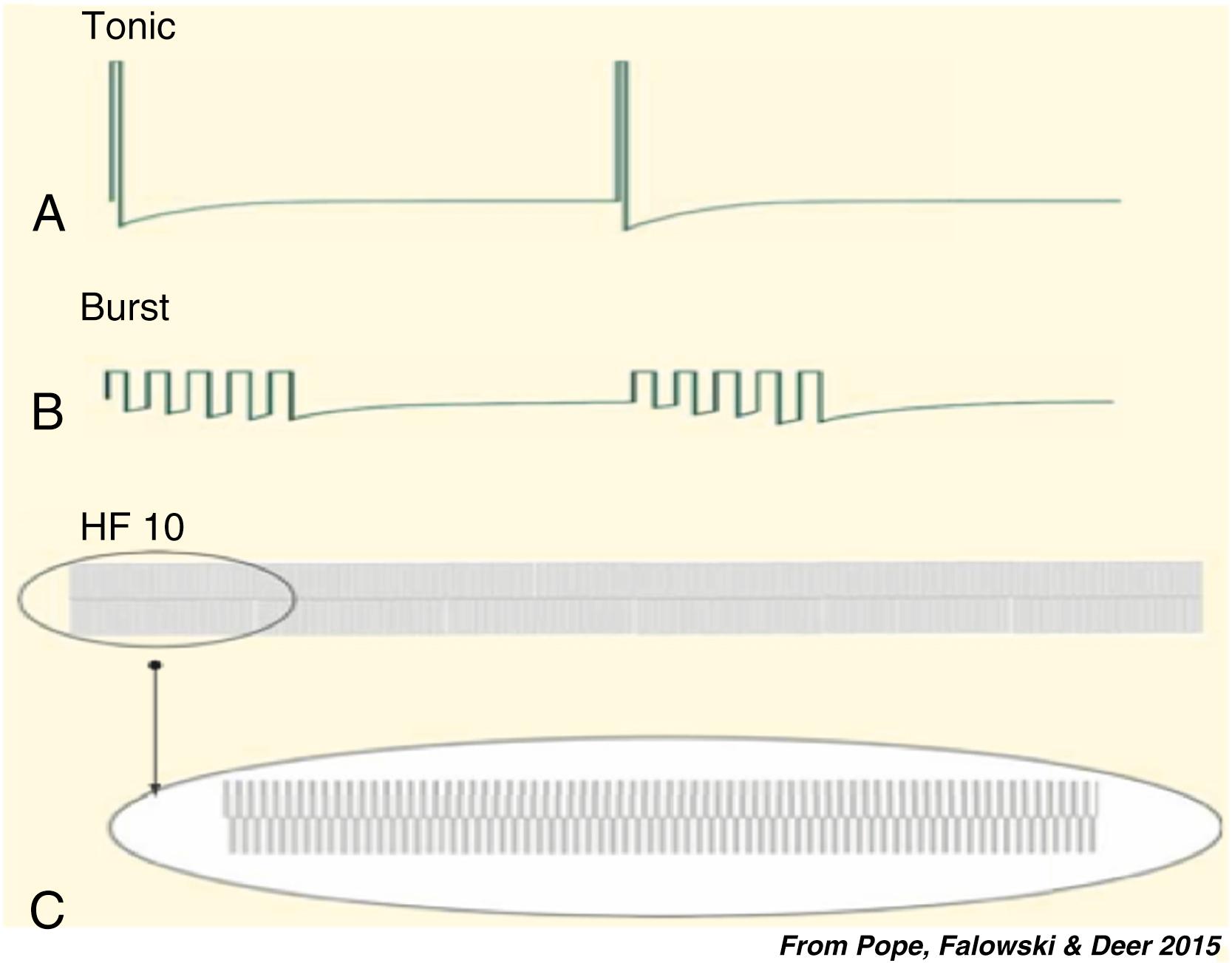
Until the first decade of the 21st century, SCS was generally applied with frequencies between 30 and 100 Hz; pulse widths between 150 and 500 μs, and an amplitude to produce comfortable paresthesia covering the painful area (conventional SCS) ( Fig. 119.1 ). In clinical application for chronic pain, a major technical goal was to place the electrodes over a “sweet spot” where stimulation-induced paresthesia covered the area(s) of pain well below the discomfort threshold.
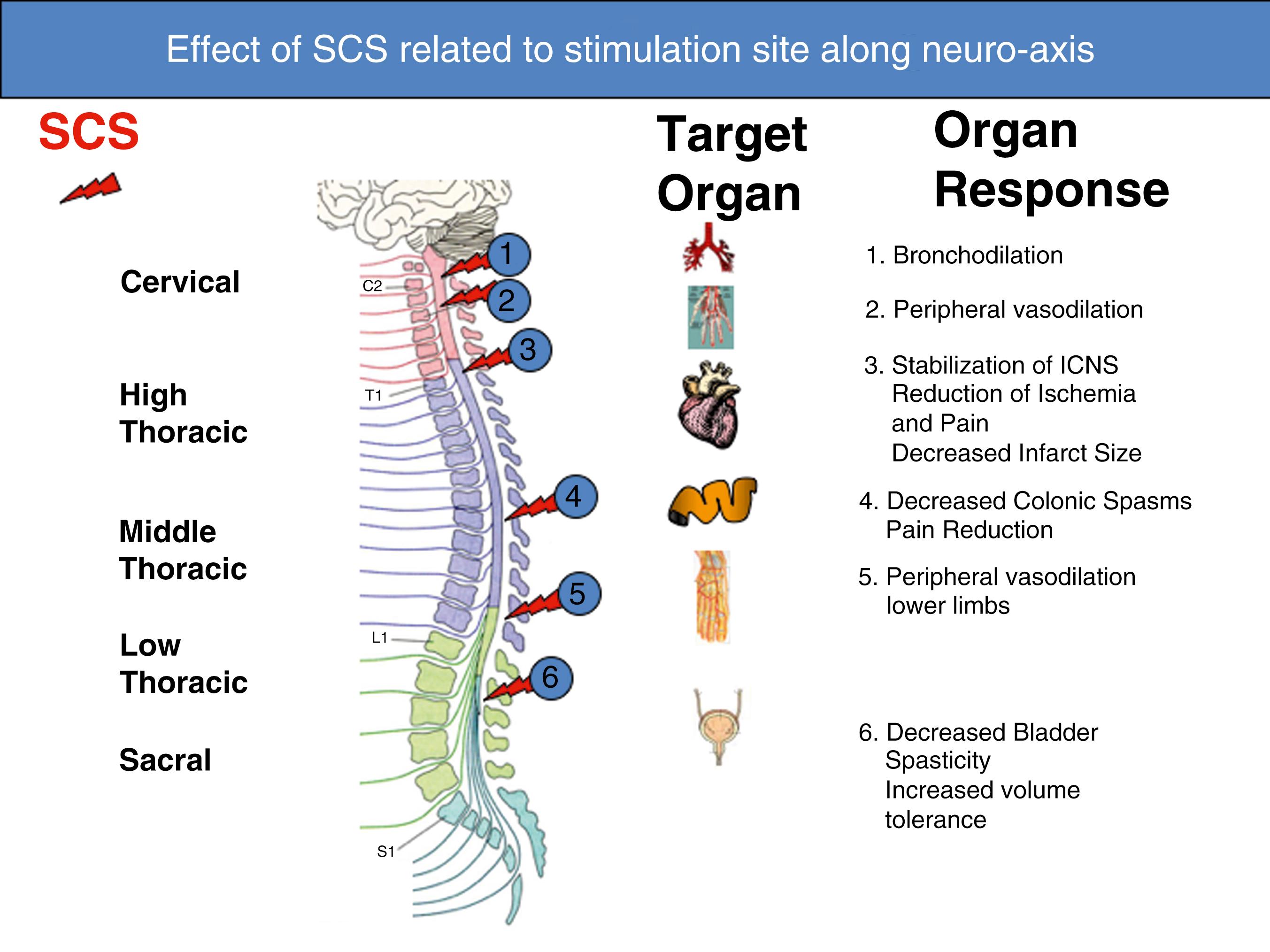
Notwithstanding predictions from the gate theory, conventional SCS has proved to be less effective for treating acute nociceptive pain conditions than for treating chronic neuropathic pain of peripheral origin, which has become its principal indication. As the 20th century drew to a close, we learned that SCS could also alleviate certain types of ischemic pain states, such as peripheral arterial occlusive disease (PAOD), vasospastic conditions, and therapy-resistant angina pectoris ( Fig. 119.2 ).
We now understand that mechanisms of pain relief with SCS differ fundamentally between neuropathic and ischemic/vasculopathic pain conditions (e.g., in ischemic pain, the primary effect of SCS appears to be alleviation of tissue ischemia, and pain reduction is a secondary effect).
During the past decade, new SCS approaches that are not based on the gate control theory have been launched: high-frequency stimulation (HF SCS): with frequencies up to 10,000 Hz (10 KHz) (75–86) and burst SCS: with bursts of five pulses (internal frequency 500 Hz) applied with a frequency of 40 Hz (89–95) (see Fig. 119.1 ). At the same time, additional peripheral targets have been identified: not only discrete peripheral nerves, but also “nerve field” stimulation and stimulation of the dorsal root ganglia. These targets outside the spinal cord per se will not be discussed in this chapter.
At present much less is known about the physiological mechanisms underlying the effects of these new techniques as compared with conventional SCS. HF SCS and burst SCS are said to provide effective treatment for nociceptive as well as neuropathic components of pain; for example, the low back pain that occurs in patients with failed back surgery syndrome (FBSS), which is one of the most common indications for conventional SCS therapy.
In the following sections we will discuss (1) conventional SCS (with the electric parameters commonly used 1970 to 2010), (2) high-frequency SCS, (3) burst SCS, and (4) SCS with slightly moderated parameters to increase transmission of the electric charge from the lead to the neural tissue without side effects.
In neuropathic pain states, activation of peripheral nerve fibers increases the sensitivity and activity of wide dynamic range neurons in the superficial laminae of the corresponding dorsal horns, which in turn causes hyperalgesia (increased sensitivity to pain) and/or allodynia (normally nonpainful stimuli cause pain). In rat models of neuropathy that employ stimulation parameters similar to those used in humans, SCS effectively suppresses this heightened activity and relieves tactile hypersensitivity as reflected by responses to innocuous stimuli (similar to clinical allodynia).
In a study that sought to determine if SCS suppresses long-term potentiation of wide dynamic range dorsal horn neurons, SCS gradually reduced the C-fiber response to the baseline level. Aβ-fibers, on the other hand, were not potentiated by the conditioning stimulus or affected by SCS. This indication that SCS affects C-fiber responses is noteworthy because the findings of previous studies supported the view that SCS primarily influences Aβ-fibers.
Investigators have used finite-element computer techniques to model the electrical fields produced in the spinal cord by SCS. These models reveal distributions of current and voltage that agree with measurements in cadaver and primate spinal cords. The models and measurements predict that an electrode’s longitudinal position is the most important factor in achieving the desired segmental effect (fibers decrease in diameter as they ascend the fasciculus gracilis), that bipolar stimulation with contacts 6 to 8 mm apart provide the greatest selectivity for longitudinal midline fibers, and that the electrical field between two cathodes that bracket the midline does not sum constructively in the midline. Clinical experience confirms that the correct position and spacing of SCS electrodes is essential and that instead of expanding the area of paresthesia, positioning electrodes more cephalad than the target area commonly elicits unwanted local segmental effects.
Psychophysical studies have found that stimulation induces a subtle loss of normal sensation in SCS patients but does not affect acute pain sensibility to an extent that could lead to undesirable side effects, such as Charcot joints. , Side effects increase with increases in stimulation amplitude and in recruitment of nerve fibers; psychophysical studies in individual patients document this with quantitative measures of stimulation adjusted over the range of amplitudes from perception to motor threshold.
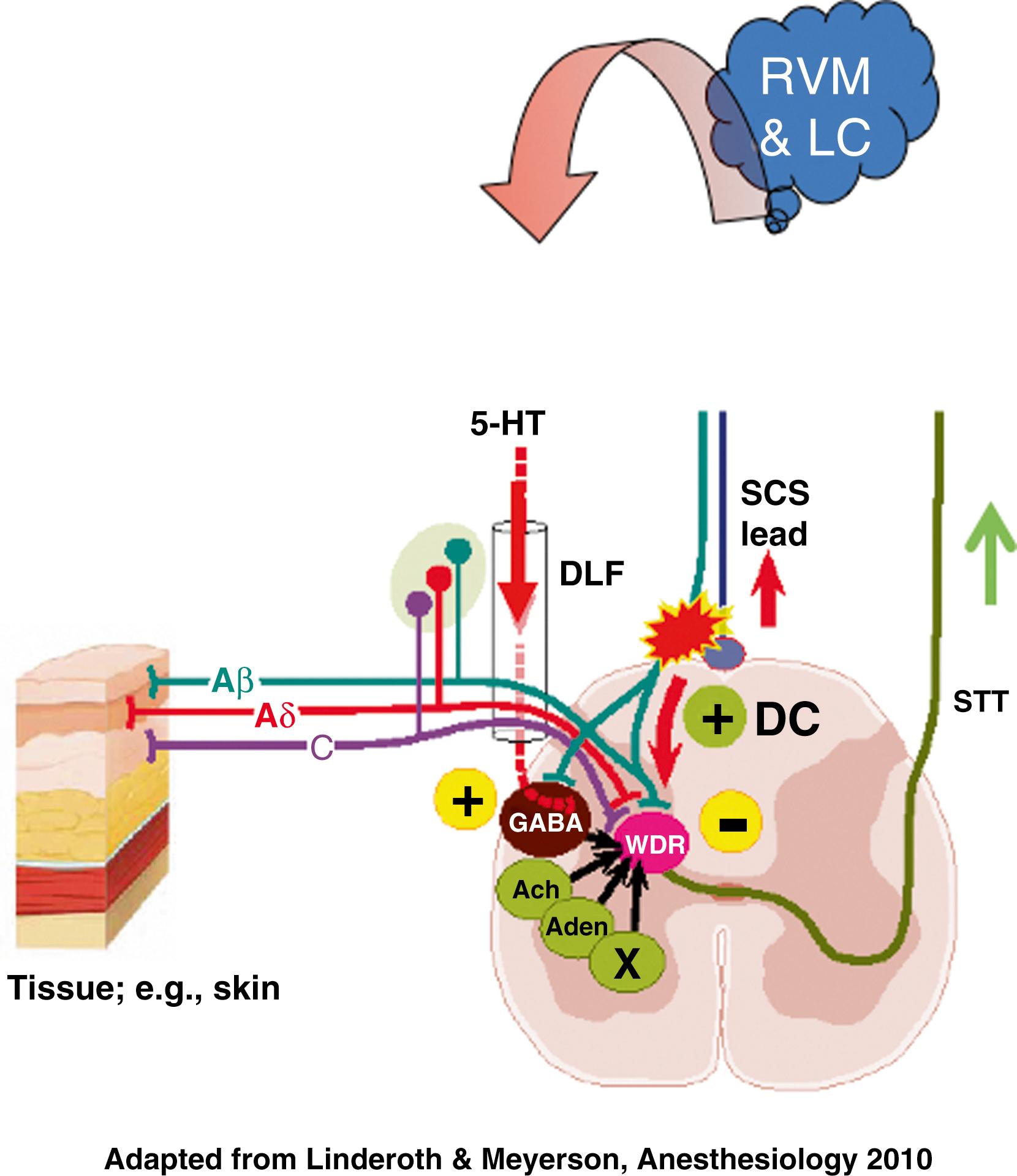
To explain the sustained pain relief (often lasting from 1 to 3 hours) that patients experience following a 30-minute period of SCS, investigators hypothesized that SCS affects the release of neurotransmitters in the dorsal horn and brain. This led to several lines of investigation, which revealed that SCS changes the concentration of neurotransmitters and their metabolites in cerebrospinal fluid , ; administration of high doses of opioid antagonists, such as naloxone, does not affect the relief of pain achieved by SCS , ; and both SCS and administration of γ-aminobutyric acid (GABA) agonists to neuropathic rats suppresses the allodynia that occurs from peripheral nerve lesions. ,
SCS induces GABA release in the dorsal horn, and the pain-relieving effect of SCS depends on activation of the GABA-B receptor. , In fact, for a period of time SCS inhibits the pathologic response properties of dorsal horn neurons often observed in allodynic rats after peripheral nerve injury (e.g., elevated firing frequency of wide dynamic range neurons, presence of after-discharge), conceivably because of an electrically induced increase in GABAergic activity.
SCS likely prompts the release of a multitude of as-yet-unidentified transmitters and neuromodulators in the dorsal horn as well as supraspinally. , , , In addition to GABA, animal and human studies indicate that SCS releases substance P, serotonin, glycine, adenosine, and noradrenaline in the dorsal horn. , , The resultant beneficial effect likely depends on a complicated interaction among several substances. Studies also indicate that the cholinergic system is involved in the SCS effect in painful neuropathy via activation of the muscarinic M4 receptors. ,
Descending inhibition via a brain stem loop was proposed in the 1980s as the principal mechanism by some research groups. Our early studies indicated that only a minor part (<10%) of the DH inhibition is relayed by a supra-spinal loop, but later observations point to a more important participation of the descending inhibitory tracts. Recent research in rodents with SCS electrodes applied both at high (cervical) and low (lumbar) levels of the spinal cord with and without transection of the dorsal columns demonstrates that up to 50% of the effects of SCS in neuropathic pain might be due to activation of supraspinal circuitry. Recent neurophysiological studies with microelectrode recordings from different brain stem regions demonstrated that both the “serotonergic cells” and the OFF-cells in the rostroventromedial medulla (RVM) are selectively activated by lumbar SCS in neuropathic rats, and this happens only in animals responding to stimulation in behavioral studies. In SCS-responding animals, the tissue concentration of 5-HT in lumbar dorsal horns increases following stimulation. Years earlier, a release of 5-HT was demonstrated in response to SCS applied with clinical parameters in decerebrated cats. The effects of 5-HT release in the dorsal horns seem to be mediated by the spinal 5-HT2A, 5-HT3, and 5-HT4 receptors, whereas the effect of the 5-HT3 activation appears to operate via spinal GABAergic interneurons.
SCS also activates neurons in the locus coeruleus, where many noradrenergic cell bodies are gathered. In contrast to the RVM where 5-HT release can be observed at the spinal level, there is no sign of direct projection of noradrenergic neurons activated by SCS to the dorsal horns (as demonstrated by several methods). However, a cascading release of neuroactive substances is probably induced or modulated by SCS both in the dorsal horns and in other sites (e.g., in the brain stem) with multiple, as yet unknown, mechanisms thereby activated.
This emerging knowledge about stimulation-induced transmitter release might be used to apply adjunct pharmacologic therapy in patients whose response to SCS is less than optimum. , In fact, the first clinical trial of adjunct pharmacologic therapy, conducted in 48 subjects who had neuropathic pain that had not responded well to SCS, found that intrathecal delivery of the GABA-B agonist baclofen in addition to SCS was beneficial in a subgroup (about 20%) of the subjects and that the effect was durable over a long time. , Other drugs, like clonidine, which partly exerts its beneficial effect via the cholinergic system, have been found to be effective in selected cases. The present knowledge of the neural circuitry and transmitters involved in conventional SCS, when applied for neuropathic pain, is illustrated in Fig. 119.3 .
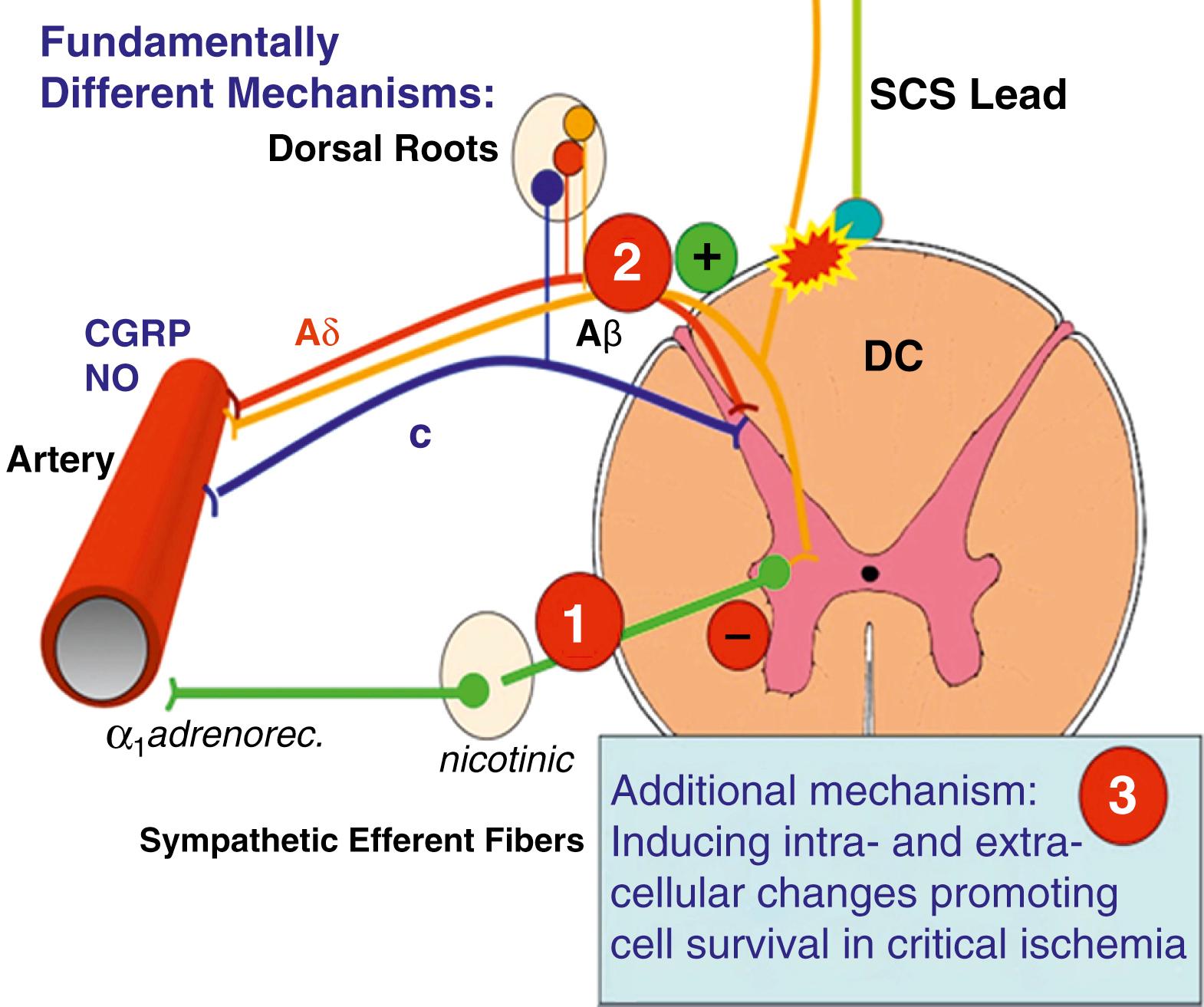
Ischemic pain is the only type of nociceptive pain known to respond to SCS, and the mechanisms involved in the stimulation-induced alleviation of ischemic pain differ fundamentally from those involved in the relief of neuropathic pain. , ,
SCS appears to exert its beneficial effect in the treatment of ischemic extremity pain by reducing tissue ischemia through increased or redistributed perfusion to the ischemic area and/or by decreasing tissue oxygen demand. In PAOD, the results of experimental studies favor the idea that SCS suppresses efferent sympathetic activity, particularly vasoconstriction maintained by nicotinic ganglionic receptors and mainly by α1-adrenoreceptors in the periphery. , This reduced peripheral vasoconstriction results in reduced ischemia and secondary relief of pain. Antidromic mechanisms might also be activated by SCS at intensities far below the motor threshold, and this might result in peripheral calcitonin gene-related peptide (CGRP) and nitric oxide release, with subsequent peripheral vasodilatation. The balance between the two mechanisms seems to depend on the level of activity of the sympathetic system, SCS intensity, and individual patient factors (e.g., genetic differences, diet), but animal studies indicate that antidromic activation might be more important during an initial vasodilative period, whereas sympathetic inhibition appears to support persistence of increased peripheral blood flow. ,
Investigation into the powerful therapeutic effect of SCS in vasospastic disorders (ischemic skin flaps induced in experimental animals ; patients with Raynaud syndrome ) shows that the mechanism of preemptive SCS might involve blocking or reducing vasospasm. This is consistent with theories that Raynaud syndrome is caused by a heightened sensitivity or increased density of α-adrenergic receptors in possible combination with dysfunction in the CGRP system. Consequently, a stimulation-induced “normalization” of function in each system could underlie the efficacy of SCS in treating this condition. Fig 119.4 illustrates the possible mechanisms of action of SCS in peripheral ischemia.
Complete lumbar sympathectomy in laboratory animals abolishes the beneficial vasodilative effects of SCS on skin and muscle tissue. In some of the animals, even an incomplete sympathetic denervation leads to partial loss of the vasodilative benefit of SCS. This supports the notion that the beneficial effect of SCS depends on its action on the sympathetic system; however, because sympathetic blocks or even surgical sympathectomies are rarely complete, SCS can be tried in patients with previous sympathetic interventions.
An interesting observation, which has not been addressed in prospective randomized clinical trials, is that increasing SCS frequency to 500 Hz in a rat model significantly increased peripheral blood flow monitored by a laser Doppler technique.
SCS therapy is often effective in complex regional pain syndrome (CRPS) accompanied by signs of dysautonomia. In principle, SCS could affect pain syndromes related to sympathetic hyperactivity by direct action on central hyperexcitability and/or by encouraging development (direct coupling) of de novo abnormal contacts between peripheral sympathetic and damaged somatosensory fibers. The indirect-coupling hypothesis, which proposes that damaged sensory neurons might become so hypersensitive to mild degrees of hypoxia that even moderate increases in sympathetic activity with peripheral vasoconstriction could excite the damaged afferents, , was not supported by the findings of a study in which SCS did not cause peripheral vasodilatation in subjects with CRPS type I.
The mechanisms underlying the effect of SCS on pain due to ischemia in the extremities, whether from occlusive vascular disease or vasospasm, seem to rely on a rebalancing of oxygen supply and demand (i.e., relief of net ischemia). The mechanisms discussed in this section are schematically outlined in Fig. 119.2 . SCS-induced vasodilation in a situation with low sympathetic vasoconstrictor tone might also occur as a result of antidromic activation, whereas with a high level of sympathetic activity, SCS-induced sympathetic inhibition could also contribute to the effect.
The mode of action of SCS in otherwise refractory angina pectoris appears to be complex, and investigators have derived conflicting data from experimental and clinical studies. Although the first experimental studies revealed that SCS has a direct inhibitory effect on cardiac nociception, the clinical studies that followed clearly demonstrated that partial resolution of cardiac ischemia seems to be a pivotal factor in the antianginal effect of SCS. Some researchers believe that a stimulation-induced increase in blood flow or redistribution of the blood supply from well-perfused to ischemic regions in the heart is the cardinal underlying factor, whereas others credit a decrease in cardiomyocyte oxygen demand. In any case, this reduced ischemia is manifest as decreased ST changes on electrocardiography as well as reversal of lactate production to extraction. However, experimental studies have been unable to demonstrate a local blood flow increase in the myocardium ; instead, preemptive SCS seems to increase the myocardium’s resistance to critical ischemia.
Another observation of possible importance is that local coronary ischemia excites the intrinsic cardiac nervous system, which consists of mixed somatosensory and autonomic ganglia located in fat pads on the exterior surface of the heart and mediates neural activity to and from the heart. This ischemia-induced excitation might lead to generalized ischemia by encouraging dysrhythmia. SCS seems to inhibit and stabilize the activity of these cardiac neurons, especially during an ischemic challenge. , , Indeed, SCS can counteract severe cardiac arrhythmia. ,
The last word on the use of SCS in cardiac ischemia remains to be written, and the effects of stimulation might extend beyond the relief of pain to provide cardioprotection before the advent of additional chest pain. ,
Whereas several preclinical studies indicate a therapeutic and protective role of SCS in ischemic cardiac conditions, clinical studies to date have not provided solid support for the effects of SCS in heart failure where only the first study (with a questionable control group) reported protective effects in several parameters reflecting halted progression of cardiac dysfunction. ,
HF SCS applied to a nerve or an axon provides a local conduction block, probably by inactivating ion channels along a short segment (e.g., Kilgore and Bhadra , ). When 10 KHz is applied to the dorsal spinal cord (HF SCS), the mode of action is not so simple. No block of transmission or activation is observed with the short pulse widths and low amplitudes used clinically.
The initial “working hypotheses” for 10 kHz SCS were:
HF SCS induces a depolarization blockade (a local reversible block; cf e.g., Kilgore & Bhadra , ).
A “membrane integration” occurs, which resembles classical temporal summation; that is, impulses within a certain time frame might depolarize a neuron and fire an action potential, although each individual impulse is insufficient to do so.
HF SCS can desynchronize neural signals from clusters of neurons firing in synchrony, so that each unit instead fires at its own rate with its own individual pattern. This hypothesis stems from observations with cochlear stimulation in patients with cochlear implants and from animal experiments; to the best of our knowledge it has not been applied to pain research.
The first two of these “working hypotheses” were challenged by a computer simulation study that demonstrated that theses mechanisms would require higher stimulation amplitudes than are used clinically in HF SCS.
In another computer modeling study, concluded that with clinical HF stimulation frequencies and pulse widths, HF stimulation seems to preferentially block large-diameter fibers and concomitantly recruit medium and smaller diameter fibers in the DCs. Thus, the large diameter fibers that would cause paresthesia with low-frequency SCS are blocked, while medium and small diameter fibers are recruited, leading to paresthesia-free relief of neuropathic pain by an inhibition of the WDR cells in the underlying dorsal horns. However, this model finding and the conclusions drawn from it are not compatible, with the observation that patients treated with 10 kHz SCS demonstrate no clinical signs of decreased large diameter fiber function (e.g., numbness or other sensory defects) even within the T9 to T10 dermatomes.
There has been only one clinical study in which SCS with slightly higher frequencies (200 to 1200 Hz) than conventionally used was applied in 20 patients who underwent quantitative sensory testing. Unlike traditional SCS, higher frequencies led to elevated pressure detection and pressure pain thresholds.
Studies by Song et al. showed that transmission in the dorsal columns is not affected by HF SCS; that is, neither fiber recruitment nor blockade occurs. Another study that used tungsten electrodes to record single DC fibers in rats with nerve injury showed no blockade with high-frequency SCS at clinically relevant amplitudes. When the authors increased the amplitude to motor threshold, 80% of the DC fibers still were not blocked. They concluded that the mechanisms involved in the effects of HF SCS are probably different from those of conventional SCS.
To date HF SCS has not been found to be superior to traditional SCS in animal studies using monophasic pulses. Since the DCs are neither activated nor blocked by HF SCS, the mechanisms might be segmental. In a rat study with biphasic stimulation Shechter et al. (2013) compared 50 Hz, 1 kHz, and 10 kHz applied at 20%, 40%, and 80% of motor threshold for 30 minutes on 3 consecutive days. For 40% motor threshold (the only amplitude of clinical interest), some effects emerged over 3 days, and 1000 Hz proved to be slightly more or equally as effective as 10 kHz SCS.
High-frequency (>800 Hz) SCS might result in uncomfortable experiences as soon as the amplitude is beyond the sensory (paresthetic) threshold.
The pulse widths used, both in the clinic and in animal studies, are necessarily very short (around 24 to 30 microseconds), and the amplitude is set so low that the patient does not feel anything. With such current parameters, one critical factor might be the amount of electric charge transmitted per unit time to the nervous tissue. Studies comparing monophasic and biphasic SCS with different pulse widths indicate this.
Preclinical studies as yet published only as conference abstracts , focusing on dorsal horn modulation with high-frequency SCS (2 to 10 kHz) have yielded interesting results. Application of 10 kHz SCS at a low amplitude (20% of motor threshold) in the rat through conductive agar from needle electrodes directly over the L5 segment demonstrated no evidence of either DC fiber conduction block or activation. Stimulation for several hours did not induce asynchronous firing in myelinated primary sensory neurons. In different pain models, as much as 135 minutes of SCS at very low amplitudes: 20% of motor threshold, which was verified to be subthreshold for activation of A-β projection neurons, induced late inhibition of A-δ and C-fibers, which occurred after 45 to 90 minutes both in superficial and in deeper laminae (lam. IV to V).
The critical issues here seem to be the very low amplitude, high frequency: 10 kHz, very short pulse width resulting in the marked latency for the appearance of the effects. It is expected that more data from these experiments will be published in the near future. ,
Based on the few published basic studies available so far and the demonstration of some effect both with mono and biphasic SCS pulses , and the absence of numbness in clinical cases (Van Buyten and others; personal comm.) as well as the demonstration of no effect on the impulse traffic in the DCs, the putative mechanisms so far are considered to be most likely segmental. This is also supported by the McMahon et al. observations discussed above.
Thus, while the definitive mechanisms of high-frequency SCS are not yet known, the direct effect of the low intensity, kHz electrical field applied to the dorsal surface of the SC acting at a population of neurons in the DH might be responsible for the beneficial effects of 10 kHz paresthesia-free SCS with a considerable latency, as observed clinically.
Several reviews summarize both hypotheses about HF mechanisms and the clinical studies up to 2018.
The principle of burst stimulation advanced by De Ridder is based upon his work with cortical stimulation. The concept is not new; in the 1970s, burst transcutaneous electrical nerve stimulation (TENS) was launched as a variant to steady-frequency TENS. At that time, hypotheses about the mechanisms for burst and high-frequency TENS were discussed; burst TENS was generally applied for nociceptive pain, and mediation by release of endogenous opioids was inferred as a possible mechanism, notwithstanding evidence to the contrary. , Recent animal studies indicate that the antinociceptive effect of low-frequency stimulation (SCS) might depend on opioid mechanisms.
Burst SCS was presented as a stimulation mode that would be effective for axial lumbosacral pain as well as for neuropathic pain. , De Ridder argues that bursting or irregular firing is more like normal nerve activity than is tonic stimulation, as delivered in conventional SCS. Burst stimulation is said to require less temporal integration to activate neurons and to be more effective as “a wake-up call to the brain.” De Ridder et al. have, on the basis of “source localized EEG” investigations of patients with burst SCS, advanced the idea that burst SCS could activate cortical areas (the dorsal anterior cingulate and the dorsolateral prefrontal cortex) involved in the modulation of pain perception (Fig.119.5). A study by Tang et al. demonstrates that there is no activation of the dorsal column Aβ fibers (as measured by recordings from the gracile nucleus in the brain stem) with burst SCS, whereas conventional SCS produces a powerful increase in firing.
Another finding is that the efficacy of burst stimulation seems to relate to the electric charge per burst—at least in a rat model of neuropathic pain.
Other studies report that burst SCS increases GABA levels in peripheral blood of the rat and does not rely on activation of GABA-B receptors, as has been claimed for traditional SCS (e.g., ; Ultenius et al. 2012).
De Ridder and Vanneste presented data from five patients undergoing tonic, burst, and sham stimulation. In a source-localized electroencephalogram subtraction and conjunction analysis they showed that burst and tonic stimulation share activation of some cortical areas such as the pregenual anterior cingulate cortex, while only burst SCS reduces the connectivity between the dorsal anterior cingulate and the parahippocampal cortices. They hypothesize that burst stimulation must modulate the medial pain pathway directly by actions onto C-fibers ending in lamina 1 connecting to the dorsomedial nucleus of the thalamus and hence to the dorsal anterior cingulum (see Fig 119.5 ). A further hypothesis presented here—linking it to the high-frequency tonic stimulation hypotheses—is that burst stimulation could disrupt synchronous firing of high threshold fibers, thus inhibiting activation directly related to pain perception.
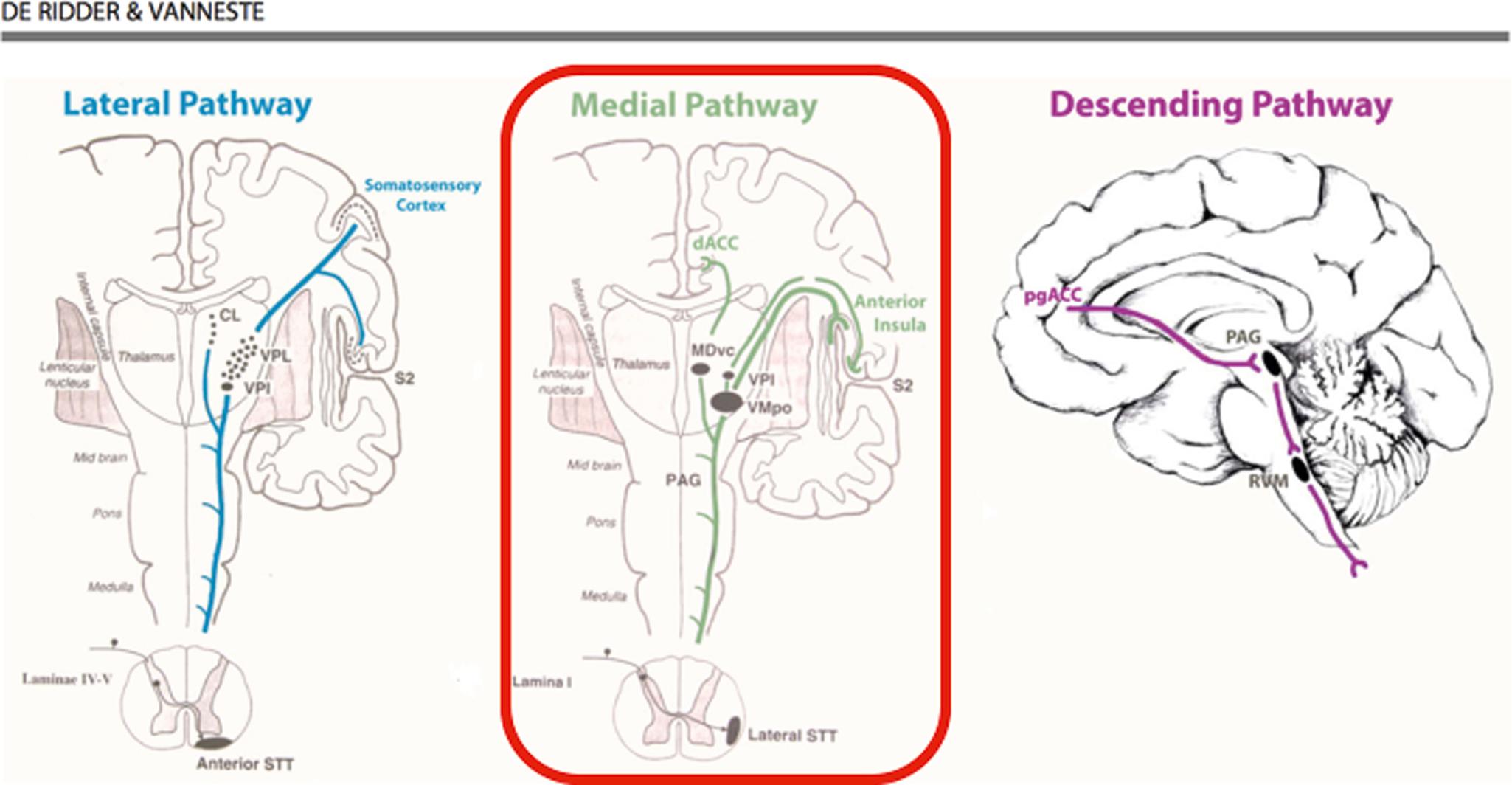
A functional magnetic resonance imaging study demonstrated that conventional SCS predominantly modulates lateral pain pathways as shown by changes in blood oxygen levels in the somatosensory cortices; however, research on eventual participation of supraspinal circuitry in burst SCS is ongoing, and the stimulation paradigm itself might be subject to change in the near future.
Most modern pulse generators can generate stimulation frequencies up to, if not above, 1000 Hz and pulse widths up to 1000 microseconds. Higher frequencies than typically used for SCS have been available for many years but have used infrequently; for example, as high cervical SCS for torticollis.
A common denominator of the new SCS algorithms is that they are applied with a relatively low amplitude and/or pulse width, so that the subject does not experience paresthesia or other cue as to whether active stimulation or placebo SCS is delivered. This admits the possibility of blinded, controlled trials.
A few studies show that conventional tonic SCS with subparesthetic amplitude might also be effective, but so far it has not convincingly been demonstrated that the outcomes of this type of SCS are equal to those of conventional paresthetic stimulation.
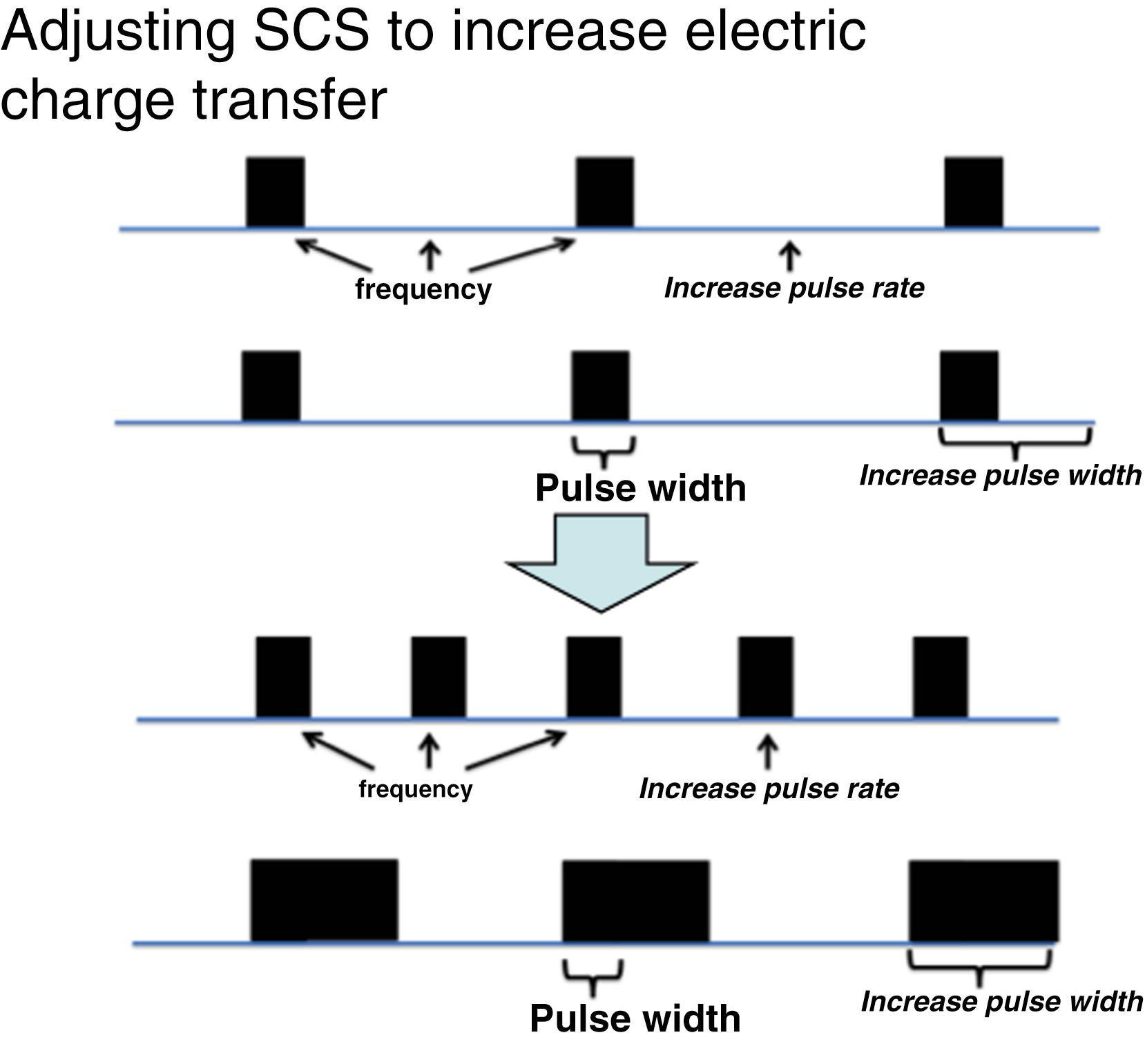
By increasing the frequency and/or the pulse width but keeping the amplitude so low that stimulation is not felt, larger amounts of electric charge can be delivered to neural tissue without discomfort or damage ( Fig. 119.6 ).
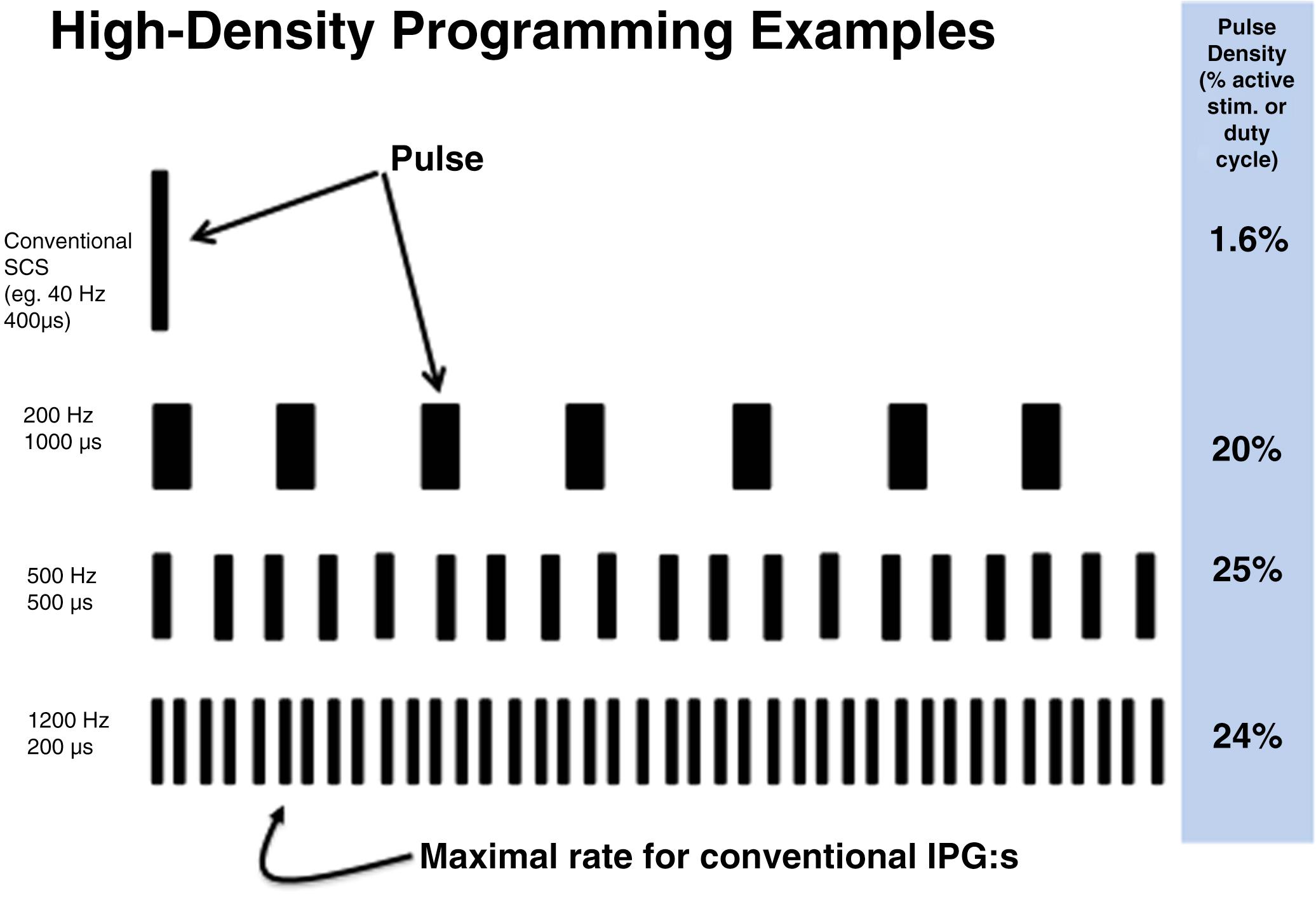
It has been demonstrated convincingly in rodent experiments that subsensory SCS can exert clear effects on models of neuropathic pain. , These studies also demonstrate that moderately increased frequencies (e.g., 1000 Hz) can be as effective at the subsensory level as 10 kHz SCS. Such changes in SCS parameters have been labeled “High-Density Stimulation” (HD) referring to the fact that pulse density (i.e., the duty cycle) can be increased from 1% to 2% up to 20% to 25% while remaining below the sensory threshold ( Fig. 119.7 ). Overall electric charge transfer is comparable to that of HF SCS and burst SCS.
In fact, an electrophysiological animal study demonstrated that compared with typical clinical SCS parameters (200 microseconds/50 Hz), 1 kHz SCS suppressed responses of more spinal neurons and/or demonstrated longer persisting suppressive effects. Thus SCS at 1 kHz might equal or exceed 10 kHz in effectiveness, as was reported by Shechter et al. in 2013 and Thomson et al. in 2018.
High-frequency SCS at amplitudes above sensory threshold (beginning above 800 Hz) generates an uncomfortable sensation rather than pleasant “tingling” paresthesia.
The first applications of SCS involved high thoracic electrode placement in an attempt to treat pain in all caudal segments ; however, this strategy commonly caused excessive, uncomfortable radicular effects before the desired segments could be recruited. When it became apparent that stimulation paresthesia should overlap the distribution of pain, clinicians adjusted the placement of electrodes to achieve this effect. For example, low thoracic electrode placement (T9 to T12) is most effective in the treatment of persistent low back and lower extremity pain following spine surgery (FBSS).
In the late 1960s and early 1970s, SCS electrodes were two-dimensional and required a laminectomy or laminotomy for introduction into the epidural, endodural, or subarachnoid space. Use of these electrodes was problematic because clinicians had no way of determining the ideal spinal level for electrode placement in any given patient and because laminotomy under local anesthesia limits longitudinal access. Furthermore, even when electrodes are placed so that paresthesia overlaps the area of pain, not all patients report pain relief. For these reasons, test stimulation with a temporary electrode is desirable.
Accordingly, in the 1970s, investigators developed percutaneous techniques using a Tuohy needle to insert temporary catheter-type electrodes for a screening trial to establish the best level for electrode placement and to determine if SCS had the desired analgesic effect. Clinicians soon applied these percutaneous techniques to the implantation of electrodes for chronic use, thus avoiding the need for laminectomy. , However, the use of a percutaneous technique to place multiple individual electrodes and achieve bipolar stimulation increased the likelihood of electrode migration, compromising stimulation, thus reducing or eliminating pain relief, and requiring surgical revision.
In the early 1980s, in response to this problem, electrode manufacturers introduced percutaneous electrodes with arrays of contacts. If such an electrode migrates slightly, its implanted pulse generator (IPG) can be reprogrammed with a different selection of stimulating anodes and cathodes to reestablish appropriate paresthesia. This noninvasive postoperative adjustment can be made with the patient in the upright or supine position (in which the device is ordinarily used, as opposed to the prone position in which it is usually implanted). Multicontact programmable systems rarely require surgical revision, and this development has led to significantly improved long-term clinical results.
New electrode designs based on computer models of SCS have facilitated steering paresthesia to cover the painful area. Clinicians are also improving results by refining the method of anchoring percutaneous electrodes.
Despite these improvements in the use of percutaneous electrodes, properly placed laminectomy electrodes offer advantages. For example, a prospective randomized, controlled technical comparison involving 24 patients—half of whom received a four-contact percutaneous electrode and half a four-contact insulated laminectomy electrode —yielded significantly superior results with the laminectomy electrode for paresthesia coverage of pain, at the same time reducing power requirements sufficiently to double battery life.
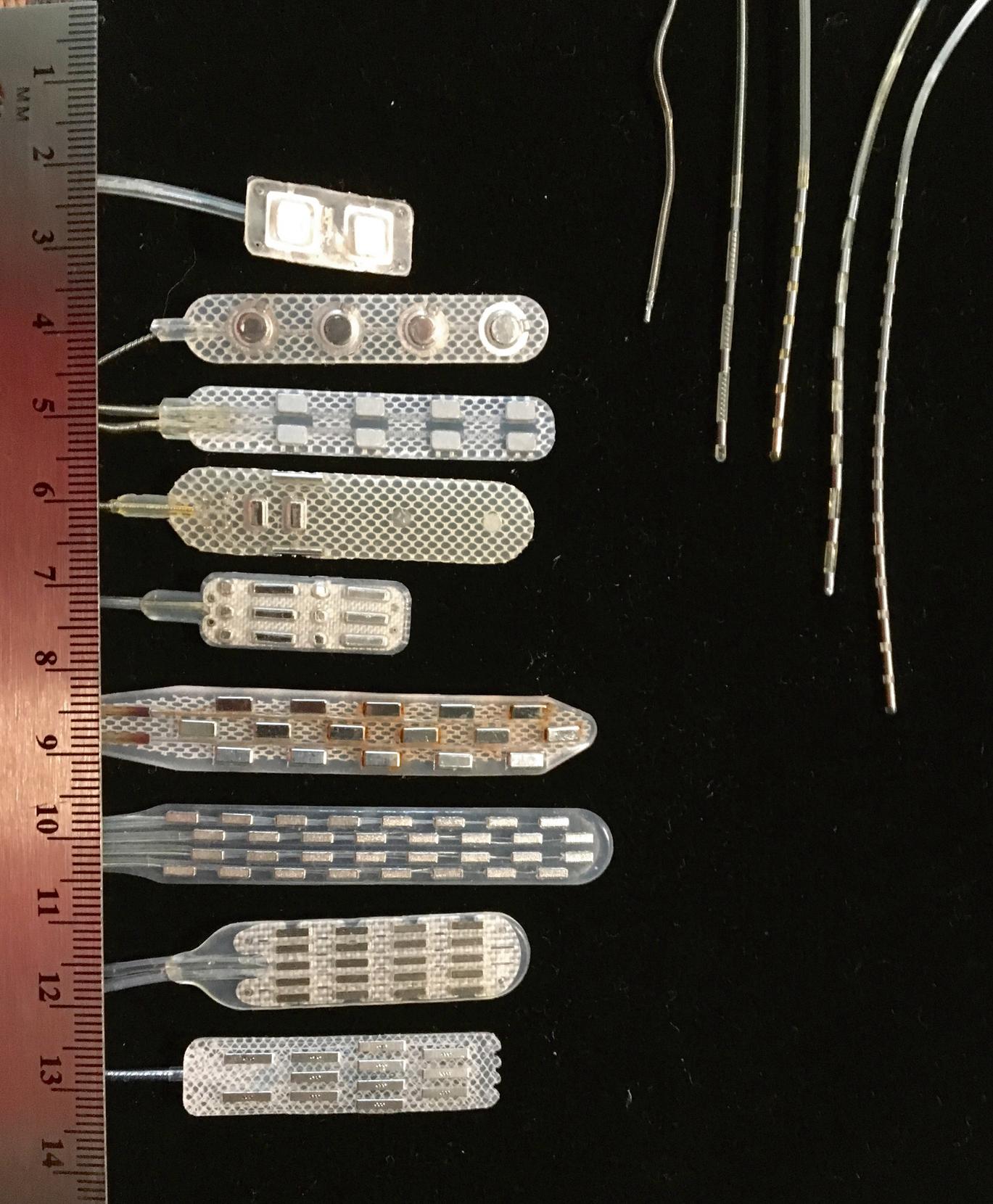
Fig. 119.8 shows a sample of percutaneous and laminectomy electrodes as well as the oblique-lateral approach used to place a percutaneous electrode and a small laminotomy opening for a laminectomy electrode. The percutaneous electrode is inserted under local anesthesia, which does not interfere with the clinician’s ability to monitor paresthesia during test stimulation. The laminectomy electrode can be implanted with local anesthesia alone, using regional anesthesia (with paresthesia achieved at a slightly higher than normal stimulation threshold to guide electrode positioning) or even with spinal anesthesia to a degree allowing intraoperative paresthesia testing. , Implantation of laminectomy electrodes under general anesthesia, using evoked potentials, has been refined and reportedly can improve outcomes.
New waveforms described above are delivered using the same electrode designs, targets, and methods of placement as those used with traditional waveforms. It has been suggested that paresthesia mapping in each patient is not necessary to optimize placement for high-frequency (HF10) paresthesia-free stimulation and that fluoroscopic guidance alone (spanning levels identified in populations of patients by paresthesia mapping) is sufficient; but affirmative evidence of this is lacking. Most studies of new waveforms have begun with electrode implantation using conventional stimulation and paresthesia mapping. If an electrode is ever to be used for conventional paresthesia-based stimulation (e.g., when a patient fails HF SCS), then implantation using paresthesia mapping (or evoked potentials as a surrogate) remains appropriate.
The prototype SCS generator, used exclusively during the first decade of experience, was a passive implant powered to deliver stimulation pulses by an external radiofrequency transmitter. Although the implant contained no life-limiting components and thus avoided the expense and potential morbidity of eventual replacement, this system was cumbersome. An IPG powered by an internal battery was subsequently developed from pacemaker technology. Patients operate these systems and control the amplitude within preset limits with an external magnet or handheld remote control. The first IPGs were powered by nonrechargeable lithium cells that required replacement approximately every 4 years. To avoid such frequent surgical replacement of the battery, with attendant expense and risk, SCS device manufacturers developed IPGs with rechargeable batteries. This, of course, increases initial cost, and comparative cost effectiveness remains to be established by long-term study. The less expensive radiofrequency systems remain in use, and miniaturization of circuitry and new antenna designs have allowed development of a system that can be inserted in its entirely through a needle.
Additional IPG technical improvements include automated amplitude adjustment to compensate for postural changes, which is based upon accelerometer data or compound action potentials. Some evidence has been reported that SCS is safe in patients with cardiac pacemakers or cardioverter defibrillators if the devices are managed appropriately.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here