Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In previous editions of this textbook, several entities of uncertain type were placed into either benign or malignant categories. Although these entities still remain an enigma with regard to line of cellular differentiation, larger clinicopathologic studies of each of these entities have revealed a better understanding of their clinical behavior. Whereas some of these entities were initially placed into the “benign” category (e.g., ossifying fibromyxoid tumor, pleomorphic hyalinizing angiectatic tumor of soft parts), these lesions clearly have a significant risk for local recurrence and can even metastasize on occasion. Similarly, other entities of uncertain type were originally categorized among the “malignant” tumors (angiomatoid fibrous histiocytoma, formerly “angiomatoid malignant fibrous histiocytoma”) but, given the lower risk for metastasis, especially with adequate therapy, behave less aggressively than the other lesions in this category. As such, we believe there is ample justification to categorize these tumors with those of intermediate malignancy, although of uncertain lineage.
Ossifying fibromyxoid tumor (OFMT) of soft tissue, first described in a series of 59 cases from the Armed Forces Institute of Pathology (AFIP) in 1989, is a rare tumor of uncertain differentiation that usually arises in the extremities. Almost 200 cases have been reported in the literature, in three large series, as well as numerous case reports or small series. Although the original description of this tumor by Enzinger et al. emphasized the bland morphologic appearance and typically benign clinical behavior, even this series indicated that exceptional examples act in a clinically aggressive fashion. Several subsequent reports described tumors with typical features that unexpectedly metastasized, or tumors with atypical or overtly malignant histologic features, some of which behaved aggressively. Although some have suggested that malignant OFMTs represent instead other tumor types, recent studies have clearly confirmed the existence of atypical and malignant forms of this tumor. As such, it seems reasonable to consider OFMT a neoplasm of uncertain differentiation, spanning a morphologic and clinical spectrum from benign-appearing lesions that almost always behave benignly to histologically malignant lesions with a high risk for aggressive behavior.
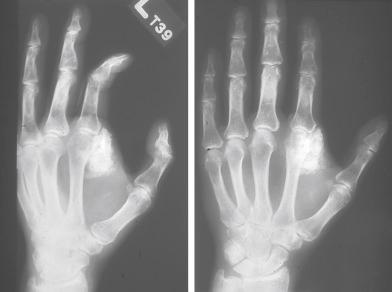
OFMT almost exclusively affects adults (mean age almost 50 years), with only rare examples documented in children. Men are affected more often than women. Most patients present with a small, painless, well-defined, often lobulated subcutaneous mass that involves the extremities in approximately 70% of cases. Less frequently involved sites include the trunk, head and neck, mediastinum, and retroperitoneum. Radiographic studies usually reveal a well-circumscribed mass with an incomplete ring of peripheral calcification and scattered calcifications in the substance of the neoplasm ( Fig. 32.1 ). Erosion of underlying bone and periosteal reaction are rarely seen.
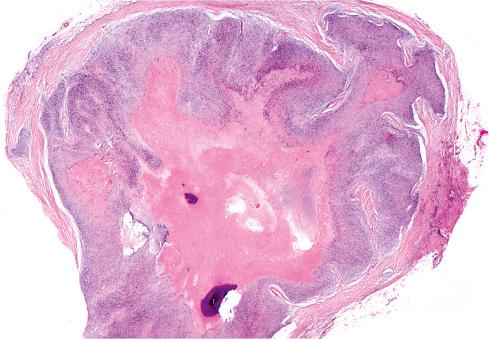
Grossly, OFMT is usually well circumscribed, spherical, lobulated or multinodular, and typically covered by a thick fibrous pseudocapsule ( Fig. 32.2 ). Most measure 3 to 5 cm, but occasional lesions are 15 cm or larger. On cut section, the tumor is tan-white and often has a gritty texture, as one would expect in a tumor that frequently has calcifications.
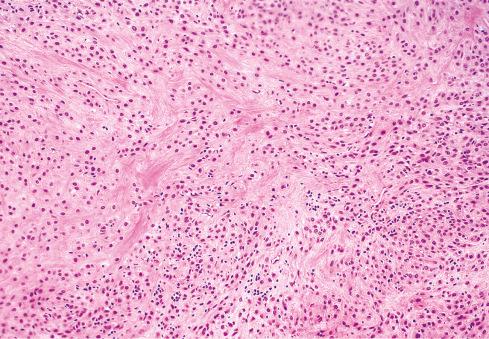
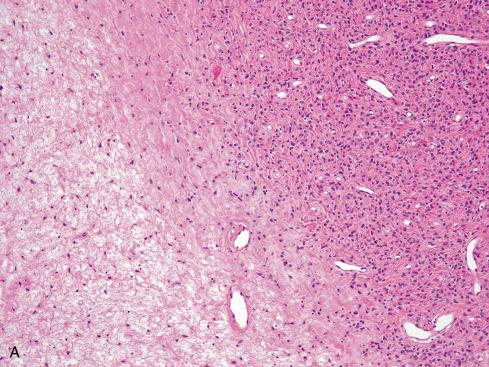
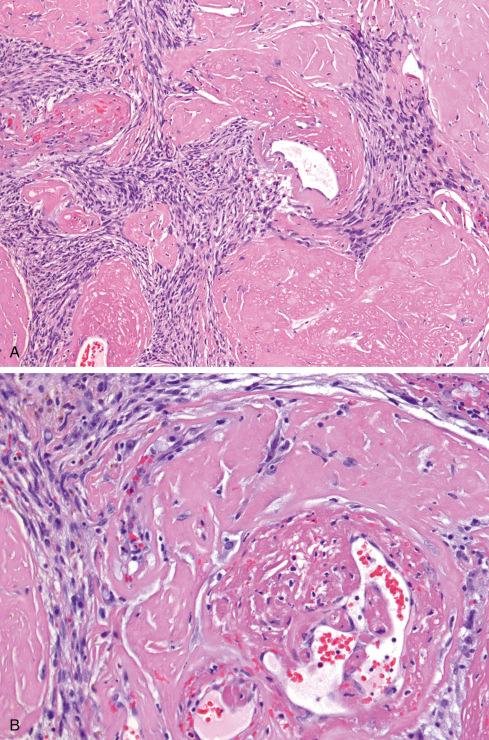
Microscopically, the majority are located in the subcutaneous tissue, but some are attached to tendons or fascia or involve the underlying skeletal muscle. A typical OFMT is composed of uniform round, ovoid, or spindle-shaped cells arranged in nests and cords and deposited in a variably myxoid and collagenous stroma ( Figs. 32.3 to 32.7 ). In approximately 70% of cases, an incomplete shell of lamellar bone is found at the periphery of the nodules, either within or immediately beneath a dense fibrous pseudocapsule and sometimes extending into the substance of the tumor ( Fig. 32.8 ). Bone is found in approximately 75% of histologically typical tumors, compared with approximately 50% of those showing atypical histologic features.
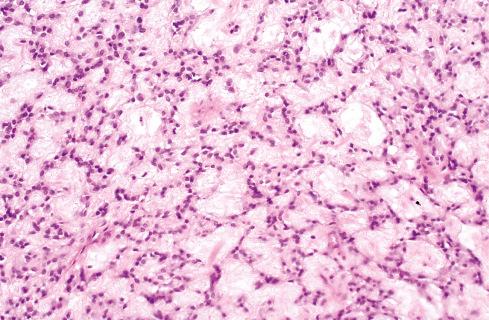
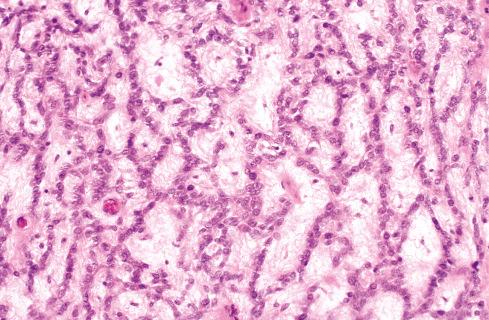
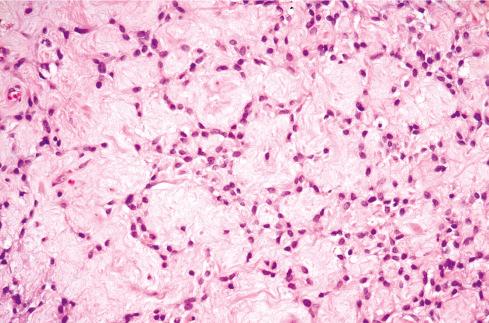
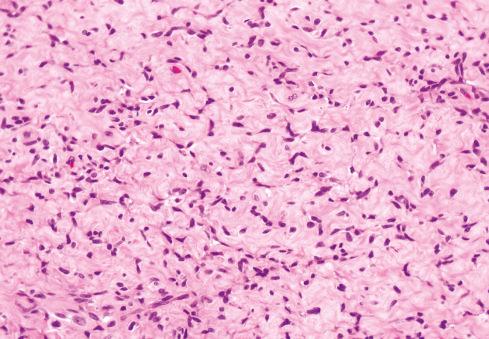
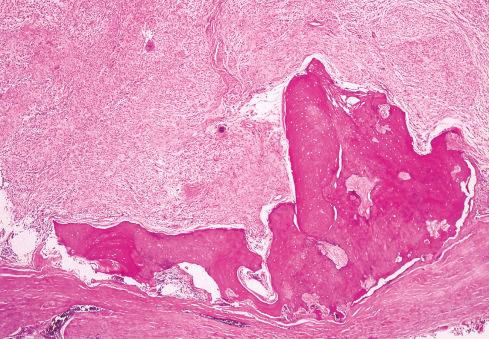
The cells, which vary minimally in size and shape, are characterized by pale-staining vesicular nuclei with minute nucleoli and small amounts of eosinophilic cytoplasm ( Fig. 32.9 ). The cells may be deposited in a variety of patterns, including cords, nests, or sheets, or may be randomly distributed in a fibromyxoid matrix. Small pseudorosette-like structures are typically present. Some lesions are predominantly myxoid with an abundant stroma of Alcian blue–positive, hyaluronidase-sensitive acid mucopolysaccharides, occasionally forming microcysts. Other tumors are predominantly collagenous. Small foci of calcification and rarely metaplastic cartilage may also be seen in the tumor nodules. Most have a rich vasculature, with many vessels exhibiting perivascular hyalinization and others subintimal fibrin deposition or thrombosis.
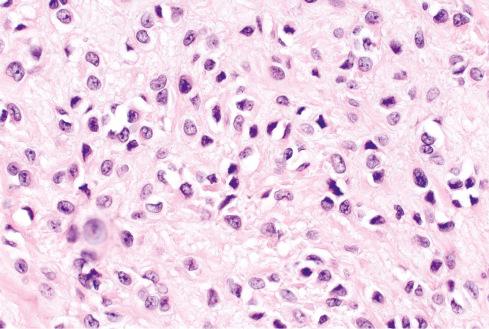
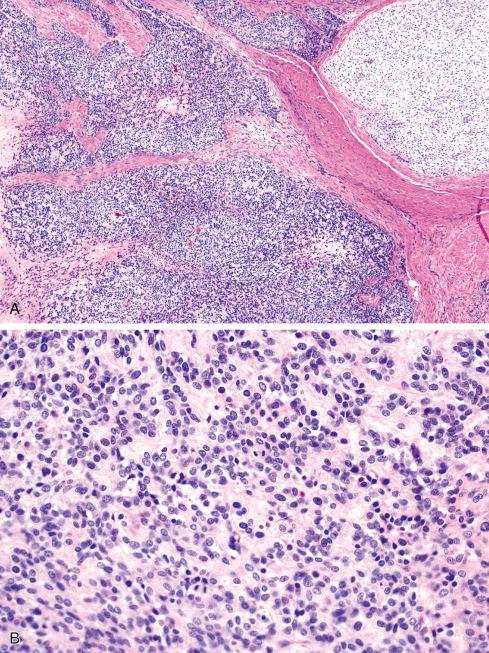
It is now well recognized that a subset of OFMTs have atypical or overtly malignant features, usually consisting of some combination of high nuclear grade, high cellularity, or increased mitotic activity (>2 mitotic figures [MF] per 50 high-power fields [hpf]). It should be emphasized that such cases maintain the overall cytoarchitectural features of typical OFMT and may be conceptualized as representing a phenomenon akin to myxoid and round cell liposarcoma rather than “dedifferentiation,” in which a stark histologic departure from the original tumor occurs. Some malignant OFMTs show areas resembling sclerosing epithelioid fibrosarcoma, with densely hyalinized collagen and epithelioid cells. Osteoid and/or woven bone may be present within the center of malignant-appearing tumors, and their presence is not indicative of osteosarcoma in a tumor otherwise showing morphologic features of OFMT. In general, confident diagnosis of malignant OFMT requires identification of areas of typical OFMT, either adjacent to malignant zones or present in an earlier manifestation of a recurrent lesion ( Figs. 32.10 to 32.12 ). Exceptionally, the tumor is composed exclusively of malignant-appearing areas. The clinical significance of these areas is discussed later.

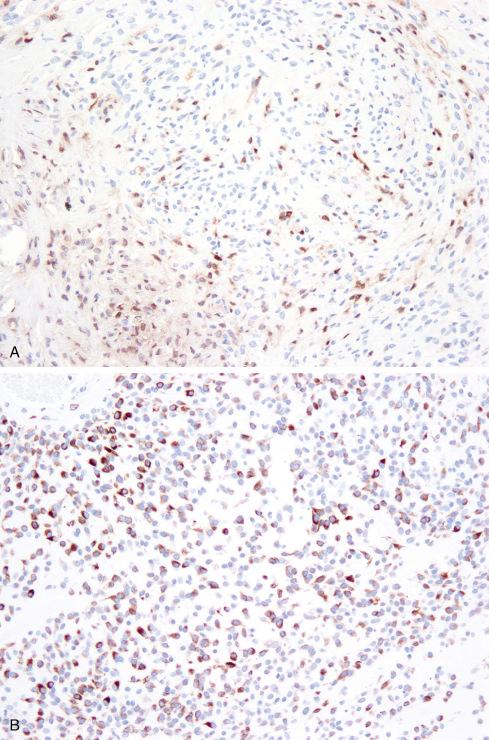
On immunohistochemistry (IHC), the cells are positive for vimentin and express S-100 protein in about 70% of cases ( Fig. 32.13 ). However, immunoreactivity for S-100 tends to be less intense and uniform than in schwannoma. Entirely typical OFMTs can show only patchy S-100 protein expression. Atypical or malignant areas express S-100 protein less often than typical areas. SOX10 expression has not been formally studied in OFMTs; in our experience it is typically negative. Scattered cells are positive for desmin and/or neurofilament protein in up to 40% and 80% of tumors, respectively ( Fig. 32.13 ). The cells may also occasionally express CD56, CD57, neuron-specific enolase (NSE), glial fibrillary acidic protein (GFAP), smooth muscle actin (SMA), or cytokeratins. Loss of expression of the SMARCB1/INI1 tumor suppressor gene product in a distinctive “mosaic” pattern has been reported by Graham et al. in approximately 70% of tested cases, reflecting hemizygous loss of the SMARCB1 locus and other regions of 22q, as determined by FISH.
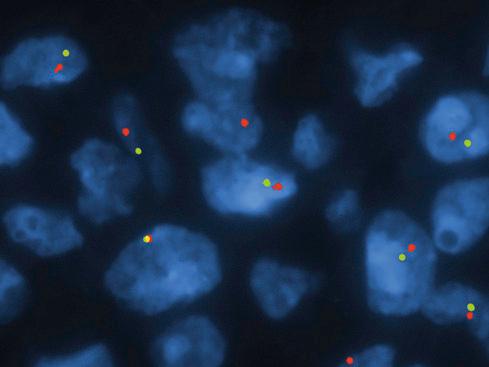
Early studies of OFMT showed a variety of chromosomal abnormalities, without a consistent aberration. In 2012, Gebre-Medhin et al. found three OFMTs to have complex karyotypes, including different structural rearrangements of chromosome band 6p21. Mapping of this breakpoint by fluorescence in situ hybridization (FISH) demonstrated rearrangements of the PHF1 gene, a transcriptional repressor previously shown to be consistently rearranged in endometrial stromal sarcomas. This finding was further confirmed by interphase FISH, showing PHF1 rearrangements in OFMT of all types ( Fig. 32.14 ). Confirmatory reverse-transcriptase polymerase chain reaction (RT-PCR) analysis of one case demonstrated an EP400/PHF1 fusion transcript. PHF1 is known to interact with polycomb group proteins, regulators of homeotic ( Hox ) genes, including PRC2 , and dysregulation of PRC2 target genes may underlie the pathogenesis of OFMT. The presence of frequent PHF1 rearrangements in typical, atypical, and malignant OFMT was subsequently confirmed by Graham et al. More recently, next-generation sequencing studies by Antonescu et al. and Kao et al. have identified a number of novel fusions in OFMTs, including ZC3H7B-BCOR , MEAF6-PHF1 , EPC-PHF1 , CREBBP-BCORL1, and KDM2A-WWTR1 . Overall, PHF1 rearrangements are most common, occurring in approximately 45% of OFMT of all types.
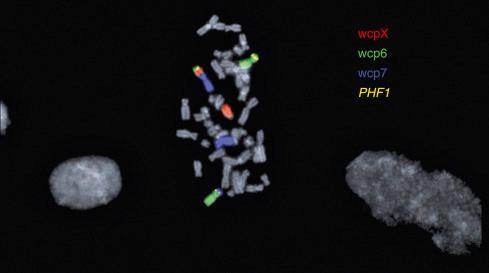
FISH analyses of OFMT have also consistently noted hemizygous loss of chromosome 22 or 22q, including but not limited to the SMARCB1 locus ( Fig. 32.15 ). It is currently unclear what role this plays in the pathogenesis of OFMT.
The differential diagnosis includes benign and malignant epithelioid nerve sheath tumors (epithelioid neurofibroma, epithelioid schwannoma, epithelioid malignant peripheral nerve sheath tumor), chondroid syringoma (cutaneous mixed tumor), myxoid chondrosarcoma, and epithelioid smooth muscle tumors.
OFMT has some features suggestive of nerve sheath differentiation, including bland, round to ovoid, S-100 protein–positive cells. However, OFMT does not arise from peripheral nerves, lacks SOX10 expression, and does not show typical morphologic features of epithelioid neurofibroma or epithelioid schwannoma (see Chapter 26 ). The cells of OFMT lack the “melanoma-like” cytologic atypia characteristic of epithelioid malignant peripheral nerve sheath tumors.
The general absence of epithelial markers in OFMT helps exclude chondroid syringoma as a diagnostic consideration. The lobulated architecture and arrangement of the neoplastic cells into cordlike structures somewhat resemble extraskeletal myxoid chondrosarcoma , but the stroma of OFMT varies between myxoid and collagenous, and the neoplastic cells have less eosinophilic cytoplasm than those of myxoid chondrosarcoma. Epithelioid smooth muscle tumors usually express myoid antigens and lack S-100 protein. Although malignant OFMT may contain areas resembling sclerosing epithelioid fibrosarcoma and show limited MUC4 expression, they contain diagnostic PHF1 rearrangements and lack EWSR1 or FUS rearrangements, the molecular hallmarks of sclerosing epithelioid fibrosarcomas and related low-grade fibromyxoid sarcomas (see Chapter 9 ).
The behavior of OFMT is highly variable. The vast majority of these tumors are histologically benign and have the characteristic features described in the original AFIP series (“typical OFMT”). Not unexpectedly, most of these pursue a benign clinical course. However, even histologically typical tumors may rarely locally recur and metastasize. For example, Yoshida et al. described a case that lacked malignant features but locally recurred, metastasized, and ultimately killed the patient. In the original report of typical OFMTs, 11 of 41 patients (27%) for whom follow-up information was available experienced one or more recurrences. In addition, one patient with three recurrences developed a similar tumor in the contralateral thigh that was presumed to be a metastasis. One additional patient developed a recurrence that showed histologic progression with increased bone production, originally interpreted as representing “well-differentiated osteosarcoma.” Based on a compilation of the largest studies, the recurrence and metastatic rates of typical OFMT are 17% and less than 5%, respectively, thereby supporting the view that it is a lesion of intermediate or borderline malignancy with a low risk of distant metastasis.
Some OFMTs have atypical histologic features, including high nuclear grade, increased cellularity, and increased mitotic activity. Such tumors show identical genetic events as do more typical tumors, establishing that typical, atypical, and malignant OFMTs represent a morphologic continuum within a single entity. Folpe and Weiss proposed a classification system for OFMT based on a combination of nuclear features, cellularity, and mitotic activity. This system has been validated in subsequent studies and correlates well with the clinical behavior of these tumors ( Tables 32.1 and 32.2 ).
| Diagnostic Criteria | |
|---|---|
| Typical OFMT | Low nuclear grade and low cellularity with mitotic activity <2 MF/50 hpf |
| Atypical OFMT | Tumors deviating from typical OFMT but not meeting criteria for malignant OFMT |
| Malignant OFMT | High nuclear grade or high cellularity and mitotic activity >2 MF/50 hpf |
| No. Cases with Follow-up | Local Recurrences (%) | Metastases (%) | |
|---|---|---|---|
| Typical OFMT | 25 | 3 (12) | 1 (4) |
| Atypical OFMT | 16 | 2 (13) | 1 (6) |
| Malignant OFMT | 10 | 6 (60) | 6 (60) |
The line of differentiation of OFMT has been the subject of considerable debate over the years, with investigators favoring cartilaginous, osteogenous, or myoepithelial differentiation. Until recently, however, the most widely accepted theory was that OFMT represented an unusual “peripheral nerve sheath tumor,” on the basis of its encapsulation, expression of nerve sheath–related markers (e.g., S-100 protein, CD57, GFAP), and some ultrastructural features.
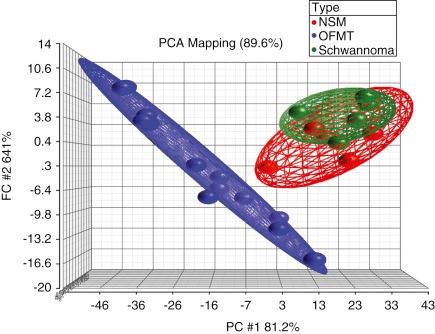
Gene expression profiling and proteomic study of OFMT suggests limited neural differentiation, with upregulation of neuron-related genes, such as EAAT4 and HuC , and downregulation of Schwann cell–associated genes, such as peripheral myelin protein 22 ( PMP22 ) and myelin expression factor 2 ( MYEF2 ). Additionally, cluster and principal component analyses show that OFMT is clearly segregated from true nerve sheath tumors, such as schwannoma and nerve sheath myxoma ( Fig. 32.16 ). Proteomic data show expression of proteins related to neuronal, schwannian, and cartilaginous differentiation. These findings, and more importantly recent molecular genetic studies showing a variety of different gene fusions in these tumors, strongly suggest that OFMT show a “scrambled phenotype” without an obvious normal counterpart, as seen in other translocation-associated soft tissue tumors.
Pleomorphic hyalinizing angiectatic tumor (PHAT), initially described in 1996 in a series of 14 cases by Smith et al., is a rare yet distinctive tumor with locally aggressive behavior. Since its initial description, fewer than 130 additional cases of PHAT have been reported, and this neoplasm likely continues to be mistaken for other entities, in particular undifferentiated pleomorphic sarcoma and schwannoma. Hemosiderotic fibrolipomatous tumor (HFLT) shows considerable clinical, morphologic, and molecular genetic overlap with PHAT, and almost certainly represents a different manifestation of the same entity. As such, these two tumors are considered together. Although we propose the alternative term “early PHAT” for HFLT, we use instead the HFLT nomenclature, for reasons of continuity with the literature. Accurate recognition of these neoplasms is of clinical importance given their propensity for local recurrence and occasional progression to myxoid sarcoma.
Hemosiderotic fibrolipomatous tumor was originally described as “hemosiderotic fibrohistiocytic lipomatous lesion” and initially regarded as reactive in nature. However, the almost 50% rate of local recurrence seen in this initial series and in subsequent studies indicates that these represent instead neoplasms, a point of view reflected in the current World Health Organization (WHO) classification, which designates them as “hemosiderotic fibrolipomatous tumor.”
HFLT most often occurs in the foot and ankle region of middle-aged adults (median age: 51). The size of reported tumors has been highly variable, but most are relative small (4-5 cm). Many are mistaken clinically for an adipocytic tumor.
Microscopically, HFLT consists of a distinctive admixture of mature fat, myxoid stroma, moderately cellular fascicles of generally bland monomorphic spindled cells with intracytoplasmic hemosiderin, iron-laden macrophages, and mixed chronic inflammatory cells ( Figs. 32.17 and 32.18 ). Other characteristic features of this lesion include small aggregates of damaged-appearing capillary-sized vessels, perivascular hyalinization, and scattered tumor cells with enlarged, pleomorphic nuclei and intranuclear pseudoinclusions. Close inspection of these lesions may disclose areas resembling “miniature” pleomorphic hyalinizing angiectatic tumors, with small, ectatic, fibrin-filled vessels ( Fig. 32.18C ).
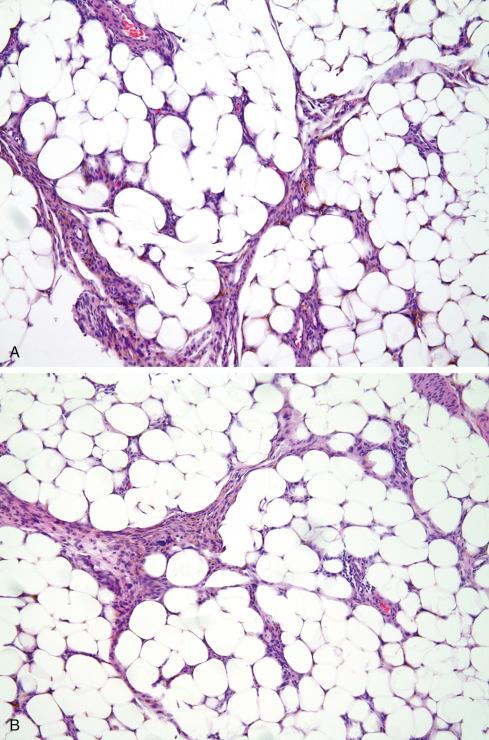
On IHC the spindled cells of HFLT are strongly positive for CD34 and negative for S-100 protein, muscle actins, and desmin. Wettach et al. first elucidated the genetics of HFLT in 2008, reporting a case with a reciprocal t(1;10) as well as a derivative chromosome involving chromosomes 1 and 3. Hallor et al. and Antonescu et al. subsequently confirmed the ubiquitous nature of this genetic event in HFLT and identified rearrangement of TGFBR3 and/or MGEA5 in 13 of 14 studied HFLTs ( Fig. 32.19 ). Others reported similar findings.
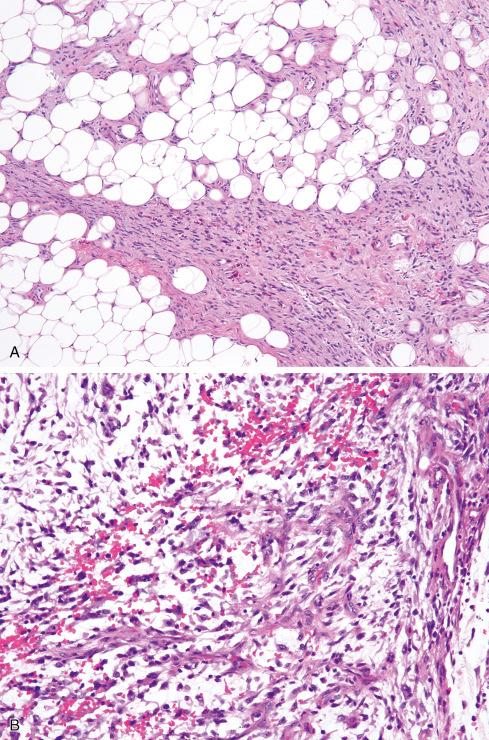
First described by Smith et al., PHAT is a distinctive hemorrhagic tumor of the subcutis characterized by ectatic fibrin-filled vessels. The tumor presents as a long-standing mass in patients ranging in age from 10 to 79 years (median: 51). There is a slight female predilection. In most cases the clinical impression is that of a hematoma, a benign neoplasm, or even Kaposi sarcoma. PHAT arises most often in the subcutaneous tissue of the lower extremity, particularly the ankle/foot, although other sites include the perineum, buttock, and arm. Rarely the tumors originate in skeletal muscle. Most average between 5 and 10 cm. Recurrences develop in approximately 30% to 50% of cases. In one unusual case, recurrences over a 25-year period ultimately necessitated amputation. In a limited number of cases, progression to a myxoid sarcoma results in subsequent recurrences. Wide local excision is recommended as the best therapeutic approach whenever possible.
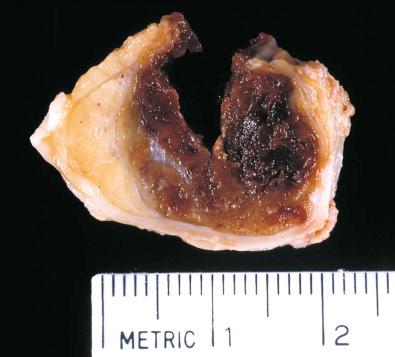
Grossly, most PHATs have a lobulated appearance with a cut surface that varies in color from white-tan to maroon ( Fig. 32.20 ). Rare examples have a prominent cystic component, and others show conspicuous myxoid change. Although having demarcated borders grossly, most actually show diffusely infiltrative margins with trapping of normal tissues at the tumor periphery.
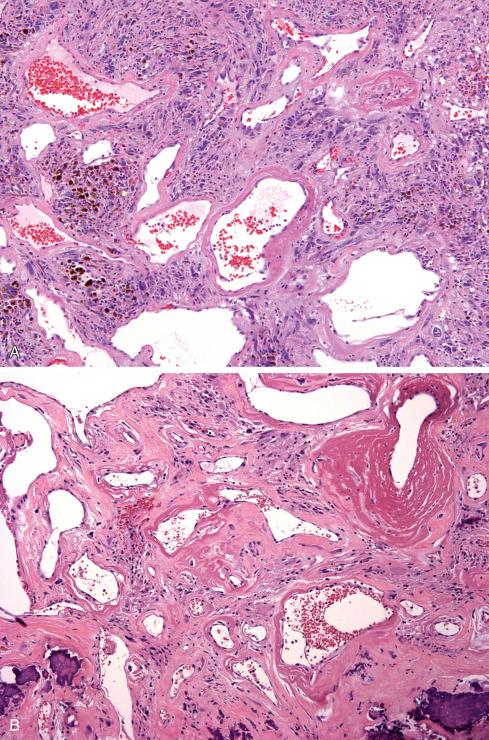
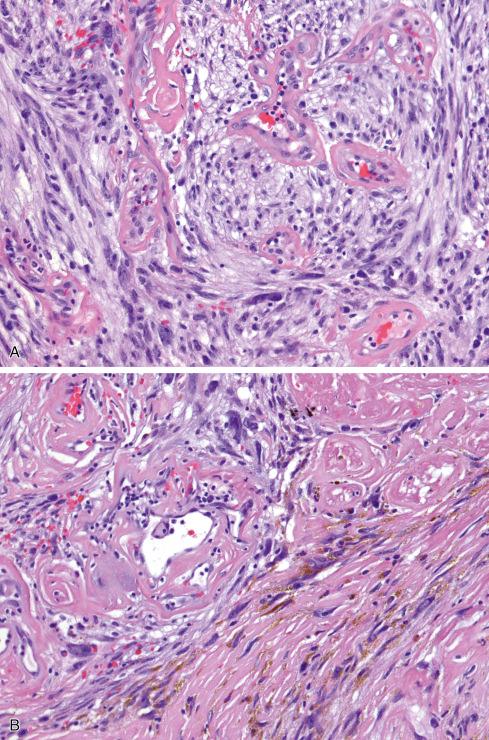
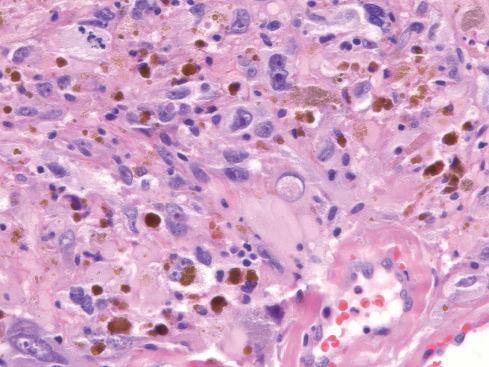
Microscopically, the most striking feature at low magnification is the presence of clusters of thin-walled ectatic blood vessels scattered throughout the lesion. The vessels range in size from small to macroscopic, and they tend to be distributed in small clusters ( Figs. 32.21 to 32.24 ). Typically, the ectatic vessels are lined by endothelium with a thick subjacent rim of amorphous eosinophilic material that is often surrounded by lamellated collagen. Some vessels contain organizing intraluminal thrombi with papillary endothelial hyperplasia. Hyaline material emanates from the vessels and extends into the stroma of the neoplasm, trapping neoplastic cells. The constituent cells are plump, spindled, and rounded with pleomorphic nuclei arranged in sheets or occasionally in fascicles reminiscent of fibrosarcoma ( Fig. 32.23A ). In general, the cells have hyperchromatic, pleomorphic nuclei and lack discernible cytoplasmic differentiation. Intranuclear cytoplasmic inclusions can be prominent ( Fig. 32.24 ). Despite the striking degree of nuclear pleomorphism, mitotic figures are scarce (usually <1/50 hpf). Occasional tumor cells, particularly those adjacent to ectatic vessels, contain intracytoplasmic hemosiderin. The tumors have a variable inflammatory infiltrate, most prominently mast cells, although lymphocytes, plasma cells, and eosinophils are conspicuous in some lesions. Foci of psammomatous calcification are occasionally present.
On IHC the lesional cells of PHAT express CD34 and vimentin, but are negative for actins, desmin, keratin, epithelial membrane antigen (EMA), von Willebrand factor, and CD31. Groisman et al. reported immunoreactivity for vascular endothelial growth factor (VEGF), a secreted protein implicated in tumor-associated angiogenesis, in both tumoral and endothelial cells.
Folpe and Weiss first suggested the hypothesis that PHAT was related to HFLT because of the presence of areas morphologically indistinguishable from HFLT at the periphery in almost 50% of classic cases of PHAT. These HFLT-like areas were labeled “early pleomorphic hyalinizing angiectatic tumor” and were thought to represent the precursor lesion to classic PHAT. In addition, as previously noted, cases only with features of HFLT sometimes displayed subtle features reminiscent of classic PHAT. These included small, damaged blood vessels surrounded by fibrin exudates and occasional pleomorphic cells with intranuclear pseudoinclusions. Others have reported cases showing mixed features of HFLT and PHAT as well. Although Smith et al. did not comment on HFLT-like areas in PHAT, such areas are illustrated in Figures 1 and 3 of their seminal description.
Until recently, little was known about the genetics of PHAT. The only conventional cytogenetic study, of a tumor showing mixed features of HFLT and PHAT, found two unbalanced translocations involving chromosomes 1 and 3 and chromosomes 1 and 10, with a karyotype of 45,XX,der(1)t(1;3)(p31;q12),-3,der(10)t(1;10)(p31;q25)[11]/46,XX[4]. These findings are similar (but not identical) to those previously reported in HFLT. Subsequently, TGFBR3 and/or MGEA5 genetic rearrangements have been identified in more than 60% of studied PHATs (all containing HFLT-like elements). We have seen in consultation other cases of PHAT showing rearrangements of both genes.
However, other studies have not identified TGFBR3 or MGEA5 rearrangements in PHAT. These differing results may represent statistical variation or the use of different diagnostic criteria for PHAT, in particular the inclusion of cases lacking a HFLT-like component. Some may also have represented “PHAT-like” pleomorphic sarcomas, which are known to lack TGFBR3 and MGEA5 rearrangements.
The differential diagnosis of HFLT includes reactive processes, such as fat necrosis with myofibroblastic proliferation and hemosiderin deposition, as well as other infiltrative, CD34-positive spindle cell tumors, in particular dermatofibrosarcoma protuberans . In general, recognition of the distinctive morphologic features of HFLT, especially the presence of intracytoplasmic hemosiderin pigment and the distinctive vascular changes, should allow these distinctions without great difficulty.
The differential diagnosis of PHAT includes schwannoma, malignant melanotic schwannian tumor (melanotic schwannoma), undifferentiated pleomorphic sarcoma, and the recently described superficial CD34-positive fibroblastic tumor (see later). Although the vascular architecture and pleomorphic cells of PHAT may suggest a schwannoma with ancient change, PHAT is not encapsulated, usually grows in an infiltrative manner, lacks distinct Antoni A and B zones, and does not express S-100 protein. PHAT with abundant hemosiderin pigment and occasional schwannomas may simulate malignant melanotic schwannian tumors , but lacks expression of S-100 protein and melanocytic markers (e.g., HMB-45, melan A). The pronounced nuclear pleomorphism in the absence of specific features of differentiation of PHAT often suggests the diagnosis of undifferentiated pleomorphic sarcoma . Despite the striking cellularity of many PHATs, this tumor lacks significant mitotic activity, displays intranuclear cytoplasmic inclusions, and expresses CD34, a constellation of findings not characteristic of undifferentiated pleomorphic sarcomas. Conversely, ectatic, fibrin-filled blood vessels may be seen in some undifferentiated pleomorphic sarcomas, and one should be very hesitant to diagnosis PHAT in lesions occurring in atypical locations, showing appreciable mitotic activity and lacking other typical features of PHAT, including HFLT-like areas. Cases of high-grade myxofibrosarcoma closely mimicking PHAT have been reported. Superficial CD34-positive fibroblastic tumor tends to occur in more proximal locations than PHAT; lacks ectatic vessels, intracytoplasmic hemosiderin, and coexisting HFLT; and shows patchy expression of keratins (see later section).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here