Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The primary structure of a protein is its linear sequence of amino acids with different side groups, which determine how the protein folds on itself (secondary and tertiary structures) and how it reacts with other molecules and cells (i.e., its molecular identity).
Methods to quantitate and fractionate proteins are based on turbidimetry, colorimetry, absorption spectrophotometry, dye binding, column chromatography, electrophoresis, and immunoassays.
Protein electrophoresis separates proteins according to their electrical charges (usually at pH 8.6).
The major proteins in plasma that contribute to the electrophoretic pattern are albumin, α 1 -antitrypsin, α 2 -macroglobulin, haptoglobin, β-lipoprotein, transferrin, complement C3, fibrinogen, and immunoglobulins.
Several minor components of plasma proteins—such as ceruloplasmin, C-reactive protein, prealbumin, and protease inhibitors—have clinical utility in diagnosing and monitoring diseases and are quantitated by immunoassays.
Patterns of protein electrophoresis in serum and urine are characteristic of specific diseases primarily involving changes in synthetic rates (liver), loss (renal), or inflammatory states.
Hereditary deficiency of some plasma proteins leads to significant diseases (e.g., α 1 -antitrypsin).
Proteins in plasma play several roles, including maintaining oncotic pressure, transporting small molecules, and promoting or inhibiting inflammatory reactions.
The major clinical use of serum and urine protein electrophoresis is to screen for monoclonal gammopathies.
Examination of the proteins in plasma can provide information reflecting disease states in many different organ systems. The most frequently performed measurement—that for total protein—is usually performed on serum, which has no fibrinogen and no anticoagulant that may slightly dilute proteins in plasma. Although total protein determination gives the physician some information as to a patient’s general status regarding nutrition or severe organ disease (as in protein-losing states), further fractionations yield far more clinically useful information.
Additional quantitation of albumin, for example, is more informative regarding nutritional status, liver synthetic capacity, and protein-losing nephropathy or enteropathy. It allows the clinician to interpret high or low calcium and magnesium levels, because albumin binds about one-half of each of those ions on a molar basis. Calculated differences between total protein and albumin represent the value of all globulins, a composite of the other fractions that individually may rise several-fold in severe disorders.
Protein electrophoresis separates the globulins from albumin and resolves the major proteins of serum into patterns that may be highly specific for some diseases. High-resolution techniques can provide a display of all components in concentrations down to about 1 g/L (0.1 g/dL in traditional units). However, at that level, quantitation by scanning of stained proteins is not highly reliable, and alternative methods should be employed. Such techniques, involving immunologic detection of individual proteins, have the dual advantages of specificity and sensitivity over electrophoresis (see Chapter 45 ).
Yet, there is much to be appreciated from visual inspection of an electrophoretogram of proteins because the human eye is highly efficient at detecting subtle variations in individual proteins as well as alterations in protein patterns. Identification of these patterns is a useful screening method to be followed by more specific confirmatory procedures to identify and quantitate aberrant protein bands. Protein electrophoresis also can be a useful tool for monitoring patients over long periods of time when marked alterations are noted in levels of particular proteins, such as in myeloma, nephrotic syndrome, cirrhosis, or extensive body burn.
This chapter reviews protein structure, methods of measurement and separation, the major plasma proteins (except coagulation factors, immunoglobulins, and the complement system, which are covered in Chapter 40, Chapter 47, Chapter 48 ), and some of the patterns encountered in particular disease states.
The backbone of all protein molecules is a continuous chain of carbon and nitrogen atoms joined through peptide bonds between adjacent amino acids. At one end (the amino-terminus) is a free amino group; at the other end (the carboxy-terminus) is a free carboxyl group. Whereas the peptide backbone is qualitatively invariant between different proteins (its total length is equivalent to the total number of amino acids in a particular protein), proteins have structural identity by virtue of the side groups or residues of the constituent amino acids. The average molecular weight of an amino acid is 120 Da. Serum proteins range in size from roughly 66 kDa to over 700 kDa. These amino acid side chains are conventionally grouped according to chemical nature: hydrogen (glycine), aliphatic (alanine, valine, leucine, and isoleucine), hydroxymethylamino (serine and threonine), aromatic (tyrosine, phenylalanine, and tryptophan), imino (proline and hydroxyproline), acidic (aspartate and glutamate), basic (arginine, histidine, and lysine), amides (asparagine and glutamine), and sulfur-containing (cysteine and methionine). These different side chains may be charged, polar, or hydrophobic, resulting in the tendency for them to be relatively soluble or insoluble in water, respectively. Writing complete amino acid sequences of peptides or proteins becomes very complicated using their common three letter abbreviations; thus, single-letter abbreviations have become standard practice for writing such primary sequences ( Table 20.1 ).
| Aliphatic | ||
|---|---|---|
| Glycine | gly | G |
| Alanine | ala | A |
| Valine | val | V |
| Leucine | leu | L |
| Isoleucine | ile | I |
| Hydroxymethylamino | ||
| Serine | ser | S |
| Threonine | thr | T |
| Aromatic | ||
| Tyrosine | tyr | Y |
| Phenylalanine | phe | F |
| Tryptophan | try | W |
| Imino | ||
| Proline | pro | P |
| Hydroxyproline ∗ | ||
| Acidic | ||
| Aspartate | asp | D |
| Glutamate | glu | E |
| Basic | ||
| Arginine | arg | R |
| Histidine | his | H |
| Lysine | lys | K |
| Amidic | ||
| Asparagine | asn | N |
| Glutamine | gln | Q |
| Sulfur-containing | ||
| Cysteine | cys | C |
| Methionine | met | M |
∗ Hydroxyproline derives from posttranslational modification of proline.
The linear sequence of amino acids in a protein is called its primary structure ; this sequence of amino acids determines the identity of a protein, what its molecular structure is, what functions it can perform, how it can bind to other molecules, and how it can participate in the processes of recognition between molecules and cells. These biological interactions are guided by reactivities between charged groups on one molecule and those on another, and similarly by hydrophobic interactions between molecules. Analytic processes such as chromatography, electrophoresis, dye binding, light absorbance, and others also depend on the primary amino acid sequence.
The secondary structure refers to specific regular three-dimensional conformations into which portions of the polypeptide chain fold. Three such structures have been identified ( ). First is the α-helix, in which the chain forms a regular helix such that the backbone C=O of the i th peptide group participates in a hydrogen bond with the N–H of the ( i +4)th peptide unit. The second is β-pleated sheets in fully extended structures, in which the chain forms a flat structure such that the side chains of adjacent amino acids point in opposite directions. In this conformation, two or more extended chains can associate so that the maximum number of C=O ●●●H–N bonds form between them. β-Pleated sheets can have their individual β-strands in parallel or antiparallel orientations. Finally, a third grouping of structures is the bend conformation, in which the direction of the polypeptide chain reverses itself, thereby allowing long primary structures to bend back on themselves and assume compact conformations.
The core of a protein molecule typically consists of combinations of α-helices and β-strands linked by loops of various lengths and shape. This inner core generally contains hydrophobic amino acids, whereas the loops and other surface portions of the protein molecule are richer in polar and charged amino acids that are hydrophilic. Upon degradation, some proteins (e.g., serum amyloid–associated protein, immunoglobulin light chains, prealbumin) release fragments rich in β-regions. These fragments are capable of coming together spontaneously in vivo to form deposits of β-sheets in fibrils that constitute amyloid. Recent work has shown an association between the genotype of apolipoprotein E (especially the allele apoE-4) and the progression of late-onset Alzheimer disease, in which cerebral plaques of amyloid form within the brain. These genetic findings suggest that Alzheimer disease may be understood and treated as a disease that has a biochemical basis in β-pleated sheet generation ( ; ).
Molecular regions with clusters of hydrophobic side groups tend to remain on the interior of a protein that is soluble in water, whereas those regions with clusters of charges or other hydrophilic moieties tend to appear on the protein’s surface. Conversely, proteins that are membrane bound usually have a distinct hydrophobic segment that protrudes to anchor the protein molecule in the lipid phase of the membrane. The actual three-dimensional structure or folding pattern of the protein, uniquely determined by its amino acid sequence, is termed its tertiary structure . Individual proteins or monomeric subunits may form more stable complexes, such as dimers, trimers, and tetramers; this is termed quaternary structure .
The sulfhydryl group on a cysteine residue can form a disulfide (covalent) bond with another cysteine within the same protein to hold different segments tightly together. This action helps stabilize the whole structure from disruption by mechanical, thermal, or other forces. These intramolecular disulfide bonds most likely form after spontaneous protein folding along the linear amino acid sequence into most thermodynamically stable conformations. Disulfide bonds can also form between cysteine residues on different molecules, thereby stabilizing multimeric molecular structures (e.g., haptoglobin, von Willebrand antigen).
The acidic and basic amino acids determine the net charge on a protein and thus its electrophoretic mobility. The charge on carboxyl and amino groups is a function of pH (i.e., whether a hydrogen ion is bound to or dissociated from the group). Combining all the different side groups and their different degrees of dissociation, the pH at which a particular protein has net charge equal to zero is called its isoelectric point (pI). Proteins with pI less than 7 are acidic and tend to have carboxyl side groups exposed, whereas those with pI greater than 7 are basic (e.g., histones that, in turn, bind to the external helical structure of DNA that is negatively charged with phosphate groups).
Proteins are synthesized from the amino end to the carboxyl end by ribosomes translating from the information encoded in messenger RNA. The initial translation product of some proteins is acted on before secretion by proteolytic enzymes that convert a preform to the mature protein by removal of a signal peptide (generally hydrophobic) that otherwise holds the new protein molecule to the endoplasmic reticulum. Release of a preprotein from the endoplasmic reticulum entails passage through a membrane pore with the participation of various translocation factors ( ). The correct assembly of a protein may critically depend on the function of so-called molecular chaperones, which are other proteins that guide the folding of nascent proteins in concert with proteases that remove selective segments to achieve functional conformations ( ). Many genetic diseases are due to harmful mutations in DNA, leading to alterations in the amino acid sequence of a protein that may block this complex assembly process or that may render any assembled protein molecules nonfunctional ( ).
Additional modifications to protein structures occur posttranslationally (i.e., after joining of the amino acids is complete) ( ). Phosphorylation consists of the enzymatically regulated attachment of phosphate groups to serine and threonine groups as well as tyrosine (e.g., by tyrosine kinases) in the peptide backbone, thereby forming phosphoproteins with a more negative charge that can alter protein functions and state of activation. Glycosylation may occur spontaneously, in the presence of sugar molecules, or in a directed manner under enzymatic control, in which oligosaccharides, frequently terminating with sialic acid (which carries a negative charge), are attached to the protein ( ). These species of molecules are termed glycoproteins . Linkages are generally to asparagine residues through N -acetylglucosamine or to serine and threonine residues through N -acetylgalactosamine. Proteolysis results in the cleavage or removal of short segments of the peptide backbone that can open up catalytic sites of a zymogen (e.g., plasminogen to plasmin) or facilitate recognition by a receptor molecule (e.g., proinsulin to insulin). Many of these posttranslational steps are unique to eukaryotic cells and do not occur in prokaryotic cells. This point is very important to the biotechnology industry, which uses cloned genes to produce human proteins in bacteria, yeast, or other artificial cell types and thus must take additional steps to synthesize accurate molecules ( ). Posttranslational changes in the structure of proteins influence their antigenicity, specific chemical or catalytic activities, abilities to bind to receptors, and electrophoretic mobilities.
Proteins that normally remain intracellular ultimately undergo degradation and clearance by the proteasome enzyme complex. This normal breakdown is essential to prevent accumulation of aging of otherwise faulty protein molecules and to allow newly synthesized fully functional proteins to take their place. Prior to degradation, protein molecules are tagged by conjugation with ubiquitin (ubiquitinylation), a small (8.5 kDa) protein that is found in all eukaryotic cells. Attachment of ubiquitin also directs proteins to other parts of the cell and modifies their molecular interactions. Conjugation with a single ubiquitin molecule is followed by attachment of further ubiquitin molecules, leading to a polyubiquitin chain that binds to the proteasome, which then degrades the protein into peptides and amino acids to be used to synthesize new proteins. This crucial proteasome action is the target of the inhibitor bortezomib, which has had clinical application for treatment of multiple myeloma and mantle cell lymphoma ( ).
Modern understanding of the protein composition of serum and plasma derives from the electrophoretic techniques introduced by Tiselius. He separated proteins dissolved in an electrolyte solution by application of an electric current through a U-shaped quartz tube that held the protein solution. At pH 7.6, four serum protein fractions, designated albumin, α, β, and γ, were identified and quantified optically by change in refractive index at the boundaries among these bands. Because separation was achieved in a homogeneous solution without solid support medium, convective forces prevented resolution into distinct zones. Hence, this technique has been termed moving boundary or frontal electrophoresis . Introduction of filter paper as an anticonvection support medium permitted separation of the protein fractions into discrete bands or zones in a process termed zonal electrophoresis . On solid support medium and at pH 8.6, the α fraction further splits into two groups of proteins: α 1 and α 2 . Other support media, such as cellulose acetate membrane, agarose gel, starch gel, and polyacrylamide gel, have been used. Cellulose acetate and agarose have predominated in the clinical laboratory because of ease of use, low cost, and commercial availability ( ).
Application of samples can be done in wells that are cut into the gel, but this process typically leaves an artifact that can interfere with the scan. A method to get around this problem involves soaking the sample into the gel by means of an overlying template. Each end of the gel is then immersed into separate buffer chambers in which electrodes are mounted. A voltage is applied between the electrodes, generating a current that passes through the gel, usually for a period of about 30 minutes, to achieve the desired resolution. The ionic strength of the buffer determines the amount of current and the movement of the proteins for a fixed voltage. If ionic strength is low, relatively more current is carried by the charged proteins. If ionic strength is high, less current is carried by the proteins, which move a shorter distance. If the electrodes are not properly aligned, the current may be denser on one side of the gel than on the other; proteins will migrate farther on the side with more current. If electrophoresis proceeds too long, the proteins may migrate off the gel into the buffer. If there is a break in the electrical circuit and no current passes, the proteins will not move from the point of application. Frequently, gels show the “smile artifact,” in which samples at the center of the gel migrate farther than those at the edges.
After electrophoresis, the gel is treated with a mild fixative, such as acetic acid, that precipitates the proteins at the positions to which they have migrated. They are then stained, and the gel is dried and cleared of excess stain. Protein patterns can be inspected visually for qualitative identification of abnormal proteins. Densitometric scanners are used to generate tracings and to quantitate the relative percentages of protein in each fraction. Those percentages are then multiplied by the total protein (separately measured) in the sample to yield the concentration of protein in each fraction.
When an electrophoretic support medium has a negative charge, the electromotive force to which it is subjected tends to move it toward the anode (positive pole; Fig. 20.1 ). However, the solid support medium is fixed; thus, it cannot move. The complementary positively charged ions in the surrounding buffer are free to move under the electromotive force; they carry with them molecules of the solvent water, which are clustered around their charges. The net result is flow of buffer toward the cathode. This buffer flow is termed electro-osmosis or endosmosis , which also carries the proteins with it to some extent by mechanical flow, not by charge. The actual distance traveled by a particular protein migrating in an electrical field is determined by the combined magnitudes of the electromotive force (a feature of the protein itself and the pH) and the electro-osmotic force (a function primarily of the support medium). When the electro-osmotic force is greater than the electrophoretic force acting on weakly anionic proteins (e.g., γ-globulins), those proteins move from the application point toward the cathode even though their charge is slightly negative.
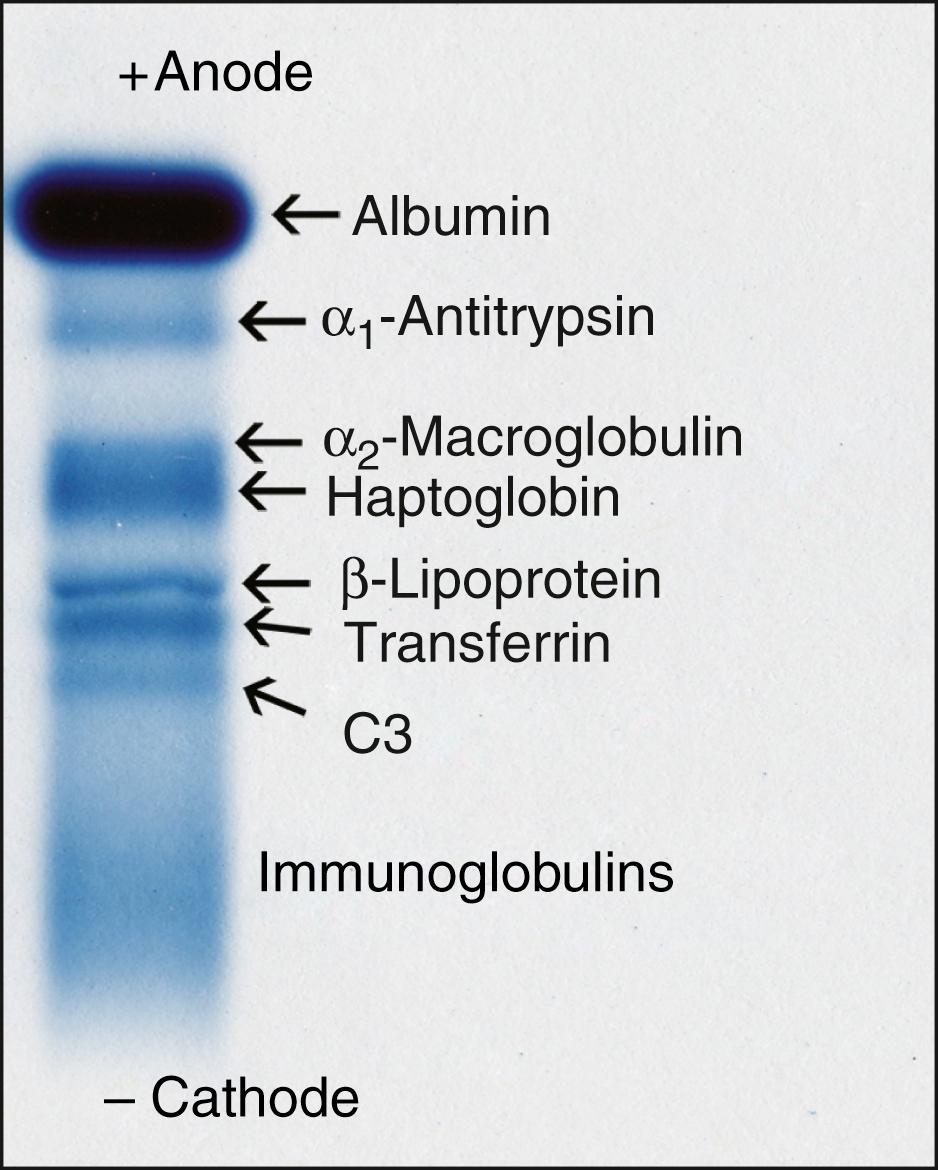
Through critical manipulations of buffer salt composition, endosmotic properties of the medium, and means of sample application, commercially available electrophoretic agarose plates now achieve consistently high-resolution quality that allows routine separation of all major serum protein species ( Fig. 20.2 ). Because of variability in the chemical formulations of gels, it should not necessarily be expected that each manufacturer’s electrophoretic system will yield identical protein separation patterns. Furthermore, optimal separation of isoenzymes generally requires different buffer and gel composition compared with the conditions for best resolution of serum proteins versus lipoproteins versus hemoglobins. A significant variation in conditions for protein electrophoresis is that for optimizing the separation of the γ-region to resolve and detect oligoclonal bands of immunoglobulin in cerebrospinal fluid (CSF). In this case, the endosmosis is set high to maximize the cathodal movement of immunoglobulins from the point of application over a span of the gel that is convenient for visual inspection.
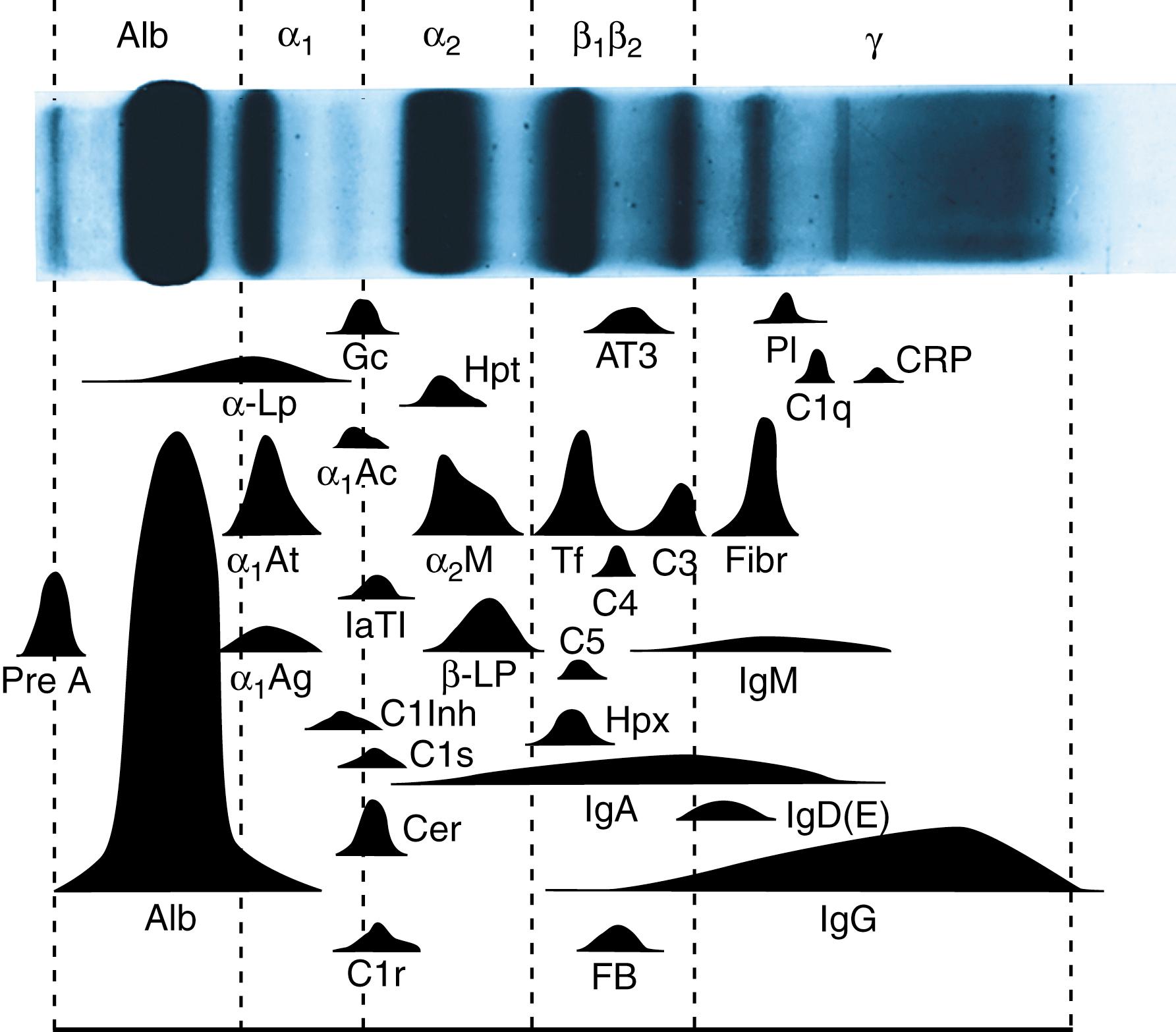
Polyacrylamide is an inert support medium whose porosity is easily adjusted by changing the composition of acrylamide before polymerization. Although polyacrylamide gel electrophoresis (PAGE) is applicable to standard separation of native proteins, it can also be used for separating proteins according to molecular weight when they are denatured in the presence of sodium dodecyl sulfate (SDS). SDS-PAGE is at present the most widely used protein electrophoretic technique for research in molecular biology. However, its very power for resolving proteins and separating them into multitudinous subunits has virtually excluded it from routine use in the clinical laboratory. Nevertheless, there is promise for clinical application of two-dimensional electrophoresis (2-DE), which uses standard separation in one direction followed by SDS-PAGE in the perpendicular direction. 2-DE results in perhaps hundreds of identifiable protein peaks, from which it may be possible to obtain important diagnostic information by sophisticated pattern analysis.
Isoelectric focusing affords superior resolution of closely migrating proteins or various forms of a single protein that differ in charge owing to minor modification (e.g., posttranslational; see Chapter 4 ). By this technique, proteins migrate through a gel containing a gradient of pH established with a mixture of ampholytes. As each protein reaches the gel location where the pH is equal to its pI, the net charge on it becomes zero. It no longer has electromotive force acting on it, and it comes to rest. Thus, the final pattern is strictly according to pI.
Chemical precipitations of serum proteins have been devised to resolve albumin and the globulins into two or more fractions that can then be measured for protein content. With the addition of sodium sulfate, sodium sulfite, ammonium sulfate, or methanol, the globulins tend to precipitate, leaving albumin in solution. By measuring total protein in the original serum and protein in the precipitate or the supernatant, values for albumin and globulin can be derived. Additionally, total protein concentration may be determined spectrophotometrically using an appropriate dye such as Coomassie blue or the biuret reagent, and the albumin concentration can likewise be determined spectrophotometrically using a dye that binds to it specifically, that is, bromocresol green or bromocresol purple. The globulin concentration can then be computed as total protein-albumin, and then the albumin to globulin (A/G) ratio can be determined. The ratio of these values (A/G ratio) has been used extensively because it accentuates abnormalities in serum protein composition, which in disease generally involves depression of albumin and elevation of one or more globulin fractions. Albumin may be depressed owing to decreased synthesis (malnutrition, malabsorption, liver failure, diversion to synthesis of other proteins) or increased loss (proteinuria, accumulation of ascites fluid, enteropathy). Globulins may be elevated owing to increased synthesis of many different proteins as part of acute or chronic reactions to disease. Lowering of albumin and elevation of globulins tend to occur simultaneously in disease, thus leading to exaggerated changes in the A/G ratio as the numerator and denominator move in opposite directions. Precipitation methods are not as accurate as zonal electrophoresis because some α-globulins may fail to precipitate, thus leading to an overestimate of the albumin fraction.
Preparative procedures for the isolation of a single minor protein constituent usually begin with a precipitation step to remove the bulk of other undesired serum proteins. The next step in protein isolation typically involves a column that separates on the basis of molecular size (gel filtration) or charge (ion exchange).
Gel filtration media such as Sephadex or agarose beads are rated according to pore size, which, in turn, determines what size molecules can pass through the interior of each bead or particle of the column. After application of a sample composed of various-sized proteins in aqueous solvent containing buffer and salt, more of the buffer is applied to drive the sample through the column. Very large molecules tend to flow through interstices of the column without entering the beads and emerge first from the bottom of the column in the void volume. Slightly smaller molecules enter the largest pores before being washed through; thus, they are slightly retarded in passing through the column. Small protein molecules pass into still smaller pores and are retained still longer. Finally, particles the size of dissolved salt penetrate farthest into the interior of gel filtration beads and come out after all of the proteins have emerged in an amount of applied buffer called the salt volume . Thus, in gel filtration, the order of protein elution is by molecular weight or size, from largest first to smallest last. Because all protein species continuously move through a gel filtration column all at the same time but at different rates, it is necessary to apply the sample in a small and uniform volume to optimize separation between peaks. Gel filtration requires that the medium be inert and not interact chemically or by charge with the proteins. It is not a method to be employed for high-resolution separation.
Ion-exchange chromatography, on the other hand, takes advantage of the charge on proteins to bind them to beads of a support medium with positively charged components such as diethylaminoethyl or quaternary aminoethyl. In anion-exchange chromatography, proteins are usually applied at a basic pH such as 8.6, at which they may be negatively charged (albumin and α 1 -, α 2 -, and β-globulins are anions) or may have no net charge (γ-globulins). The neutral proteins pass immediately through an anion-exchange column, whereas the anionic ones stick to the positively charged column matrix. If a buffer with a higher salt concentration is washed through, anions of the salt displace the anionic proteins and exchange for them by binding to the support medium. The proteins then elute from the column. By using a steadily increasing gradient of salt concentration in the eluting buffer, the proteins can be resolved according to charge. The ones with a small amount of charge will elute first, whereas those with the greatest charge (e.g., albumin) elute only when displaced by higher salt levels.
Alternatively, if pH is lowered while salt concentration is held low, anionic proteins acquire a net neutral or slightly positive charge and pass through the column. A gradient of falling pH can be used to resolve anionic proteins, with the order of elution being roughly β-, α 2 -, and α 1 -globulins, and albumin. Note that this order of elution is the reverse order of electrophoretic migration at pH 8.6, because in anion-exchange chromatography, mobility is retarded according to net negative charge, whereas in electrophoresis, mobility is enhanced by that charge.
Cation-exchange chromatography begins at an acid pH, with the proteins having positive charge (cations) and adhering to a negatively charged column matrix such as carboxymethylcellulose. They can be displaced by the cations of high salt in an eluting buffer or by increasing the pH, which will reverse the charge on the proteins to negative. By cation exchange, albumin should elute first, followed by α 1 -, α 2 -, β-, and γ-globulins.
Another separation modality by column is hydrophobic chromatography, in which samples are applied at high salt and are eluted with low salt. The support medium interacts with proteins with a hydrophobic nature; this is a good complementary technique to follow ion-exchange chromatography, in which the sample was eluted with high salt.
Affinity chromatography is based on specific binding between a protein of interest and another protein that has been covalently linked to the solid support medium of a column. For example, coagulation factor VIII complexed with von Willebrand factor (vWF) can be selectively removed from the other plasma proteins by passing plasma through a column that contains monoclonal anti-vWF antibody linked to the solid-phase matrix. The factor VIII-vWF complex selectively binds to the column as other plasma proteins wash through. The factor VIII is then dissociated from the vWF, thereby allowing it to elute in a purified fraction suitable for transfusion therapy. Such antigen–antibody interactions may be disrupted by high salt concentration, change in pH, or a chemical denaturant, such as urea, in different applications. Other affinity chromatography gels use a binding phenomenon that mimics naturally occurring molecular interactions. Thus, some dyes coupled to agarose are able to bind albumin, thereby removing it selectively from serum. Therapeutic antibodies or their Fab portions are concentrated with affinity columns containing their intended target antigens (e.g., Digibind for digoxin, CroFab for crotalid snake venom). Immunoglobulins can also be absorbed from a sample by staphylococcal protein A coupled to the gel matrix. Many other separation schemes can effect a high degree of purification in a single step with affinity chromatography medium coupled to dyes, drugs, nucleotide cofactors, and sugars. A clinical test using affinity chromatography is quantitation of glycosylated hemoglobin using a dihydroxyboronate affinity matrix that selectively binds molecular species of hemoglobin to which glucose has been covalently attached while allowing the nonglycosylated forms to pass through the column. The glycosylated hemoglobin is then separately eluted and quantitated.
Capillary electrophoresis is a separation method based on flow through a capillary tube that can be tailored to resolution of different molecules based on size, hydrophobicity, or stereospecificity. It is applicable to large molecules such as DNA or proteins, as well as to small ones such as hormones or therapeutic drugs. Physically, the method is similar to high-performance liquid chromatography (HPLC), in which solvent is pumped through a column that retains or passes solutes according to chemical interactions. Capillary electrophoresis for serum proteins employs a column with properties similar to agarose; thus, the separation of proteins is comparable with that from electrophoresis. This analysis can be automated with a detector at the effluent end that detects and quantitates protein bands without the need for staining and separate scanning. Although equipment costs are relatively high for capillary electrophoresis, reagent and labor costs are low, and the procedure is fast and very quantitative, leading to the promise of more widespread clinical applications in the future ( ; ). When a monoclonal immunoglobulin band is suspected by capillary electrophoresis, further treatment of the specimen with anti-IgG, anti-IgA, anti-IgM, anti-κ, and anti-λ antisera is done in separate aliquots prior to performing the electrophoresis again; the monoclonal protein type is identified as the band that disappears with that antiserum. This approach is satisfactory for relatively high concentrations of monoclonal proteins, but it does not have good detection and quantitation power at lower concentrations of those proteins ( ).
The ultimate reference method for determining concentration of protein is the analysis for nitrogen content. Nitrogen is present uniformly along the peptide bonds throughout the length of a protein and more irregularly in the side groups, wherever tryptophan, arginine, lysine, histidine, asparagine, or glutamine is present. The Kjeldahl technique consists of acid digestion to release ammonium ions from nitrogen-containing compounds. The ammonium can then be quantitated by conversion to ammonia gas and titration as a base, or by nesslerization, in which double iodides (potassium and mercuric) form a colored complex with ammonia in alkali. Although determination of nitrogen content can be extremely precise, its use for calculation of protein concentration depends on the exact protein composition of a sample because each protein has a somewhat different nitrogen content according to amino acid composition. However, for a sample of a purified protein, nitrogen content is highly accurate for estimating protein concentration when the nitrogen content on a molar basis is already known for that purified protein. Knowledge of a protein’s exact amino acid sequence allows an accurate calculation of what the nitrogen content should be. Because clinical samples consist of unpredictable mixtures of different proteins and measurement of nitrogen content is not a simple procedure, it is not commonly used in clinical laboratories.
The refractive index can be accurate for measuring serum protein concentration as dissolved solute for levels above 2.5 g/dL. Hemolysis, lipemia, icterus, and azotemia produce erroneously high results. The refractive index cannot be used for urine protein measurements because of excess amounts of solutes in relation to the protein.
Specific gravity (and thus, by inference, protein content) can be estimated by pipetting drops of serum or blood into a graded series of copper sulfate solutions. A protein–copper shell forms about the drop to prevent dissolution for a short interval, during which time the drop falls to the bottom, remains stationary, or rises to the top. The protein concentration of a sample is estimated from the specific gravity of the copper sulfate solution in which the drop remains stationary. This technique is simple and has been used widely as a screening test for hemoglobin concentration in whole blood.
Proteins in solution absorb ultraviolet light at 280 nm (A 280 ), owing mostly to tryptophan but also to tyrosine and phenylalanine ( ). For accurate conversion of A 280 readings to protein concentration, the molar absorptivity must be used because each protein contains a different amount of these three amino acids. However, the A 280 of a mixture of proteins is not a perfect measure of protein content because molar absorptivities vary greatly among different proteins. Because nucleic acids (which absorb strongly at 260 nm and also somewhat at 280 nm) may be present in protein preparations, a better estimate of protein concentration in the presence of nucleic acids is given by the following formula:
Direct measurements of absorbance can be used for quantitating proteins in the range of 0.05 to 1.5 mg/mL.
Turbidimetric methods are often used for a similar concentration range in CSF or urine. Protein forms precipitate on the addition of trichloroacetic acid, sulfosalicylic acid, or other acid reagent. The resulting turbidity can be used for protein quantitation by increment in optical density in comparison with similarly treated standards. However, these techniques are not specific to proteins, because other acid-insoluble substances, such as nucleic acids, can also precipitate.
A colorimetric technique highly specific for proteins and peptides is the biuret method, by which copper salts in alkaline solution form a purple complex with substances containing two or more peptide bonds. Interferences are minimal, although ammonium ion may acidify the reaction, while hemoglobin and bilirubin absorb in the same region as the biuret complex (540–560 nm). The biuret method is extensively used in clinical laboratories, particularly in automated analyzers in which protein concentration can be measured down to 10 or 15 mg/dL.
Greater sensitivity can be obtained using the Folin-Ciocalteu reagent (or phenol reagent, phosphotungstomolybdic acid), which oxidizes phenolic compounds such as tyrosine and, in addition, tryptophan and histidine to give a deep-blue color.
Lowry and colleagues (1951) used the biuret method followed by the phenol reagent, which greatly enhanced color formation, because the phenol reagent can react with biuret complexes involving all peptide bonds. The Lowry assay has been extensively used for consistently accurate determinations of protein concentration. Further sensitivity for detection down to 1 μg of protein can be obtained using Coomassie brilliant blue dye, which is free of interferences from a very wide range of substances.
Comparable sensitivity is obtained with ninhydrin, which develops a violet color by reacting with primary amines. This reagent is widely used for detection of peptides and amino acids after paper chromatography and amino acid analyses from ion-exchange columns as well as for detection of drugs on toxicology screens using thin-layer chromatography (see Chapter 24 ).
Quantitation of albumin in the presence of other proteins is possible by virtue of the specific binding of albumin to certain dyes such as bromophenol blue, methyl orange, hydroxybenzeneazobenzoic acid, bromocresol purple, and bromocresol green (BCG). BCG is used extensively in automatic analyzers for determining serum albumin in parallel with biuret reagent for total protein. Dyes bound to albumin absorb maximally at slightly different wavelengths, thus allowing direct spectrophotometric quantitation of the albumin.
The standard dyes used for staining electrophoresis are Coomassie brilliant blue, Ponceau S, and amido black. For detection of minor components in high-resolution gels, silver staining is very sensitive down to nanogram quantities ( ). In addition, special dyes, such as Oil Red O and Sudan Black, stain lipoproteins, and periodic acid–Schiff stains glycoproteins separated in special electrophoretic applications.
Because electrophoresis followed by staining does not afford explicit identification of serum proteins, immunologic measurements have been instituted for quantitation of individual proteins ( ). Nephelometry detects the turbidity produced usually within minutes or less by the precipitation of a reagent antibody with its target protein in a serum sample ( ). The major serum proteins are now widely measured by this method on automated immunochemistry analyzers that have supplanted former measurements by radial immunodiffusion. Owing to the specificity of the antibody reagent, nephelometry has great specificity for quantitating individual proteins, even in the presence of others. Proteins present in lower concentrations may be quantitated by specific immunoassays ( Chapter 45 ).
The major serum proteins are those components that are readily resolved and detected on electrophoretic gels stained by conventional clinical laboratory techniques ( Table 20.2 , and see Fig. 20.1 ).
| Protein | Concentration Range (g/L) | Molecular Weight | Actions |
|---|---|---|---|
| Prealbumin | 0.15–0.36 | 62,000 | Binds thyroxine; transports vitamin A |
| Albumin | 39–51 | 66,000 | Oncotic pressure; amino acid reservoir; carries small molecules |
| α 1 -Antitrypsin | 2.0–4.0 | 54,000 | Protease inhibitor |
| α 2 -Macroglobulin | 1.5–3.5 | 725,000 | Protease inhibitor |
| Haptoglobulin | 0.4–2.9 | 100,000 (Type 1-1) | Binds hemoglobin |
| β-Lipoprotein | 2.7–7.4 | 380,000 | Lipid transport |
| Transferrin | 2.0–4.0 | 80,000 | Transports iron |
| C3 | 0.6–1.4 | 185,000 | Component of complement system |
| Fibrinogen | 1.0–4.0 | 340,000 | Clot formation |
| Immunoglobulin A | 0.4–3.5 | 160,000 | Surface immunity |
| Immunoglobulin D | 0.1–0.4 | 180,000 | |
| Immunoglobulin E | 50–600 (μg/L) | 180,000 | Binds to mast cells; hypersensitivity reactions |
| Immunoglobulin G | 7–15 | 150,000 | Humoral immunity |
| Immunoglobulin M | 0.25–2.0 | 850,000 | Humoral immunity primary response |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here