Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The first clinical report of spinal cord stimulation (SCS) was published in 1967. These devices were the logical progression of the concept of gate control theory proposed by Wall and Melzack in 1965. This theory proposed that “control of pain may be achieved by selectively activating the large, rapidly conducting fibers.” Since this first report of the use of dorsal column stimulation, the use of implantable devices for the management of chronic pain has become well established in the United States, Europe, and Australia, and around the world approximately 35,000 to 50,000 patients undergo implantation of a spinal cord stimulator yearly. Electrical stimulation of the spinal cord has proven efficacy for the relief of pain in patients with arachnoiditis, pulmonary venoocclusive disease (PVOD), peripheral nerve injury, complex regional pain syndrome (CRPS), angina pectoris, and radiculopathy and pain of spinal origin.
Indications for SCS device implantation are not always clear. Experienced clinicians can be found debating the relative efficacy of implantation for specific diseases or in unique and complex patients. Despite the absence of randomized, controlled trials in many cases to prove the efficacy of SCS for specific conditions, strong recommendations for most patients can be made because the results of multiple observational studies can be considered overwhelming for major conditions. To date, the conditions with the strongest evidence as to the benefit of SCS are the failed back surgery syndrome (FBSS) and type 1 and 2 of the CRPS.
FBSS refers to patients who have new or persistent pain after spinal surgery for back or leg pain. One systematic and comprehensive review regarding the effectiveness of SCS in treating chronic spinal pain showed that there is a clear (level I–II) role for conventional low-frequency SCS as a treatment for otherwise intractable lumbar FBSS. Another recent meta-analysis detailing conventional SCS for chronic back and leg pain demonstrated that more than half of the patients experienced remarkable pain relief, independently from previous spinal surgery the patients possibly underwent. Moreover, in this study, pain remission was maintained during a mean follow-up period of 24 months. Finally, in an extensive 2004 review of the literature, an overall success rate of 62% was demonstrated among the 747 patients affected by FBSS and treated with SCS.
CPRS is a form of chronic pain that usually affects an arm or a leg and typically develops after an injury, a surgery, a stroke, or a heart attack. It is defined by pain out of proportion to the severity of the initial injury. Although its incidence is significantly less than that of FBSS, its indications are also well documented in the literature. One randomized controlled trial (completed among 56 patients), which compared SCS plus physical therapy with physical therapy alone, showed that at 6 months, the SCS group pain was reduced by 3.6 on the visual analogue scale (VAS). In comparison, among the group receiving physical therapy alone, VAS pain was increased by 0.2 ( P < .001). A recent randomized prospective trial has compared SCS with another promising neuromodulation technique, dorsal root ganglion stimulation (DRGS), which involves the percutaneous placement of a lead in the epidural posterosuperior space of the intervertebral foramen. Both methods of treatment were proven effective in this trial; however, higher statistical significance was associated with DRGS when focusing on the specific metrics of pain relief, postural stability, and mood improvement.
General indications for SCS implantation can be applied to all potential candidates. It is usually accepted, but not proven, that patients who have objective findings related to their pain complaint will have better outcomes after implantation than patients who do not have such evidence. A clear exception to this rule is patients with CRPS for whom there is often not objective evidence of their pain complaint but who have been reported to respond well to SCS.
Patients who have clear non-SCS surgical options with a good probability of success for relief of their pain should undergo the definitive surgical procedure rather than treatment with an implantable device. For example, a patient with an L5 radiculopathy and a magnetic resonance imaging scan that shows a herniated nucleus pulposis that compresses the L5 nerve root should be treated with a discectomy rather than implantation of an SCS system. In addition, patients who underwent surgical spine procedures may still suffer from unrecognized persistent compression of the neural elements. For this reason, a preoperative spinal magnetic resonance imaging should routinely be performed to search for an organic substrate of the pain before SCS may be proposed as the next therapeutic option.
Implantation, although a relatively low-morbidity procedure, is a surgical procedure, and less invasive or dangerous alternative treatments should be exhausted before consideration of implantation of these devices. These include physical medicine approaches, psychological approaches, anesthesia procedural approaches such as epidural steroid injections, and pharmacologic approaches. A trial with and failure to respond to oral opioid therapy before consideration for implantation of an SCS device is controversial, and there are scarce data to help with this decision. Variables include physician and patient preferences, history of substance abuse, patient tolerance, type of pain, and family concerns, among others. Of note regarding this subject is a recent systematic review of randomized controlled trials and meta-analyses which demonstrated that, in patients with intractable spine/limb pain, SCS was associated with increased odds of reducing pain medication consumption. In addition, in the largest randomized controlled trial to date comparing different SCS techniques, SCS demonstrated significant improvement in pain, function, and quality of life in addition to reduced opioid consumption.
A psychiatric or psychological evaluation should be obtained before implantation. Patient outcome may depend on confounding coexisting psychiatric comorbidity. It is extremely important to assess patients for psychiatric disorders, including substance abuse, pain disorder, somatization disorder, depression, factitious disorder, malingering, and personality disorder. Psychiatric comorbidity is a controversial and difficult topic to resolve. The presence of one or more of these disorders should not exclude the patient entirely from consideration for implantation but should be a strong vector in the decision-making process.
The final general indication for implantation is response to a trial of SCS. A survey of academic pain centers in the United States revealed that all respondents indicated that trials are done before implantation. The average duration of a trial for SCS is 6.6 days. Two programs report that trials for SCS are done on the day of implantation in the operating room. Although some practitioners may believe that this is an inadequate trial, no evidence currently exists to compare duration of trial and outcome for SCS. There is general consensus that a trial of approximately 1 week with good pain relief is the best predictor of success with subsequent implantation. A unique phenomenon regarding the trial period was examined by a retrospective chart review which showed that placement of trial stimulator decreased pain scores and reduced pain scores more significantly more than permanent spinal cord stimulator did. This phenomenon may place patients in a challenging psychological situation in which they are left hoping for and expecting long-term relief that might not always arrive.
Patient selection remains the biggest source of error in implantable therapies. There is no equation to define how to consider the effects of ongoing litigation, history of substance abuse, unrecognized psychological disease, lack of employment, lack of a support system at home, disruptive sleeping patterns, abuse of medication, poor or good coping skills, cultural variables, family history of chronic pain, and so on. In addition, there will still be placebo responders to such dramatic interventions as an SCS implant.
Cost-effectiveness analysis of SCS versus best medical and conventional treatment for chronic pain shows that the cumulative 5-year costs for SCS were $29,123 per patient compared with $38,029 for patients treated with conventional methods. In addition, 15% of patients on SCS returned to work compared with none of the patients in the conventional care group. A lifetime cost analysis of SCS versus costs and outcomes before SCS in patients with CRPS showed not only decreased pain and improved quality of life but also a savings of $60,000 per patient. Similar outcomes were obtained when comparing patients treated for intractable angina pectoris with and without SCS. ,
Transcutaneous electrical nerve stimulators are believed to reduce pain by stimulating large myelinated afferent nociceptive fibers in the periphery. This increase in large-fiber activity theoretically stimulates inhibitory interneurons in the substantia gelatinosa, thereby “closing the gate” on the transmission of painful information carried on small fibers. The first SCS implantation was performed based on this theory. This theory has been criticized based on the facts that only chronic, and not acute pain, is reduced by SCS; SCS works for neuropathic pain but not consistently for somatic pain (except for ischemic pain in peripheral vascular disease and angina pectoris, which cannot be characterized as neuropathic); paresthesias must overlap the painful area; several minutes are required before pain is suppressed; weeks of stimulation may be needed to reach maximal pain relief; and relief may outlast stimulation by long periods of time. Pain relief cannot be explained based solely on the “gate control theory” of pain. The SCS mechanism of action is unclear. Some support can be found for 10 different specific mechanisms or proposed mechanisms of action, and the reader is referred for further discussion to a review by Oakley and Prager.
Neurochemical data suggest that SCS may reduce dorsal horn neuronal hyperexcitability. SCS induces dorsal horn release of γ-aminobutyric acid, substance P, serotonin, glycine, and adenosine while inhibiting the release of glutamate and aspartate. Evidence of a neurochemical mechanism of action is limited but suggestive. Neurophysiologic evidence suggests that SCS can cause suppression of wide-dynamic-range A-β fibers normally responsive to mechanical, chemical, and thermal stimulation. In animal models with dorsal horn lesions, these effects are not suppressed. In addition, if the spinal cord is transected above the level of effective SCS, the effect of SCS is reduced. These experiments suggest that the dorsal columns may mediate the effect and that supraspinal mechanisms may be necessary to see an analgesic effect of SCS. Further suggesting the involvement of supraspinal pathways of pain control and transmission are neuroimaging studies demonstrating that tonic SCS mainly acts by modulating the lateral pain ascending pathway and by interfering with the electrical and metabolic activity of the cingulate gyrus, lateral sensory thalamic nuclei, prefrontal cortex, and postcentral gyrus. Additional work has demonstrated that the analgesic properties of SCS could be hampered by the use of opioid antagonists, thus suggesting that SCS might be also effective through the activation of the descending opioid pathway. Animal experiments also show the involvement of the anterior pretectal nucleus, which is an origin of descending pain-inhibitory pathways.
SCS, either directly or indirectly, alters autonomic activity. These observations may be due to stimulation of release of vasoactive substances in the central or peripheral nervous system. Attempts to isolate the autonomic nervous system effects as a direct consequence of SCS and a definitive mechanism of action have been contradictory, and it is an oversimplification and inaccurate to ascribe nociceptive, antianginal, or antiischemic effects of SCS to an alteration in autonomic nervous system activity alone.
In summary, although information assisting understanding of the SCS mechanism of action is accumulating, the precise mechanism of action seems to be complex and may vary depending on the clinical condition for which the device was placed. A single, simple, unifying mechanism of action of SCS is not evident at this time.
Improvement of equipment has increased efficacy and decreased complication rates for implanted SCS systems. Complication rates have declined to approximately 8%, and reoperation rates may be as low as 4%. Early SCS systems used a single monopolar electrode that connected to a percutaneous externalized power source. The current standard of care involves implanted electrodes that are typically quadripolar but that can have dual octapolar electrodes. The number of potentially usable electrode combinations in a dual octapolar system is in the tens of millions. Programming of such a stimulator has not evolved to a point where one can select from among all these combinations, but supporters contend that the large numbers of possible combinations, the closer proximity of electrodes, and the longer area of possible coverage improve the success rate and quality of relief.
The typical implanted electrode array is a quadripolar array. Each electrode can be programmed as either “on” or “off” or as an anode or a cathode. In using a quadripolar system, there are approximately 50 potentially usable combinations. The power systems are either via an implanted generator with a battery or an implanted device with a radiofrequency-coupled receiver transducer system. Implanted generators need to be replaced approximately every 3 to 7 years, whereas the receiver transducer does not have to be replaced. Patients with receiver transducer systems must wear an external device pasted to the skin to receive paresthesia.
A complete description and comparison of electrodes, generators, programming options, and devices can be found at the following websites:
The most common indication for SCS in the United States is for back or neck radiculopathic pain. Patients presenting with conditions such as lumbar or cervical radiculopathy, postlaminectomy syndrome, spondylolisthesis, spondylosis, or even persistent and refractory axial low back pain of uncertain etiology may respond to treatment with SCS and should be assessed. Topography of pain that is amenable to overlap by paresthesia of the spinal cord–stimulating device is a requirement for successful treatment. Radicular patterns tend to be more easily obtained than axial patterns, and for this reason, patients with pain predominantly in the extremities may be better candidates than patients with predominantly axial symptoms. This should not exclude a trial in an otherwise good candidate who has primarily axial symptoms. Although it is technically more difficult to obtain coverage of patients with predominantly axial back or neck pain, newer, more compact electrodes with multiple arrays sometimes placed bilaterally and triangularly might be useful in providing relief to patients with axial pain, and these arrays should be considered. Long-lasting relief is a reasonable goal. A large series of patients with follow-up of 2 to 20 years demonstrated that 52% of patients continued to have a 50% reduction in pain. The authors noted that even among patients who did not claim a 50% reduction of pain, most patients continued to use their devices.
The evidence of efficacy of SCS for back and neck pain is not lacking. A literature review was performed on studies of patients with SCS for chronic leg and back pain and included all relevant articles identified through MEDLINE search from 1966 through 1994. Fifty-nine percent of patients reported in these studies had greater than 50% pain relief at a time between 1 and 45 months after surgery. These authors noted the lack of a randomized trial at that time. Subsequent to this, a prospective, randomized comparison of SCS and reoperation in patients with persistent radicular pain with or without low back pain after lumbosacral spine surgery was conducted. The primary outcome measure of this study was frequency of crossover to the alternative procedure. This study showed a statistically significant advantage of SCS over reoperation. A literature review on SCS for chronic pain of spinal origin was conducted by North and Wetzel, who concluded that SCS is a valuable treatment option, particularly for patients with chronic pain of predominantly neuropathic origin and a topographic distribution involving predominantly the extremities. Efficacy may be better for spontaneous than for evoked pain.
Pain associated with spinal cord injury has been treated with SCS. Most authors report satisfactory responses in a smaller percentage of their patients ranging between 20% and 40%. Case reports supporting the use of SCS for whiplash and idiopathic acute transverse myelitis have been published. , Sindou and colleagues reported the predictive value of somatosensory-evoked potentials assessing neural conduction in the dorsal columns. These authors suggest that if central conduction time is abolished or significantly altered, then the success rate of SCS is dramatically less than in patients with normal preoperative central conduction times.
The usefulness of SCS for the treatment of postherpetic neuralgia has recently been described in a prospective case series involving 28 patients with either acute herpes zoster or postherpetic neuralgia studied over 29 months. Eighty-two percent of patients with intractable pain for longer than 2 years had a median decrease in their VAS score from 9 to 1 (0 to 10 scale) as well as improvement in their pain disability index. Four patients with acute herpes zoster had prompt and dramatic improvement in their pain symptoms.
The treatment of PVOD with SCS is the most common indication for this modality in Europe. For no other indication is the early evidence of efficacy more compelling and yet SCS for PVOD is not a common treatment in the United States. One study reported 37 patients receiving adequate pain relief after a trial in 41 patients with end-stage peripheral vascular disease. Seventy-eight percent of patients reported substantial pain relief at a mean follow-up of 25 months. Another investigator reported the results of SCS in 20 patients with ischemic rest pain and ulcers. Eighteen of 20 patients in this study had immediate relief of pain after electrical stimulation. These authors also reported improved skin nutritional flow leading to healing of ulcers of moderate size and increased salvage of feet with limb-threatening ischemia at 2-year follow-up. They report an increase in capillary density and an increase in red blood cell velocity and peak red blood cell velocity after arterial occlusion by intravital capillary microscopy.
More recently, SCS has been investigated by the European Peripheral Vascular Disease Outcome Study. Patients with nonreconstructable critical leg ischemia showed increased limb survival when compared with patients receiving usual treatment without SCS, as well as improved pain relief. Patients received an implant if their transcutaneous oxygen pressure measurement was 10 to 30 mm Hg before SCS trial or if it was less than 10 mm Hg but improved to 20 mm Hg or more during the SCS trial. Limb amputations were 20% at 12 months in the SCS group compared with 46% in the matched no SCS group. These and other authors suggest the routine use of transcutaneous oxygen pressure both before and during the trial phase as a predictor of success. A useful predictor of success in patients with diabetes with PVOD is the absence of autonomic neuropathy. Twenty-five of 28 patients with autonomic neuropathy failed SCS treatment, whereas all 32 patients without autonomic neuropathy had successful outcomes.
Evidence exists of the efficacy of SCS in severe Raynaud phenomenon and Buerger disease. SCS has shown remarkable effectiveness and should be considered as a treatment for patients with severe PVOD that is not amenable to surgical intervention.
Despite the lack of an identifiable objective lesion, SCS appears to be quite effective for treatment of CRPS. One report retrospectively reviewed experience with SCS and found 12 patients with CRPS who were followed up for an average of 41 months after implantation. Eight of these patients reported excellent pain relief, and four patients reported good pain relief. Another study looked at 18 patients with CRPS. Fourteen patients had a successful trial and subsequently had their systems internalized. Follow-up varied from 4 to 14 months. Six of 14 patients had good pain relief. Five had moderate pain relief. One had minimal and three had no pain relief. Of note is that pain relief was limited to the body parts covered by the paresthesia.
In a randomized trial comparing SCS plus physical therapy with physical therapy alone in 54 patients with CRPS, the SCS group had a mean reduction in pain of 2.4 cm (on a 1- to 10-cm scale) compared with an increase of 0.2 cm in the physical therapy alone group. Health-related quality of life also improved in the SCS group, but, interestingly, there was no difference between the groups in functional status.
A recent report has demonstrated that response to sympathetic blockade can be predictive of a better outcome from SCS for patients with CRPS. Thirteen of 13 patients with a positive response to sympathetic blockade had pain relief during the trial period, and 87% had greater than a 50% reduction in pain at 9 months. Only 3 of 10 patients with a negative response to sympathetic blockade had good relief and went on to permanent implantation, and only 33% had a 50% reduction in pain at 9 months.
It appears that at this time there are very good data to support the use of SCS for the treatment of CRPS if it is possible to obtain paresthesia coverage that overlaps the painful area.
The treatment of postamputation phantom or residual limb pain with SCS is still a controversial area. Contradictory reports for this and for brachial plexus avulsion injury pain exist, and it is not possible to make definitive recommendations for treating or not treating phantom limb or brachial plexus avulsion injury pain with SCS based on these data.
A large body of data supports the use of SCS for intractable angina pectoris. The complexity and implications of severe coronary artery disease as an indication for SCS requires discussion. The first published report of the use of SCS for angina was in 1987. These investigators described 10 patients with intractable angina in whom SCS devices were implanted, which led to a decrease in the frequency and severity of angina and a major reduction in the use of antianginal medications. Other investigators followed with a report on 10 patients and added bicycle ergometry as a measure of exercise tolerance and showed an increase in work capacity, increased time to angina, decrease in recovery time, and a decrease in the magnitude of ST segment depression associated with angina. By 1991, it was shown that reductions in angina attacks continued over 9 months, and a persistence in increase in rate-pressure product (a measure of the ability of the heart to do work). Between 1993 and 1994, results of a randomized, prospective study demonstrated improved exercise capacity, improved quality of life, and a decrease in ischemic episodes as measured by Holter monitor. Complications were few and of a technical nature, and a surgical algorithm that did not involve a trial before implantation was described. It has been shown that SCS does not conceal myocardial infarction that may occur in patients with implanted devices. A decrease in hospital admissions for patients with implanted SCS devices compared with a control group has been demonstrated. In 80% of patients, the beneficial effect has been reported to last 1 year, and in 60%, improvement in exercise capacity and quality of life lasted as long as 5 years. There are no data showing harmful or nontherapeutic effects of SCS for angina. The use of SCS may be beneficial even in critically ill patients. Janfaza and colleagues reported the 6-week survival in such a patient, whereas other investigators have reported an effect insufficient to overcome the severity of ischemic disease, albeit relief of ischemic symptoms, until death occurred.
A prospective, randomized trial of SCS versus coronary artery bypass grafting in 104 patients with severe angina, increased surgical risk, and no prognostic benefits from revascularization demonstrated long-lasting improvement in quality of life and comparable survival and symptom control in both groups. Benefit after discontinuation of SCS has been shown for as long as 4 weeks after discontinuation. No difference in anginal complaints, nitroglycerin intake, ischemia, or heart rate variability was shown. SCS has been shown to decrease hospitalization in patients with angina, and there is evidence that modern SCS devices are compatible with modern pacemaking devices. ,
In our practice, patients who meet selection criteria are instructed not to eat or drink anything after midnight the night before their implantation trial. They are instructed to continue their nonsteroidal antiinflammatory drugs, but care is taken to discontinue other anticoagulants or platelet inhibitors for proper durations of time. The procedure is done in two stages. Both the trial phase (phase 1) and the generator implantation phase (phase 2) are done in the operating room with an anesthesiologist present. The trial phase (phase 1) involves either the placement of a percutaneous cylindrical electrode or a paddle-type electrode placed via minilaminectomy. The electrodes are either removed after a period of trial stimulation or are attached to a connecting cable during phase 1, allowing the electrode to remain under the skin entirely. In this case, the connecting cable can be removed and the electrode attached to a new sterile cable during phase 2.
Patients with chronic pain may have central changes that amplify painful signals. It is necessary to have the patient awake and able to answer questions appropriately to assess the location of paresthesia during the trial phase. Patients may require laminectomy for placement of a paddle-type electrode in the thoracic or cervical region. Patients with chronic pain often have multiple comorbidities, particularly patients with PVOD or intractable angina pectoris. These factors create an anesthetic challenge and require a preoperative consultation between surgeon and anesthesiologist to define goals and needs. Our typical anesthetic involves intravenous fentanyl and midazolam for analgesia, anxiolysis, and amnesia and propofol for sedation. The propofol can be turned off and the patient awakened for questioning regarding paresthesia topography at appropriate intervals. We also use large volumes of local anesthetic, typically 0.5% lidocaine without epinephrine. A dose of 4 mg/kg (56 mL for a 70-kg person) can be safely administered even as a single dose and more can be administered after 1 or 2 hours. Another option is intravenous remifentanil plus local anesthetic, but this requires an anesthesiologist experienced in the use of this drug because the therapeutic window is often so narrow. Despite the best efforts of experienced practitioners, the implanter should be prepared for oversedation and the need for airway control during the procedure or suboptimal ability to describe the paresthesia topography ( ). The patient should be advised that this is a possibility and that if it occurs, the electrode can still be placed without paresthesia guidance, but success rate might be reduced. The patient should also be educated about information needs that will occur intraoperatively before getting to the operating room. A recent report describes the successful implantation of an SCS device in 19 of 19 patients undergoing laminectomy with 12.5- to 20-mg bupivacaine intrathecal spinal anesthesia. In all patients with spinals and anesthesia throughout the surgical procedure, SCS paresthesia was readily appreciated by the patients.
There is no description of the absolute need for alertness and ability to communicate during surgery. It is generally accepted that, due to uncertainty about anatomic and physiologic overlap, simply placing the lead at the proper anatomic location will not reliably produce a paresthesia that will be effective in reducing pain. There are published case series in which successful implantation has been done under general anesthesia.
A retrospective, nonrandomized review of surgical experience concluded that patients with flat electrodes placed via laminectomy had better pain relief that was longer lasting than patients who had percutaneously placed cylindrical electrodes. A prospective, randomized, controlled trial has recently shown that electrode arrays implanted via laminectomy had lower amplitude requirements and better patient ratings of paresthesia coverage. The decision as to whether to place a flat electrode via minilaminectomy or a percutaneously placed cylindrical electrode is a controversial and emotionally charged issue because anesthesiologists, who place most of these devices, use a percutaneous approach and many neurosurgeons place leads via laminectomy. To further complicate matters, anesthesiologists should not place leads via laminotomy because they do not have the training, experience, or ability to manage complications and many pain specialist anesthesiologists derive substantial income from placing SCS devices (see ). In addition, other specialists such as physiatrists and neurologists are receiving training and are eligible for board certification in pain medicine and are implanting cylindrical leads. At this time, there is no overwhelming evidence of the advantage of placing electrodes via a laminectomy compared with percutaneously placed leads, but there is at least one compelling study.
For percutaneous placement of a cylindrical electrode, the procedure is as follows. Patients are placed prone on a fluoroscopy table in the operating room and prepared for a surgical procedure that can last hours. We do not place a Foley catheter. Standard surgical preparation is done after identifying important landmarks fluoroscopically and marking the skin to identify them. The skin and subcutaneous tissue are anesthetized with local anesthetic before insertion of the 15-gauge epidural needle. The epidural space is identified using a loss of resistance to air or saline technique ( Fig. 120.1 ). For a patient with an L5 radiculopathy pain pattern, the epidural space is entered at approximately T12–L1, at which point the cylindrical electrode is advanced through the needle and positioned using fluoroscopic guidance to the desired level at approximately T9–T10, at which time trial stimulation is carried out ( Figs. 120.2 and 120.3 ) . For placement of a device for stimulation of the upper extremity, entry is made at T1–T3 with the electrode tip to arrive at C3–T4 approximately. The target site for the electrode tip for a patient with angina is approximately T4–T5. For a unilateral syndrome, the electrode should be positioned approximately 3 mm ipsilaterally off midline. The electrode can be directed and “steered” using an angled stylet. Once the target anatomic site is reached, the electrode is anchored and attached to a temporary screening cable and a trial screener. The awakened patient is then questioned about the presence or absence and location of paresthesia coverage. Parameters that are available for adjustment include amplitude and pulse width and frequency, the same as with an implanted generator (see ).

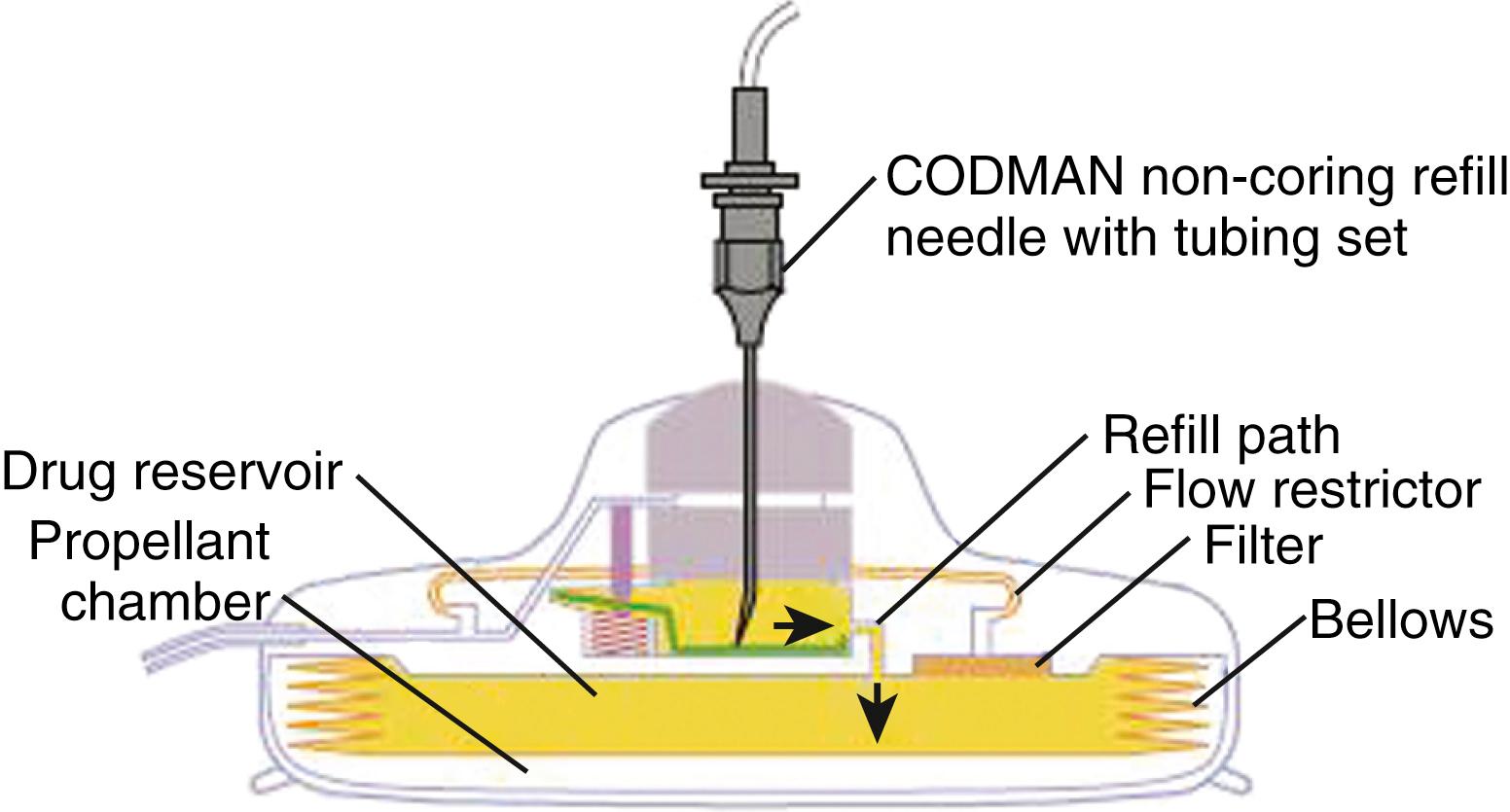
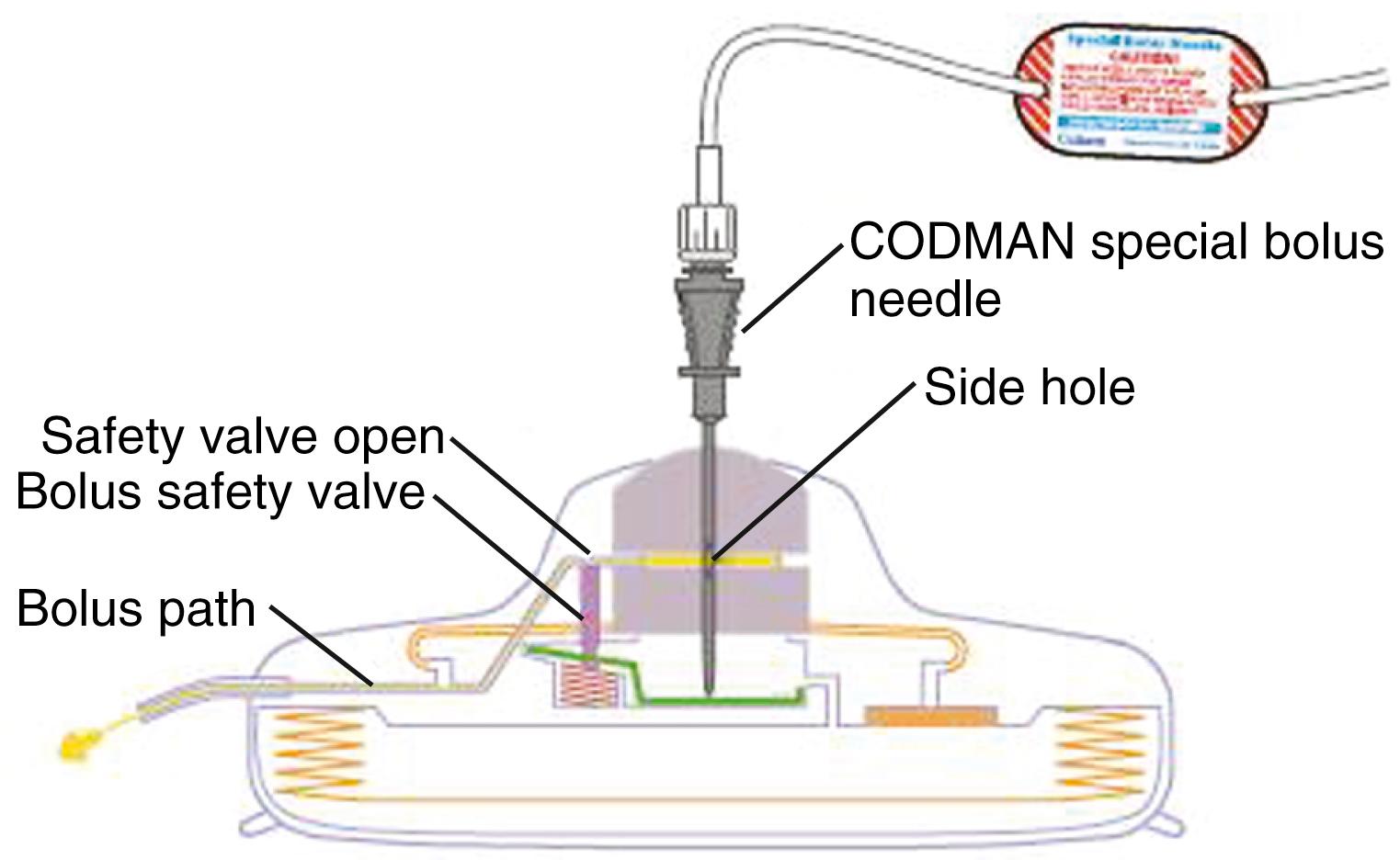
Once good paresthesia overlap to pain topography is obtained, the electrode is disconnected from the cable. A 5-cm vertical incision over the site of epidural needle placement is made. The subcutaneous tissue is undermined at the level of the lumbodorsal or supraspinous fascia, the epidural needle is carefully removed along with the electrode stylet, and the lead is anchored using an anchoring device that will prevent the electrode from migrating ( Fig. 120.4 ). The electrode is attached to a percutaneous extension lead, and a tunneling device is used to exit the skin at a site approximately 10 cm lateral to the incision. The electrode is placed within the incision with only the temporary connecting cable externalized. This allows the electrode to remain beneath the skin throughout the entire trial period. Care should be taken to avoid contact of the electrocautery device with the electrode. A defect in the electrode could result in a line of continuity between the externalized lead and the epidural space. If the patient has a successful trial, then it is necessary to remove only the extension lead, and the electrode, which has already been positioned and has not been exposed to a contaminated external environment, can remain in place. The skin and subcutaneous tissue are closed with interrupted Vicryl sutures and skin staples (see and 120.2).

Placement of a paddle-type electrode or flat lead is done via a standard laminotomy procedure. The electrode is placed in the epidural space using the same anatomic targets as described for cylindrical electrode placement. A dual laminotomy is sometimes required to properly position the electrode. Paresthesia overlap with topography of pain should be tested with the awake patient intraoperatively. The electrode cable is anchored to fascia, and the electrode is attached to a temporary percutaneous connecting wire as described previously.
At the conclusion of the phase 1 of implantation, the procedure is the same for both percutaneous and flat electrode management. The percutaneous connecting cable is attached to a trial screener, and the patient is screened at home for 4 to 7 days. When the patient returns, he or she is questioned about success of pain reduction. A general rule of thumb is that a reduction in pain of 50% or more is necessary to proceed to phase 2, but there are many exceptions and a careful assessment by the implanting surgeon is required for proper decision-making. For patients with intractable angina pectoris, a reduction in the frequency, intensity, and duration of anginal episodes should be documented.
For phase 2, the patient is taken to the operating room, and under local anesthesia with monitored anesthesiology care, the electrode and cable are removed if the trial was not successful. Patients who have had a successful trial will have their incision reopened, and the generator will be implanted in the buttock. A site selected below the belt line and cephalad enough to allow comfortable sitting should be selected. Generators can be implanted in a variety of anatomic sites, including the abdomen, lateral thorax, and subclavicular and subaxillary space.
After local anesthetic infiltration, a horizontal incision is made in the skin planes large enough to enable placement of the generator. The pocket is created by sharp and blunt dissection and is made at a depth no greater than 2 cm to enable easy programming transcutaneously.
A tunneling tool is used to connect the midline incision to the pocket, and the extension wire is threaded through the tunnel. The system is connected, and the incisions are approximated in two layers using 3-0 Vicryl sutures for the deeper tissues and staples for skin closure. Familiarity with all the equipment is necessary before proceeding to the operating room.
Intraoperative problems peculiar to SCS implantation include an inability to properly position the electrode in the epidural space, inability to obtain paresthesia overlap of the painful area, and unanticipated events. We have described the inability to thread a percutaneous catheter in a patient with unidentified epidural lipomatosis. The catheter was readily placed under direct vision after laminotomy. We use a dual laminotomy technique in those cases in which a proper anatomic position cannot be achieved through a single laminotomy to enable proper anatomic and physiologic positioning of a flat electrode and have estimated that unanticipated anatomic complications occur as often as 18% of the time in revision flat electrode placements. Finally, it is uncommon, but it is sometimes impossible to obtain satisfactory paresthesia overlap of the painful area, particularly with angina pectoris and axial low back or neck pain, despite an unimpeded ability to manipulate the electrode(s). Even distal extremity paresthesia coverage can sometimes be impossible to obtain for unproven but theorized reasons such as epidural scarring or any impediment to the flow of electricity to and into the spinal cord.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here