Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The most frequently encountered soft tissue lesion is the dermal scar. It is commonly seen in a postsurgical setting (e.g., re-excision of a tumor) but may also reflect nonsurgical trauma or result from a ruptured cyst or hair follicle. A keloid is a distinct subtype of scar in which the scarring process is exuberant and ultimately extends beyond the focus of injury. Keloids have a predilection for patients of African descent, but they can occur in any race. Keloids most commonly occur on the head and neck and upper trunk; the ear is an especially common site.
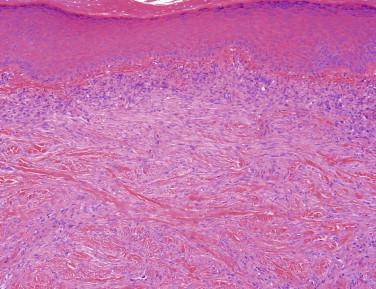
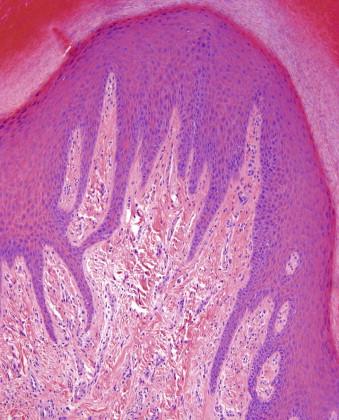
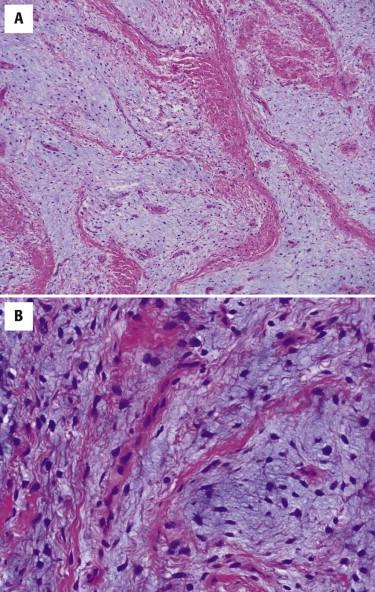
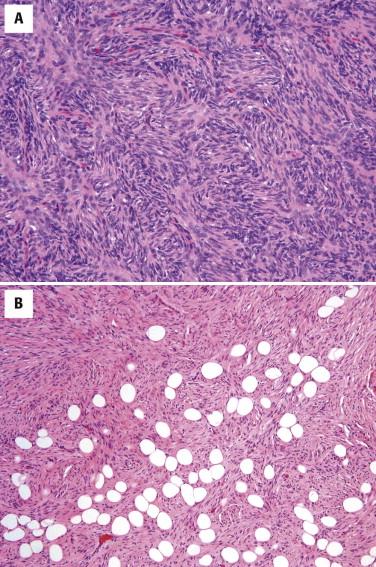
Dermal scars are composed of a variably cellular proliferation of bland fibroblasts in a collagenous stroma. As the scar becomes more organized, the cellularity decreases, and the fibroblasts tend to be arranged parallel to the epidermis ( Fig. 13-1, A ). A reactive vascular proliferation, characterized by irregular, thin-walled vessels, accompanies the fibroblasts. The vessels tend to be oriented perpendicularly to the overlying epidermis. Depending on the cellularity and size of a scarring reaction and its clinical manifestation as a nodule, a scar may be designated as hypertrophic.
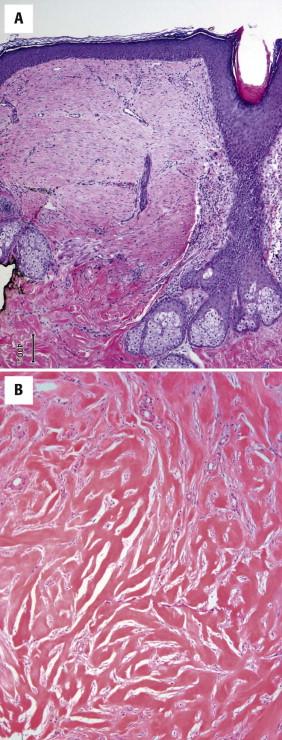
Keloids are characterized by widely spaced fibroblasts associated with broad, glassy collagen fibers ( Fig. 13-1, B ). The vasculature of keloids is less conspicuous.
The differential diagnosis of dermal scars and keloids is rarely difficult. Rarely, fibromatosis (see the following) may be difficult to distinguish from a hypertrophic scar; however, fibromatosis tends to have long sweeping fascicles as opposed to shorter, more haphazardly arranged fascicles of a scar. The vasculature of fibromatosis is also different, showing uniformly distributed, slender vessels in contrast to the perpendicular arrangement of the reactive vessels in a scar. With the exception of palmar, plantar or penile variants, fibromatosis rarely presents as a superficial mass. A more challenging problem is to recognize that a scarlike process may reflect the presence of a malignant tumor with desmoplastic stroma. Careful attention to clinical (no history of trauma; clinical suspicion for tumor) and histologic (sun-damaged skin, associated atypical intraepidermal melanocytic proliferation or squamous dysplasia) context is needed to avoid overlooking the presence of a desmoplastic carcinoma or melanoma.
Scars may need revisions for cosmetic reasons. The treatment of hypertrophic scars and keloids is problematic. No single treatment regimen is highly successful. A combination of intralesional steroid injection and surgical excision is the most common approach. Regardless of the therapeutic intervention, keloids frequently recur, and the recurrence may be more problematic than the original lesion.
Dermatofibroma or benign fibrous histiocytoma is a common benign mesenchymal tumor of the skin, which may show a spectrum of differentiation toward fibroblasts, myofibroblasts, or histiocytes.
Dermatofibroma is most often found on the extremities of adults, but the tumor can manifest at any age and site. Typically, the tumor manifests as a small slowly growing, painless, white to red-brown, slightly elevated nodule.
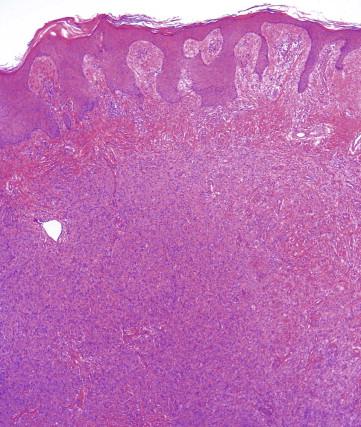
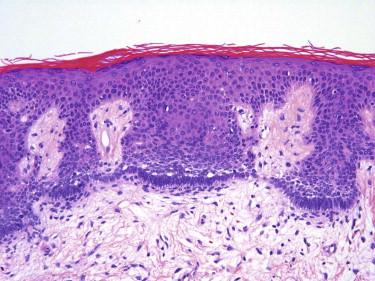
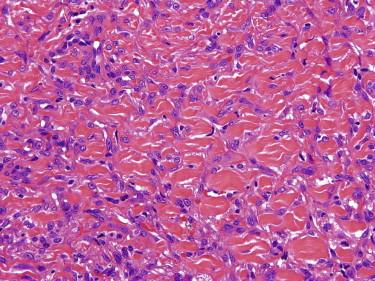
Dermatofibroma typically appears as a somewhat circumscribed dermal spindle cell tumor ( Fig. 13-2 ). A thin rim of uninvolved dermis separates most tumors from the overlying epidermis, which frequently shows hyperplasia and basilar hyperpigmentation. Such a “grenz zone” may be lost when a dermatofibroma has been traumatized. The dermal-epidermal junction overlying a dermatofibroma may show a trichoblastic proliferation that can be mistaken for a basal cell carcinoma (BCC) on a superficial shave biopsy ( Fig. 13-3 ). Most dermatofibromas are restricted to the dermis, but extension into the superficial subcutaneous fat is not uncommon. At the periphery of the lesion, the tumor cells interdigitate around preexisting collagen fibers of the reticular dermis ( Fig. 13-4 ). This characteristic “collagen trapping” is a very useful diagnostic feature and is present in most variants of this tumor. The growth pattern of the lesional cells may appear random to vaguely storiform. Cytologically, a dermatofibroma is composed of a heterogeneous population of oval to fusiform fibrohistiocytic cells ( Fig. 13-5 ). Foamy histiocytes, multinucleated giant cells, and hemosiderin-laden macrophages may be present ( Fig. 13-6 ). Mitotic figures can be seen.
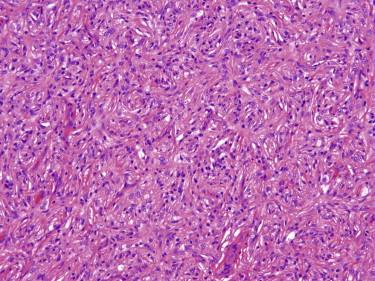
There is a broad spectrum with regard to the size and cellular density of dermatofibromas, as well as with regard to the cytologic appearance of the constituent cells (fusiform or epithelioid cell shape, clear or granular cytoplasm, and the presence of nuclear atypia) and secondary features, such as hemorrhage and foamy macrophages. This is reflected in a number of proposed variants of dermatofibroma (or benign fibrous histiocytoma), including cellular, aneurysmal, epithelioid, clear cell, palisading, granular cell, and atypical variants. Often, different histologic variants are combined in a single tumor.
Cellular dermatofibroma, also known as cellular benign fibrous histiocytoma, is a more densely cellular and fascicular tumor compared with classic dermatofibroma ( Fig. 13-7, A ). It is typically a deeper seated, stellate-shaped tumor, which frequently involves subcutaneous tissue, growing along fibrous septa. Cytologically, the tumor is composed of relatively monotonous spindle cells with tapered nuclei and amphophilic cytoplasm, resembling those seen in dermatofibrosarcoma protuberans (DFSP) and may show mitotic activity or focal necrosis ( Fig. 13-7, B ). However, in contrast to DFSP, cellular benign fibrous histiocytoma displays typical collagen trapping at the periphery and lacks the diffuse infiltrative “honeycomb” growth pattern of the former. This variant of fibrous histiocytoma may occasionally show focal CD34 immunoreactivity, particularly at the periphery of the tumor where the tumor cells intercalate between dermal fibroblasts.
Flesh-colored or brown, firm papule
Dermal spindle cell tumor with storiform growth pattern
Plump spindle cells that wrap around collagen bundles at the periphery
Foamy histiocytes, multinucleated cells, hemosiderin may be present
Associated epidermal hyperplasia is common
Associated trichoblastic proliferation not uncommon
Positive for FXIIIa; may be positive for CD68 or SMA
Negative for S100, usually negative for CD34
Dermatofibrosarcoma protuberans
Amelanotic sclerosing melanocytic nevus
Dermal scar
Dermatomyofibroma
FXIIIa, Factor XIIIA; SMA, smooth muscle actin.
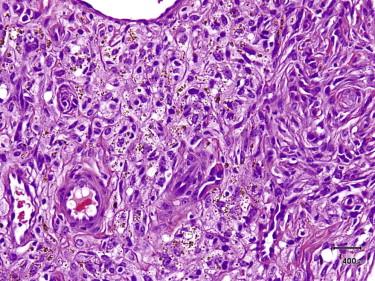
Aneurysmal dermatofibroma is characterized by large blood-filled spaces lined by compressed tumor cells in conjunction with otherwise typical histologic features of a dermatofibroma ( Fig. 13-8 ). Sometimes the blood-filled spaces are so prominent that the true nature of the tumor can be inconspicuous. Hemosiderin-laden macrophages are often found in this variant.
Epithelioid dermatofibroma is composed of cells with round to oval, vesicular nuclei and abundant eosinophilic cytoplasm ( Fig. 13-9 ). Areas resembling more typical dermatofibroma may be present focally, but in contrast to other variants, epithelioid dermatofibroma is relatively well circumscribed and polypoid in appearance and less commonly displays peripheral collagen trapping. The differential diagnosis of an epithelioid dermatofibroma is with other epithelioid cutaneous tumors, including melanocytic nevi, malignant melanoma, and myoepithelial tumor. Immunohistochemical stains for S100, melanocytic markers, and cytokeratins, which are expected to be negative in an epithelioid dermatofibroma, will aid in this distinction. Epithelioid dermatofibromas may sometimes express epithelial membrane antigen (EMA), a diagnostic pitfall.
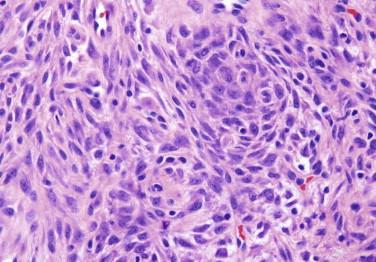
Atypical fibrous histiocytoma, also referred to as dermatofibroma with monster cells, shows features of typical dermatofibroma but contains a subpopulation of cells with large, hyperchromatic and pleomorphic nuclei and multinucleated cells ( Fig. 13-10 ). Mitotic activity may be high, and atypical forms may be present.
The lesional cells of dermatofibroma are often immunoreactive for factor XIIIa, although the staining for this marker can be quite variable and therefore of limited diagnostic utility. Cells with a histiocytic appearance (foamy cytoplasm, multinucleated cells) are often positive for CD68. Dermatofibromas, especially cellular variants thereof, may also contain tumor cells immunoreactive for smooth muscle actin (SMA), reflecting fibroblastic–myofibroblastic differentiation. The tumor cells in dermatofibromas do not express CD34 or S100 protein. However, one should be cautious interpreting immunoreactivity for these markers because entrapped non-neoplastic cells within a dermatofibroma may express either of these antigens.
This distinction is usually straightforward but may be difficult in a small biopsy sample. The most helpful diagnostic feature is the presence of peripheral collagen trapping in a dermatofibroma as opposed to a diffusely infiltrative growth pattern of a DFSP. Other features typical of dermatofibroma and arguing against DFSP include relative circumscription, cellular and nuclear polymorphism, associated epidermal or trichoblastic hyperplasia, and the presence of foamy histiocytes. Immunohistochemically, dermatofibromas are typically negative for CD34 and express factor XIIIa and CD68.
A dermatofibroma may be confused with an amelanotic blue nevus or a spindle cell melanoma. A nested rather than storiform growth pattern and more oval or rounded nuclei with occasional intranuclear inclusions typical of melanocytes are helpful in this distinction. Blue nevi also have more uniform cytologic features compared with dermatofibromas. An immunostain for S100 protein or Melan-A would readily distinguish a melanocytic proliferation from a dermatofibroma.
Atypical fibrous histiocytoma (dermatofibroma with monster cells) needs to be distinguished from atypical fibroxanthoma, pleomorphic fibroma, and an undifferentiated pleomorphic sarcoma of the skin. Age may be helpful because atypical fibroxanthoma and pleomorphic sarcoma more often arise in older adults, typically on sun-damaged skin of the head and neck. Histologically, as with other variants of dermatofibroma, key to the diagnosis is recognizing the conventional growth pattern of more typical dermatofibroma with characteristic collagen trapping. Pleomorphic fibroma may be distinguished from atypical fibrous histiocytoma by its far lower cellularity and absence of dermatofibroma-like areas and foam cells. Pleomorphic fibroma, atypical fibroxanthoma, and undifferentiated pleomorphic sarcoma typically extend to the overlying epidermis and lack the “grenz zone.”
Dermatofibromas may also be confused with a scar. The fibroblasts in scars are usually aligned parallel to the skin surface and lack the typical collagen trapping seen with dermatofibroma. The vasculature of a dermatofibroma is typically thin, branching, and evenly distributed in contrast to the irregular, reactive vessels arranged perpendicular to the epidermis in a scar.
Dermatofibroma and its variants are benign tumors. Most dermatofibromas do not need to be excised except for cosmetic reasons. However, for the cellular and atypical variants, conservative complete excision is worth considering given their propensity for local recurrence (the local recurrence rate of these variants is ≈20%) and less favorable cosmetic result after continued growth and local recurrence. Very rare cases of visceral metastases from fibrous histiocytoma have been reported, but metastatic lesions appear to behave in an indolent fashion and maintain the histologic appearance of the original tumor.
Dermatomyofibroma manifests as a flesh-colored or hypopigmented plaque, most commonly on the upper trunk or neck of young adults.
| Dermatofibroma | Dermatofibrosarcoma protuberans |
| Epidermal hyperplasia with or without trichoblastic proliferation | No epidermal hyperplasia |
| Relatively circumscribed | Infiltrative |
| Heterogeneous cell population, often including foamy histiocytes | Monomorphic cell population; no foamy histiocytes |
| Collagen trapping at the periphery | No collagen trapping |
| Loose storiform growth pattern | Tight storiform growth pattern |
| Usually confined to dermis, but may extend into subcutis typically along fibrous septa | Extensive involvement of subcutis Entrapment of individual adipocytes |
| IHC, CD34- or focal, usually at the lesional periphery | IHC, CD34+, strong and diffuse |
Dermatomyofibroma is composed of bland spindled cells resembling superficial fibromatosis arranged in fascicles with a distinctly parallel orientation to the overlying epidermis ( Fig. 13-11 ). The tumor is usually restricted to the reticular dermis but may show limited involvement of the subcutis in a manner similar to dermatofibromas. The individual tumor cells have elongated vesicular nuclei and faintly eosinophilic cytoplasm.
The tumor cells variably express muscle specific actin and may express SMA. Dermatomyofibromas are typically negative for CD34 and also negative for S100 protein, desmin, or factor XIIIa.
Dermatofibrosarcoma protuberans can be distinguished by its more cellular nature, storiform growth pattern, and immunoreactivity for CD34. Dermatofibromas have a more varied population of tumor cells and a storiform growth pattern, show collagen trapping, and usually demonstrate immunoreactivity for factor XIIIa. Dermal scars can be confused with dermatomyofibroma because of the parallel arrangement of spindled cells. Dermal scars usually show evidence of epidermal injury with effacement of the normal rete peg architecture and reactive vasculature oriented perpendicularly to the overlying epidermis. Dermatomyofibromas tend to spare adnexal structures while the latter are not spared in a scar. Superficial fibromatosis (Dupuytren contracture) shows significant cytologic overlap with dermatomyofibroma, but the clinical setting (involvement of tendons on the palmar surfaces of hands and feet) is quite distinct. Desmoid-type fibromatosis may also show considerable overlap with dermatomyofibroma histologically, but it usually presents as a larger, deeply situated, and more infiltrative lesion. Desmoid-type fibromatosis does not typically occur in the dermis. Finally, the differential diagnosis from a fibroblastic connective tissue nevus (discussed in the following) may be difficult. Knowledge of the clinical setting (presence since childhood) is helpful. Histologically, fibroblastic connective tissue nevus is composed of haphazardly arranged bundles of bland spindle cells involving the dermis and subcutaneous tissue in contrast to more orderly arrangement of spindle cells in dermatomyofibroma. By immunohistochemistry, fibroblastic connective tissue nevi express CD34 and typically lack SMA immunoreactivity.
Dermatomyofibroma is a benign tumor. Excision may be necessary for definitive diagnosis when only a small portion of the tumor has been sampled for histology.
Fibroblastic connective tissue nevus is a rare benign cutaneous proliferation of CD34-positive fibroblastic or myofibroblastic cells first described by de Feraudy and Fletcher in 2012. The age range at presentation is broad, although the lesion appears to be most common in children (median, 10 years). The lesion presents as a solitary, slowly growing firm plaque-like or nodular skin lesion, up to 2 cm in size, most often on the trunk, but the extremities and head and neck area may also be involved.
Fibroblastic connective tissue nevi are poorly circumscribed and unencapsulated lesions composed of fascicles of bland spindle cells growing in an irregular, ramifying pattern through the dermis and entrapping adnexal structures ( Fig. 13-12 ). The stroma is loose or collagenous. The overlying epidermis often shows distinctive papillomatous hyperplasia. Another distinctive feature of fibroblastic connective tissue nevus is the presence of adipose tissue very superficially in the reticular dermis. Extension into the subcutis is not uncommon but usually occurs along the fibrous septa. Cytologically, the cells show features of bland fibroblasts or myofibroblasts with oval tapering nuclei and pale amphophilic cytoplasm. There is no cytologic atypia or mitotic activity.
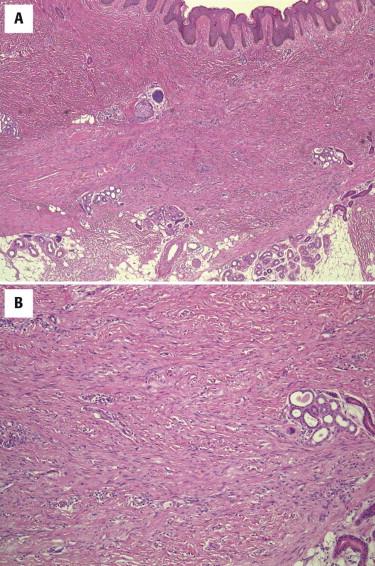
Most fibroblastic connective tissue nevi express CD34, although it is usually patchy and variable in intensity. Rare cases express SMA and factor XIIIa. Desmin, S100, cytokeratins, and CD31 are negative.
The differential diagnosis of fibroblastic connective tissue nevi includes DFSP, particularly plaque-like variant, dermatomyofibroma, pilar leiomyoma, and occasionally fibromatosis. DFSP is the most important differential diagnosis of fibroblastic connective tissue nevus. It can be distinguished based on the presence of storiform rather than fascicular growth pattern; more prominent cytologic atypia; and diffuse, strong CD34 immunoreactivity. Identification of giant cell fibroblastoma-like areas and more diffuse infiltration of the subcutis are also helpful to distinguish DFSP from fibroblastic connective tissue nevus. Dermatomyofibroma (discussed earlier) similarly presents as a fascicular dermal-based lesion. However, it typically presents in an older age group, and the fascicles of spindle cells tend to run distinctly parallel to the epidermis in contrast to the more haphazard appearance of the fibroblastic connective tissue nevus. Immunohistochemically, dermatomyofibroma consistently expresses SMA and is only rarely positive for CD34. Although there is certainly some overlap in clinical presentation, pilar leiomyomas also tend to occur in the older age group, are most often extremity based, and can be painful. Histologically, pilar leiomyoma is more nodular in appearance, and the spindle cells show the features of smooth muscle differentiation: elongated nuclei with blunt ends and brightly eosinophilic fibrillary cytoplasm. By immunohistochemistry, pilar leiomyomas express SMA, desmin and H-caldesmon and are typically negative for CD34. Superficial fibromatosis may resemble fibroblastic connective tissue nevus histologically but occurs in a distinctly different clinical setting (involvement of tendons on the palmar surfaces of the hands and feet).
The available data suggest that fibroblastic connective tissue nevus does not recur and does not metastasize and thus does not require a complete excision unless indicated for cosmetic reasons. Distinguishing this lesion from DFSP is the most important task to ensure appropriate patient management.
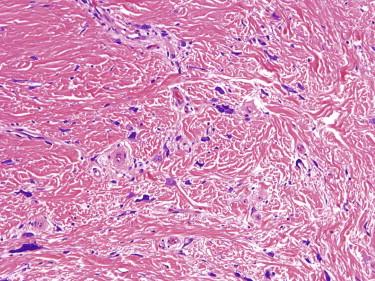
Pleomorphic fibromas present in adult patients as a slowly growing, dome-shaped papule. The extremities are the most common location followed by the trunk and head and neck. Clinically, they can resemble a skin tag, a nevus, or a neurofibroma.
The tumors are well-circumscribed, polypoid, and paucicellular lesions composed of widely scattered spindled, stellate, and pleomorphic multinucleated giant cells ( Fig. 13-13 ), which are thought to represent degenerative atypia. Mitotic figures are rare, and atypical mitotic figures are almost never seen. The stroma is usually densely collagenous, but myxoid change may be seen and rarely is a prominent feature. Adnexal structures are absent.
The tumor cells are CD34 positive and variably positive for SMA. Immunostains for S100 protein, cytokeratin, and desmin are negative.
The differential diagnosis primarily includes atypical fibroxanthoma and atypical fibrous histiocytoma. Both of these entities are much more cellular than pleomorphic fibroma. Atypical fibroxanthoma usually occurs on sun-damaged skin of elderly patients. Atypical fibrous histiocytoma shows areas of more typical benign fibrous histiocytoma with peripheral collagen trapping.
Pleomorphic fibromas, despite their alarming histologic features, are benign tumors and do not typically recur.
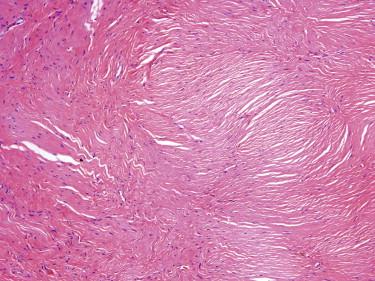
This benign fibrous lesion usually presents as a solitary flesh-colored papule or nodule but may be multiple in Cowden's disease.
Sclerotic fibromas are paucicellular, circumscribed dermal nodules composed of bland spindled to stellate cells embedded in a dense collagen matrix. The collagenous stromal background is the most distinctive feature. It has a whorled, wood grain–like or a plywood-like pattern with prominent intervening clefts ( Fig. 13-14 ). Occasional multinucleated cells may be present. By immunohistochemistry, the lesional cells are diffusely positive for CD34 and negative for S100, actin, and EMA. The differential diagnosis of sclerotic fibroma includes a perineurioma, which can be very similar morphologically but can be distinguished by expression of EMA (negative in sclerotic fibroma).
Sclerotic fibroma is a benign lesion that does not require treatment.
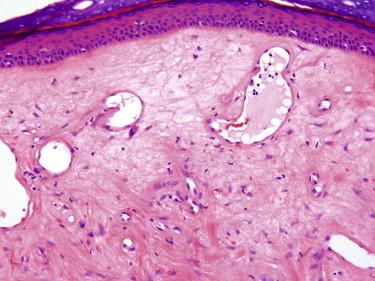
Angiofibromas are a group of lesions with similar histologic features that present as solitary or in some cases multiple firm, usually flesh-colored papules. In the autosomal dominant neurocutaneous syndrome (tuberous sclerosis), angiofibromas (adenoma sebaceum) typically manifest in childhood as multiple small papules or nodules on the central face, especially the nasolabial fold. Fibrous papules, another type of angiofibroma, are solitary acquired lesions of adulthood. They commonly occur on the central face, especially the nose. They may be confused clinically with BCC or melanocytic nevus. Pearly penile papules are another type of angiofibroma that occurs in groups on the penis, primarily the coronal margin and sulcus in up to 10% of young adults. Clinically, they are often mistaken for warts. Acral fibrokeratomas may also be considered a form of angiofibroma. They can be multiple in tuberous sclerosis but usually present as solitary acquired lesions on the digits, especially around the nail fold.
Angiofibromas are characterized by ectatic vessels embedded in a collagenous stroma with scattered spindled to stellate fibroblasts, including multinucleated cells ( Fig. 13-15 ). Variants have been described referring to differences in cell density and cytology of the fibroblasts, including cellular, epithelioid, granular, pleomorphic, and clear cell variants of fibrous papules ( Fig. 13-16 ). In the acral fibrokeratoma variant, there is often prominent acanthosis of the epidermis, and the fibroblasts and collagen fibers are often vertically oriented ( Fig. 13-17 ).
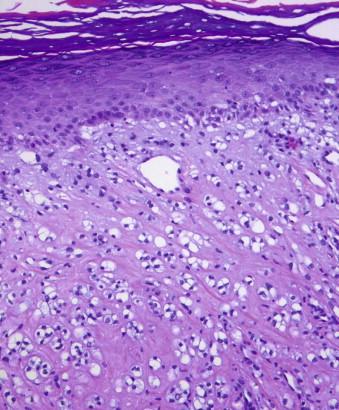
Trichodiscomas and fibrofolliculomas are tumors of perifollicular mesenchyme that can be confused with angiofibromas. The stroma in these two tumors tends to be more myxoid, and the fibroblasts are more slender. In trichodiscoma, there is often a folliculosebaceous collarette surrounding the fibroblastic proliferation. In fibrofolliculoma, there is a proliferation of follicular mantle epithelium.
The primary entity in the differential diagnosis of acral fibrokeratoma is a supernumerary digit, but acral fibrokeratomas lack the vestiges of neural structure seen in supernumerary digits. Fibrous papules of the nose may at times be associated with a slight hyperplasia of intraepidermal melanocytes. It has been suggested that some of these lesions may represent regressed nevi (melanocytic angiofibromas). A small superficial biopsy can raise concern about the possibility of an associated lentigo or lentigo maligna. Careful attention to the cellular density and distribution of melanocytes as well as clinical correlation is critical for the correct diagnosis. Cellular variants of fibrous papule may be mistaken as a melanocytic tumor. Clear cell fibrous papule may be confused with a sebaceous neoplasm or even metastatic renal cell carcinoma, but immunohistochemical stains can resolve this issue readily.
Angiofibromas are benign lesions that do not require treatment, except for cosmetic reasons.
Nodular fasciitis is a self-limiting neoplasm that typically presents as a rapidly growing subcutaneous nodule most often on the extremities (especially forearm) of young to middle-aged adults. However, all age ranges may be affected as well as a wide variety of anatomic sites. Antecedent history of trauma is present in the minority of cases. Genetic studies identified MYH9-USP6 gene fusion as a recurrent event in nodular fasciitis, confirming its neoplastic nature.
Most cases of nodular fasciitis are centered in the subcutis, but rare cases occur primarily in the dermis. Nodular fasciitis is usually a circumscribed or only focally infiltrative mass composed of a loose arrangement of spindled myofibroblasts arranged in a random, tissue culture–like growth pattern ( Fig. 13-18, A ). The spindle cells are usually admixed with scattered lymphocytes, extravasated red blood cells, and occasional osteoclast-type giant cells. Most cases show varying cellularity and myxoid stroma, which sometimes shows cystic breakdown. Numerous plump, reactive-appearing vessels are present. Occasional osteoclast-type multinucleated giant cells may be identified. As it involutes, nodular fasciitis can become paucicellular and hyalinized. Cytologically, the lesional cells have the appearance of myofibroblasts with uniform oval nuclei, fine chromatin, and small nucleoli ( Fig. 13-18, B ). Nuclear pleomorphism is not observed. Mitotic activity is brisk, but atypical mitotic figures are absent.
Rapidly growing nodule
Nodular proliferation of myofibroblasts
Usually located in the subcutis
Vague fascicular or loose, tissue culture appearance
Myxoid edematous background, cystic degeneration
Extravasated red blood cells, lymphocytes
Variably positive for SMA, muscle-specific actin
Spindle cell sarcomas
SMA, Smooth muscle actin.
The fascial variant of nodular fasciitis is similar in appearance except for the pattern of growth, extending along fascial planes of the subcutaneous septae and imparting an infiltrative appearance. The vasculature is usually more prominent than in typical cases of nodular fasciitis.
Intravascular fasciitis shows an intravascular myofibroblastic proliferation similar to nodular fasciitis. The intravascular proliferation results in a multinodular appearance. Multinucleated osteoclast-type giant cells are usually more prominent in this variant.
The myofibroblasts of nodular fasciitis show membranous immunoreactivity for SMA and muscle-specific actin. Nodular fasciitis is typically negative for desmin, cytokeratin, and S100 protein. In rare, difficult cases, genetic testing for the MYH9-USP6 fusion can be performed.
Benign entities in the differential diagnosis include cellular dermatofibroma (cellular benign fibrous histiocytoma) and fibromatosis. Dermatofibromas are typically dermal based, have a more storiform pattern of growth with peripheral collagen trapping, and have a more heterogeneous population of cells. Fibromatosis is usually less cellular but far more monotonous in appearance than nodular fasciitis. Storiform cell arrangements are less common. More typical are long fascicles of slender fibroblasts. Malignant entities in the differential diagnosis primarily include spindle cell sarcomas, namely, low-grade myofibroblastic sarcoma or, in prominently myxoid lesions, myxofibrosarcoma. The degree of nuclear hyperchromasia and the presence of atypical mitotic figures may help to distinguish nodular fasciitis from a low-grade myofibroblastic sarcoma, although sometimes this distinction can only be made in retrospect when a lesion recurs. Myxofibrosarcoma typically has a far more infiltrative, multinodular growth pattern and shows a greater degree of nuclear atypia and characteristic curvilinear vessels.
Nodular fasciitis is a self-limiting lesion; excision is unnecessary.
Proliferative fasciitis is very similar in its clinical manifestation to nodular fasciitis. It tends to occur as a subcutaneous mass at sites of trauma on the extremities, especially the forearm. It occurs in middle-aged to older patients.
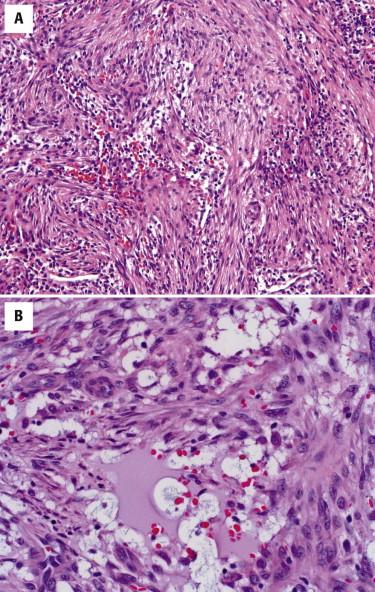
Unlike typical cases of nodular fasciitis, proliferative fasciitis has a less circumscribed, more infiltrative pattern of growth similar to the fascial variant of nodular fasciitis. Proliferative fasciitis, similar to nodular fasciitis, shows a tissue culture–like proliferation of bland myofibroblasts. The hallmark of proliferative fasciitis is ganglion-like myofibroblasts characterized by their large vesicular nuclei, prominent nucleoli, and abundant cytoplasm ( Fig. 13-19 ). These cells are typically absent in nodular fasciitis. Pediatric cases of proliferative fasciitis may be almost exclusively composed of these ganglion-like cells and show greater cytologic atypia and even focal necrosis, mimicking a malignant neoplasm.
The immunophenotype of proliferative fasciitis is essentially identical to that of nodular fasciitis. The spindled and ganglion-like cells are positive for actins reflecting their myofibroblastic differentiation; stains for cytokeratin, desmin and S100 protein are typically negative.
Because of the large ganglion-like myofibroblasts, pleomorphic sarcomas, especially pleomorphic rhabdomyosarcoma, are usually considered in the differential diagnosis. However, rhabdomyosarcoma typically shows a greater degree of nuclear atypia; may demonstrate cross-striations; and may express desmin and myogenin, a specific marker of skeletal muscle differentiation.
Proliferative fasciitis is self-limiting process that recurs very rarely and requires no therapy.
Fibroma of the tendon sheath presents as a slow-growing firm nodule of the hands or feet that is firmly attached to the underlying tendon sheath. It usually occurs in young to middle-aged adults, most commonly involving the fingers, hands, or wrists.
The tumors are well circumscribed to lobular proliferations attached to the tendon sheath. They are composed of widely spaced bland spindled to stellate cells in a dense collagenous and sometimes myxoid stromal matrix ( Fig. 13-20 ). Although most lesions are paucicellular, hypercellular areas resembling nodular fasciitis may be seen. Characteristic cleftlike vasculature is often present at the periphery of these lesions.
The clinical presentation suggests the possibility of a localized tenosynovial giant cell tumor (giant cell tumor of the tendon sheath). However, histologically, giant cell tumor of the tendon sheath is a very different lesion composed of rounded mononuclear cells, osteoclast-type giant cells, foamy histiocytes, and lymphocytes in variable proportions. Hemosiderin deposits are often present. Superficial fibromatosis may enter the differential diagnosis with fibroma of the tendon sheath; however, fibromatosis is typically a more cellular and infiltrative lesion and is composed of long fascicles of monotonous fibroblastic cells. More cellular variants of fibroma of the tendon sheath may resemble nodular fasciitis. However, nodular fasciitis rarely arises in the hands and feet, clinically is a rapidly growing lesion, and usually shows myxoid zones with tissue culture–like appearance of spindle cells and reactive vasculature.
Fibroma of the tendon sheath is a benign entity with a tendency for local recurrence in up to one fourth of cases. Simple excision is the therapy of choice.
Although not technically a fibrous or fibrohistiocytic tumor, it is presented here because of its clinical similarities to fibroma of the tendon sheath.
There are two main subtypes of tenosynovial giant cell tumors, which have the same histologic appearance but are divided based on the growth pattern. The localized, extraarticular type of tenosynovial giant cell tumor is also referred to as giant cell tumor of the tendon sheath. This tumor generally occurs in young to middle-aged adults, more frequently in women, and most commonly affects the hands. The great majority arises in the fingers, usually at interphalangeal sites. Less common sites include the feet, knees, or wrists. The tumors typically present as slow-growing masses fixed to the underlying tendon sheath. Diffuse-type tenosynovial giant cell tumor, also termed pigmented villonodular synovitis, is a soft tissue equivalent of the intraarticular process and most often occurs in periarticular soft tissues, often around the knee region. Both forms of tenosynovial giant cell tumor are considered to represent a true neoplasm and are characterized by a recurrent translocation t(1;2)(p13;q37) resulting in CSF1-COL6A3 fusion.
Tenosynovial giant cell tumors are usually lobular, partially encapsulated, exophytic masses attached to the tendon sheath. There are several major cell types present in variable proportions: round to oval mononuclear cells with oval eccentric nuclei, inconspicuous nucleoli and amphophilic cytoplasm, multinucleated osteoclast-type giant cells, and chronic inflammatory cells ( Fig. 13-21 ). Cellular density can be variable. The multinucleated cells are usually scattered throughout the tumor, but they can be quite sparse. Secondary changes, including xanthoma cells and hemosiderin deposition, are commonly seen.
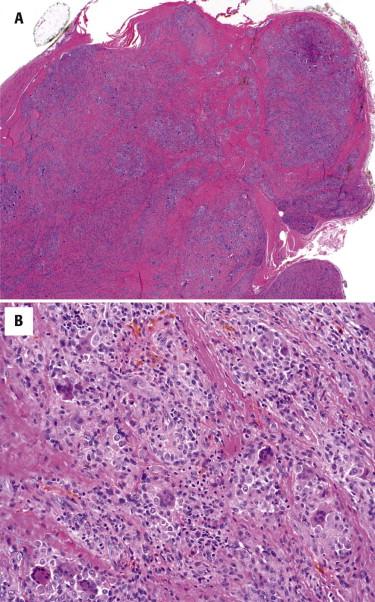
The giant cell tumor of soft tissue (also known as giant cell tumor of low malignant potential; see the following) has some similarities with tenosynovial giant cell tumor. However, the former entity typically does not involve the hands, has a distinctly multinodular growth pattern, more prominent spindle cell component, and often a shell of metaplastic bone. Osseous metaplasia is distinctly uncommon in tenosynovial giant cell tumor. Tendinous xanthomas could be considered in the differential diagnosis in tumors in which xanthoma cells are particularly prominent. However, xanthoma cells are essentially the sole component of tendinous xanthomas. Fibroma of the tendon sheath is similar clinically, but histologically, this is a far less cellular lesion composed of cytologically bland spindle myofibroblasts growing loosely in collagenous stroma. Characteristic thin, slitlike vessels are typically present at the periphery of this lesion.
Tenosynovial giant cell tumor is a benign tumor but prone to local recurrence. The risk of local recurrence is particularly high for the diffuse-type tumors. Surgical excision is the treatment of choice.
Myofibromas may present as solitary (myofibroma) or multiple lesions (myofibromatosis) involving the skin, skeleton, central nervous system (CNS), or visceral organs. Although initially described in infants, they can occur at any age. Myofibromatosis is generally restricted to infants, with almost half of the cases presenting at birth. When solitary, they are most frequently found in the skin of the head and neck followed by the trunk. Tumors involving the skin often have a violaceous appearance and may clinically be mistaken for a vascular neoplasm.
Myofibromas involving the skin are well-circumscribed, nonencapsulated tumors. Frequently, they have a multinodular appearance. They typically have a characteristic biphasic appearance with spindled, myoid cells arranged in fascicles or nodules and more primitive-appearing round cells growing around a hemangiopericytoma-like vasculature ( Fig. 13-22 ). The fascicles of spindled cells are often at the periphery of the tumor and the round cell component located centrally. Solitary myofibromas in adults often have a less distinct zonal pattern. The mitotic rate is generally low. Coagulative tumor necrosis as well as vascular invasion may be seen in myofibromas and can cause the mistaken interpretation of this tumor as a sarcoma.
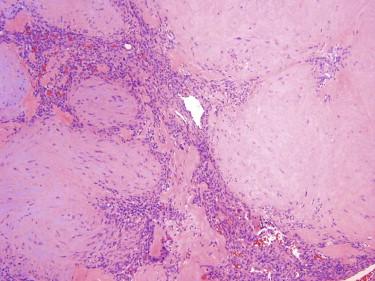
The tumor cells of myofibroma generally show some immunoreactivity for actins, indicative of myofibroblastic differentiation. They are negative for desmin.
The differential diagnosis varies depending on which component is most prominent. When the spindled cells predominate, potential confusion may occur with nodular fasciitis, cellular dermatofibroma, leiomyoma, desmoid-type fibromatosis, and low-grade myofibroblastic sarcoma. None of these lesions shows biphasic architecture and hemangiopericytomatous features, which help to identify a myofibroma. When the hemangiopericytoma-like areas dominate, the differential diagnosis would include myopericytoma; solitary fibrous tumor; and, in particularly cellular examples, synovial sarcoma. Myopericytoma may represent a morphologic continuum with a myofibroma. Immunolabeling for CD34 would help to distinguish a solitary fibrous tumor from hemangiopericytoma-like areas in myofibroma where CD34 would only highlight the vasculature but not the spindle cells. Synovial sarcoma typically shows a greater degree of cellularity and monotonous fascicular arrangement, features not encountered in a myofibroma. Immunolabeling for TLE1 protein or identification of SS18 ( SYT ) gene rearrangement by molecular methods may help in particularly challenging cases.
Myofibromas are benign tumors that may spontaneously resolve. Conservative complete excision is the treatment of choice. Rarely, solitary lesions recur. Despite the occasional presence of worrisome histologic features such as necrosis or vascular invasion, there is no risk of metastasis. Although myofibromas are benign, myofibromatosis is prone to local recurrences and rarely can be lethal secondary to visceral involvement.
Cutaneous myxomas and superficial angiomyxomas represent a spectrum of the same tumor. They present as small dermal nodules most commonly on the trunk, lower extremities, and head and neck regions. They may be sporadic or associated with Carney's complex in which they often present as multiple lesions on the ears or eyelids in early adulthood. Carney's complex is an autosomal dominant condition associated with abnormalities in chromosomes 2p16 and 17q, involving mutations in the PRKAR1A gene. In addition to cutaneous myxomas, patients with Carney's complex also frequently develop cardiac myxomas, spotty pigmentation, endocrine overactivity, psammomatous melanotic schwannomas, and large-cell calcifying Sertoli cell tumors. Cutaneous myxomas can be the first manifestation of Carney's complex.
Sporadic myxomas and myxomas associated with Carney's complex are essentially identical. They are characterized by a paucicellular proliferation of bland, oval to spindled cells embedded within an abundant myxoid matrix ( Fig. 13-23 ). Angiomyxomas usually have a well-developed vasculature accompanied by an inflammatory infiltrate with frequent neutrophils ( Fig. 13-24, A ). Another interesting feature often encountered is the presence of epithelial elements that can range from epidermoid cysts to strands of compressed basaloid epithelium ( Fig. 13-24, B ). The cystic epithelial elements often show induction of follicular epithelium.
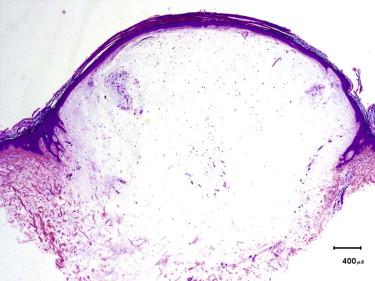
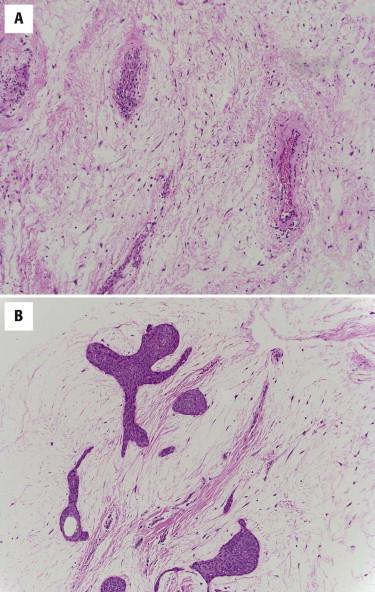
The tumor cells of myxoma may show focal immunoreactivity for CD34, and are typically negative for S100 protein, desmin, and SMA.
The benign entities on the differential diagnosis include focal dermal mucinosis, digital mucous cyst, and myxoid nerve sheath tumors. Focal dermal mucinosis is a bland, hypocellular myxoid nodule that is cytologically similar to myxomas but lacks supporting tumor vasculature. Digital mucous cysts have similar morphology as focal dermal mucinosis but typically occur at the nail base of fingers and toes and may be related to ganglion cysts. Myxoid neurofibromas tend to be more cellular than myxomas; show wavy, slightly hyperchromatic neural-type nuclei; and are immunoreactive for S100 protein. Dermal nerve sheath myxoma (myxoid neurothekeoma) shows a characteristic multinodular growth pattern and is also diffusely positive for S100 protein (discussed in the following). If myxomas are cellular, they need to be distinguished from myxoid sarcomas, in particular low-grade myxofibrosarcoma. The latter is a usually a larger, more infiltrative lesion arising in the subcutis characterized by curvilinear vessels and the presence of at least scattered large pleomorphic spindle or epithelioid cells.
Myxomas are benign lesions but may persist or recur locally.
Superficial acral fibromyxoma is a distinctive benign myxoid tumor, which most commonly arises in periungual regions of fingers and toes. Rare cases were reported on the palm, heel, and nonacral sites. This tumor typically affects adults and is more common in men. Clinically, this is a solitary, slow-growing nodule that may be painful.
Superficial acral fibromyxoma is centered in the dermis and may abut the epidermis, resulting in a polypoid, exophytic appearance or may infiltrate the subcutaneous adipose tissue. The margins are usually ill defined. Morphologically, it is composed of spindle or stellate cells growing loosely or in a vague fascicular pattern. The stroma characteristically shows alternating myxoid and collagenous areas ( Fig. 13-25, A ). The vasculature is conspicuous, showing small, regular vessels. Cytologically, the cells show bland nuclear features with small oval nuclei, inconspicuous nucleoli, and amphophilic cytoplasm ( Fig. 13-25, B ). Mast cells are conspicuous, but other inflammatory cells are typically not seen in this lesion. Nuclear atypia of the “degenerative” type and occasional multinucleated cells as well as cartilaginous and osseous metaplasia were reported. Mitotic figures are usually inconspicuous, and necrosis or vascular invasion is not seen.
The tumor cells are immunoreactive for CD34. A smaller proportion of cases may express EMA and SMA. S100, desmin, cytokeratins, and MUC4 are negative.
The differential diagnosis of superficial acral fibromyxoma includes a variety of myxoid lesions. Superficial angiomyxoma is more uniformly myxoid and typically shows more conspicuous inflammatory infiltrates. Other forms of cutaneous myxoma are less cellular and less vascular than superficial acral fibromyxoma. Myxoid nerve sheath tumors can be distinguished based on more hyperchromatic and wavy (neural) appearance of the lesional nuclei and immunoreactivity for S100 protein. Acquired digital fibrokeratoma (acral fibrokeratoma) has a similar clinical presentation but is a smaller, less cellular, and more heavily collagenized lesion showing vertically oriented spindle cells. The presence of myxoid stroma and infiltration of subcutis are not typical for acquired digital fibrokeratoma. Low-grade fibromyxoid sarcoma may occur in superficial locations, although it is very rare for this tumor to arise on fingers and toes. The fibrous zones of low-grade fibromyxoid sarcoma show swirling arrangement of the lesional cells, a feature not usually seen in superficial acral fibromyxoma. Immunolabeling for MUC4 may be helpful because it is positive in low-grade fibromyxoid sarcoma and negative in superficial acral fibromyxoma. Myxoid DFSP may enter the differential diagnosis of superficial acral fibromyxoma based on the presence of diffuse CD34 immunoreactivity; however, the clinical presentation (DFSP rarely arises in acral locations) and identification of more typical storiform areas help distinguish DFSP from superficial acral fibromyxoma.
Superficial acral fibromyxomas are benign but have a propensity for local recurrence in about one quarter of cases, particularly in incompletely excised lesions. No systemic metastases were reported.
Fibromatoses can be broadly subdivided into superficial and deep categories. Superficial fibromatoses include palmar (Dupuytren's contracture), plantar (Ledderhose's disease), and penile fibromatosis (Peyronie's disease). Palmar and plantar fibromatosis usually presents as a diffuse thickening or a nodule. Flexion contractures may occur. Peyronie's disease usually manifests as a plaquelike induration on the dorsal or lateral penis. Patients with superficial fibromatoses have an increased incidence of epilepsy and diabetes mellitus, the mechanism of which is unknown. Patients with one form of superficial fibromatosis are at increased risk of developing other forms of superficial fibromatosis. Desmoid-type or deep fibromatosis includes abdominal and extraabdominal forms occurring in deep soft tissue and practically do not occur as a primary lesion on the dermis. They are beyond the scope of this chapter.
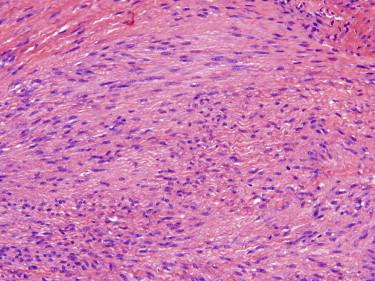
All forms of fibromatosis are histologically similar. The lesions are composed of long sweeping fascicles of bland fibroblasts in a collagenous background ( Fig. 13-26 ). The growth pattern is nodular and infiltrative. The fibroblasts have bland nuclear features and often have a small, discernible nucleolus. Mitotic figures may be present and occasionally numerous, but atypical mitotic figures are not seen. Other features that could be encountered in fibromatosis include focal hemorrhage and scattered inflammatory cells, keloidal collagen and multinucleated giant cells, especially in plantar fibromatosis.
Indurated plaque, nodule, flexure contraction
Nodular and infiltrative growth
Long sweeping fascicles or sheets of low cellular density
Cytologically bland fibroblasts
Stroma may be collagenous or myxoid (or both)
Variably positive for actins
Negative for S100 protein, desmin, and CD34
Scar
Fibroma
Desmoplastic melanoma
Fascicular spindle cell sarcomas: synovial sarcoma, fibrosarcoma, MPNST
MPNST, Malignant peripheral nerve sheath tumor.
The fibroblasts show variable expression for actin. Immunostaining for β-catenin has been suggested to be helpful for the distinction from histologic simulants, but we found that this stain is not particularly useful in this setting.
Fibromatosis may have significant morphologic overlap with a scar, especially in a partial biopsy sample. The presence of long, sweeping fascicles and lack of reactive vasculature will favor the former. Dermatomyofibroma shows considerable histologic overlap with fibromatosis but may be distinguished based on the clinical presentation (trunk, proximal extremities of young adults) and characteristic arrangement of the fascicles running parallel to the epidermis. Cellular dermatofibroma (cellular benign fibrous histiocytoma) will have a fascicular growth pattern similar to fibromatosis but can be distinguished based on collagen trapping at the periphery of the lesion typical of all dermatofibromas. Particularly cellular and mitotically active examples of superficial fibromatosis may have to be distinguished from sarcomas with a fascicular growth pattern such as fibrosarcoma, synovial sarcoma, or malignant peripheral nerve sheath tumor (MPNST). These sarcomas are very rare in superficial locations; display a greater degree of nuclear atypia and pleomorphism; and may show necrosis and atypical mitotic figures, features not characteristic of superficial fibromatosis.
Fibromatoses have a tendency for local recurrence. They do not metastasize, but they can cause significant local damage and morbidity. In superficial fibromatosis, surgery is generally only recommended for symptomatic relief (pain or functional impairment). Margins of surgical excisions are often positive but a positive margin does not require further surgery in the absence of symptoms.
Fibrous hamartoma of infancy typically occurs in the first 2 years, with a significant proportion presenting at birth. Boys are more frequently affected than girls. The most common location is the axilla and shoulder region, but fibrous hamartoma of infancy can occur in a wide range of locations.
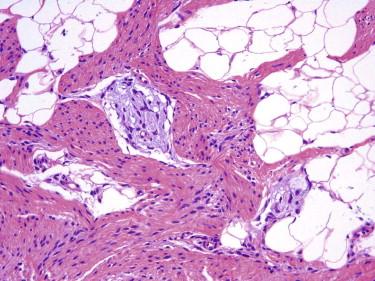
Fibrous hamartoma of infancy has four components in variable proportions: (1) fascicles of myofibroblasts, (2) aggregates of small round to fusiform or stellate primitive mesenchymal cells in a myxoid stroma, (3) mature adipose tissue, and (4) strips of fibroconnective tissue with or without inflammatory cells ( Fig. 13-27 ). The constellation of findings is unique and pathognomonic.
Fibrous hamartoma of infancy is a benign tumor but may locally recur. A simple excision is the treatment of choice.
Infantile digital fibromatosis manifests as a solitary or multiple firm nodules on the fingers or toes of infants and young children. Only rarely does this entity present after the age of 3 years, and many are present at birth. Frequently, more than one digit is involved.
The tumor is composed of fascicles of uniform fibroblasts in a densely collagenous stroma ( Fig. 13-28, A ). Intracytoplasmic eosinophilic inclusions, often perinuclear, are characteristic of the entity. These can be highlighted by a trichrome stain ( Fig. 13-28, B ).

This tumor frequently recurs. However, most spontaneously regress over time. Therefore, treatment should be conservative and driven by functional and cosmetic outcomes. Excision is recommended in cases with significant interference with joint function.
Elastofibroma presents as a slow-growing mass usually involving the subscapular fascia of elderly patients. There is often a history of intense and repetitive manual labor, and it is thought the development of elastofibroma may be related to repetitive stress.
The tumor is composed of swollen collagen and elastic fibers and some entrapped adipocytes. The elastic fibers exhibit degenerative change and have a characteristic beaded or serrated appearance ( Fig. 13-29 ), which can be highlighted with an elastic stain.
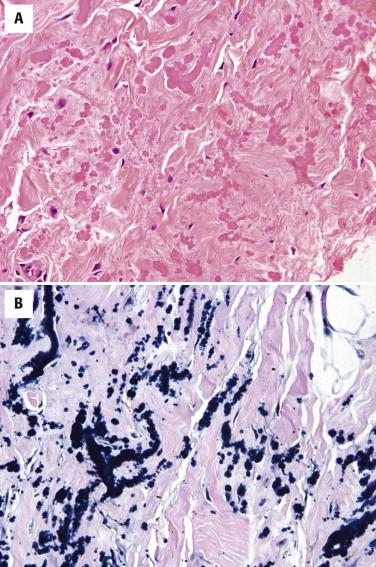
Because of its distinctive appearance, elastofibroma is rarely confused with other entities. Fibrolipoma may be confused with elastofibroma, but it has a more prominent adipose tissue component and does not have the characteristic degenerated elastic fibers.
Elastofibroma is a benign tumor that may be treated with conservative excision for cosmetic reasons.
Dermatofibrosarcoma protuberans most commonly presents in early to mid adult life. However, a wide age range may be affected, and cases in the pediatric population are increasingly recognized. DFSP most commonly arises in the trunk or proximal extremities. The initial presentation is as a plaque that may subsequently develop into a nodular or multinodular mass. Plaque lesions may clinically cause confusion with sclerosing dermatoses such as morphea or scleroderma.
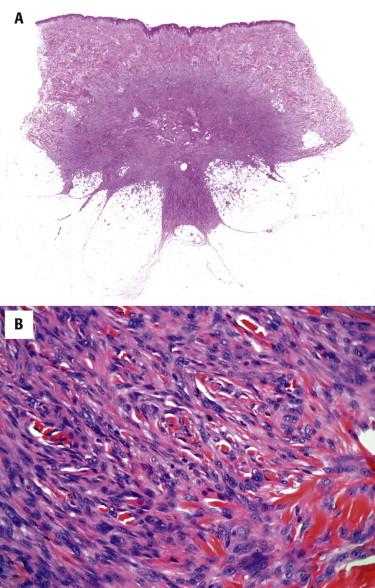
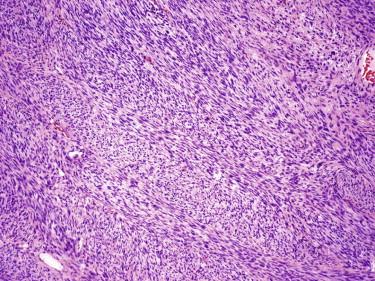
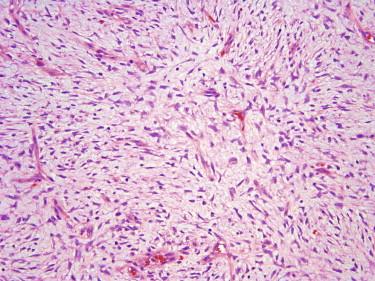
In its classic form, the tumor is composed of a storiform proliferation of monotonous, slender spindled cells with dark nuclei and lightly staining cytoplasm, typically showing diffuse infiltration of the subcutaneous adipose tissue (honeycomb pattern) ( Fig. 13-30 ). However, small subsets may deviate morphologically from this classic appearance and may show such features as giant cell fibroblastoma–like areas, plaque-like appearance, fibrosarcomatous change, myxoid change, and myoid nodule formation. Giant cell fibroblastoma is discussed in the following. Fibrosarcomatous change is characterized by a change in morphology to a more fascicular, herringbone pattern of growth with more prominent nuclear atypia and mitotic activity ( Fig. 13-31 ). In the myxoid variant, the tumor cells take on a more stellate appearance, and the underlying vasculature is accentuated and may resemble the plexiform vasculature of a myxoid liposarcoma ( Fig. 13-32 ). Myoid nodules are characterized by a smooth muscle or myofibroblastic proliferation surrounding vessels within the tumor. Another variant is the pigmented variant (so-called Bednar tumor) in which spindled cells containing melanin pigment are present in an otherwise typical DFSP ( Fig. 13-33 ).
![FIGURE 13-33, Bednar tumor (pigmented dermatofibrosarcoma protuberans [DFSP]). A, DFSP with scattered pigmented cells. B, A Fontana stain confirms the presence of melanin pigment. FIGURE 13-33, Bednar tumor (pigmented dermatofibrosarcoma protuberans [DFSP]). A, DFSP with scattered pigmented cells. B, A Fontana stain confirms the presence of melanin pigment.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/SoftTissueTumorsandTumorlikeReactions/32_3s20B9780323261913000137.jpg)
The tumor cells of DFSP are strongly immunoreactive for CD34. The lesional cells are usually negative for factor XIIIa, but focal staining may on occasion be seen. DFSP with fibrosarcomatous transformation often shows loss of CD34 expression or weaker expression in the fibrosarcoma component.
Dermatofibrosarcoma protuberans harbors a supernumerary ring chromosome derived from portions of chromosomes 17 and 22 or an unbalanced t(17;22) translocation. This translocation results in a gene fusion whereby the platelet-derived growth factor β ( PDGFβ ) gene is placed under the control of the collagen type 1 A1 ( COL1A1 ) gene promoter.
Dermatofibrosarcoma protuberans is usually easily distinguished from dermatofibroma if an adequate biopsy is provided. If an excisional biopsy is available, the distinction can usually be made at scanning magnification. DFSP has an infiltrative pattern of growth and extensively permeates the subcutaneous fat lobules. In contrast, dermatofibroma is centered and usually confined to the dermis. If it does involve the superficial subcutis, it usually follows the fibrous septa and spares the center of fat lobules. DFSP also lacks the epidermal hyperplasia frequently seen over dermatofibroma. DFSP is a much more uniform tumor with a more prominent storiform pattern than dermatofibroma. Foamy macrophages are also very helpful: they are common in dermatofibroma but absent in DFSP. In superficial biopsies, it may still be a difficult differential diagnosis. In such cases, immunostain for CD34 may be helpful, but it is important to remember in this context that positive labeling for CD34 may be seen in both DFSP and dermatofibroma. Whereas DFSP tends to show uniform, strong expression of CD34 in most or all tumor cells, dermatofibroma typically reveals a mixed population of CD34-positive cells, particularly at the periphery admixed with S100 protein-positive dendritic (Langerhans) cells and CD68-positive histiocytes. In our experience, factor XIIIa immunolabeling is helpful in evaluating an epithelioid variant of dermatofibroma but far less so in evaluating other variants.
Diffuse neurofibroma may also show features similar to DFSP, especially the myxoid variant. Although it often involves subcutaneous tissue, it does not have the storiform growth pattern of DFSP. The tumor cells of diffuse neurofibroma have more wavy nuclei. On the other hand, DFSP can have features similar to diffuse neurofibroma, and immunostains for S100 protein may be necessary for the correct diagnosis in selected cases. It should be highlighted that neurofibromas may have significant immunoreactivity for CD34.
Dermatofibrosarcoma protuberans may have to be distinguished from a perineurioma, a neoplasm with perineurial differentiation that is common in subcutis and may occur in the dermis. The most helpful histologic features to distinguish a perineurioma from DFSP are relative circumscription of the former and the presence of long fascicles composed of very slender cells with long processes arranged in a lamellar pattern (see later discussion of perineurioma). CD34 immunostaining may not help because approximately two thirds of perineuriomas are diffusely positive for this marker, but expression of EMA, Claudin-1, and glucose transporter 1 (GLUT1) will help to differentiate them from DFSP.
The differential diagnosis of plaque-like DFSP may include fibroblastic connective tissue nevus (discussed earlier). Both lesions demonstrate fascicles of bland spindle cells percolating the dermis and extending into the subcutis. DFSP can be distinguished based on the presence of a whorled growth pattern; more prominent cytologic atypia; and diffuse, strong CD34 immunoreactivity. Identification of giant cell fibroblastoma-like areas and more diffuse infiltration of the subcutis are also helpful to distinguish DFSP from fibroblastic connective tissue nevus.
There is a relatively high rate of local recurrence (up to 20%) of DFSP with standard wide local excision. Recurrences are usually related to positive or narrow margins of the primary excision. Metastases may occur (<5%) but typically only after repeated local recurrences. Evidence indicates that Mohs micrographic surgery can reduce the risk of local recurrence, but there may be a selection bias in the success rates of Mohs surgery because this treatment modality is typically used for smaller tumors that are more amenable to complete excision. The COL1A1-PDGFB molecular pathway can be targeted by tyrosine kinase inhibitors in locally advanced or metastatic DFSP.
Indurated plaque
Nodule or multinodular mass
Uniform spindle cells in dermis and subcutis with storiform growth pattern
Infiltration of subcutaneous lobules in a diffuse pattern
Positive for CD34
Negative for S100 and FXIIIa
Dermatofibroma (positive for FXIIIa and negative for CD34)
Neurofibroma (positive for S100 and variably positive for CD34)
Perineurioma (positive for EMA and variably positive for CD34)
EMA, Epithelial membrane antigen; FXIIIa, factor XIIIA.
The significance of fibrosarcomatous DFSP is controversial. Although some have suggested no associated increased risk of local recurrence or metastasis if the tumor was completely excised, it is now generally accepted that fibrosarcomatous transformation does confer more aggressive behavior with a metastatic rate of 10% to 15%.
Giant cell fibroblastoma is the juvenile form of DFSP. First supported by reports of giant cell fibroblastoma with histologic and clinical overlap with DFSP, this relationship is further supported by the description of tumors with composite features of both by their identical immunophenotype and by cases of giant cell fibroblastoma that have recurred as DFSP and vice versa. Giant cell fibroblastoma also harbors the same cytogenetic abnormality as DFSP (see the preceding).
Giant cell fibroblastoma usually occurs in children, with the majority of patients in the first decade of life. Cases in adults are uncommon. Giant cell fibroblastoma typically presents as a dermal or subcutaneous mass, which most frequently involves the trunk, thigh, or inguinal region.
Histologically, giant cell fibroblastoma shows varying cellularity and is composed of spindled to stellate cells with mild to moderate nuclear pleomorphism loosely arranged in an abundant myxoid to hyalinized stroma ( Fig. 13-34, A ). Typically, these spindled cells infiltrate around adnexal structures and through fat in an identical fashion to DFSP. Most characteristically, this stroma displays prominent cracking artifact, with the formation of pseudovascular spaces lined by a discontinuous layer of enlarged, multinucleated tumor giant cells ( Fig. 13-34, B ). The presence of such cells may result in an incorrect diagnosis of a pleomorphic sarcoma.
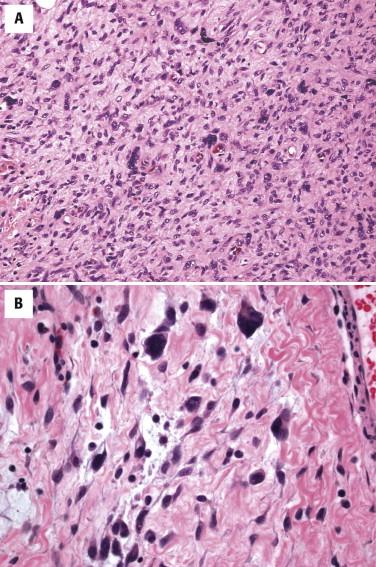
Similar to the closely related DFSP, the tumor cells of giant cell fibroblastoma are diffusely positive for CD34 but negative for factor XIIIa. Giant cell fibroblastoma has the same cytogenetic abnormality as DFSP (see above).
The differential diagnosis of giant cell fibroblastoma usually centers on sarcomas such as myxofibrosarcoma or pleomorphic sarcoma. Unlike giant cell fibroblastoma, myxofibrosarcoma typically presents in older adults and shows more distinctive myxoid nodules with enlarged, pleomorphic cells clustered around curvilinear vasculature. Pleomorphic sarcomas typically present in older adults and show greater degrees of cytologic atypia, pleomorphism, and mitotic activity and lack the pseudovascular spaces characteristic of giant cell fibroblastoma.
The tumors have a tendency for local recurrence. No cases of metastatic giant cell fibroblastoma have been reported. Treatment is wide local excision.
Plexiform fibrohistiocytic tumor (PFHT) most commonly presents as a deep dermal or subcutaneous mass of the extremities in children or young adults. Any anatomic site may be involved, but involvement of the head and neck region is very uncommon.
Subcutaneous nodule
Children and young adults
Plexiform growth pattern
Nodules of histiocytic cells
Fibromatosis-like fascicles
Histiocytic cells: positive for CD68
Myofibroblastic cells: positive for actin
Dermatofibroma
Cellular neurothekeoma
Fibromatosis
Reactive or reparative process
Plexiform fibrohistiocytic tumor displays as a poorly circumscribed, plexiform growth pattern ( Fig. 13-35, A ). It has characteristically a biphasic appearance, being composed of multiple small nodules of histiocytoid cells in association with bandlike fascicles of spindled cells. The nodules are composed of an admixture of round to oval mononuclear histiocytoid cells and osteoclast-like giant cells. Fascicles of fibroblastic- or myofibroblastic-appearing spindled cells mingle with the histiocytic cell nodules ( Fig. 13-35, B ). There can be significant variation in the relative proportion of the spindle cell and histiocytic components in any given tumor. In the original description of the tumor, approximately 40% of cases showed the classic bimorphic appearance; another 40% showed a predominance of the histiocytic component, and 20% showed primarily a fibroblastic growth pattern. Mitotic activity is typically low.
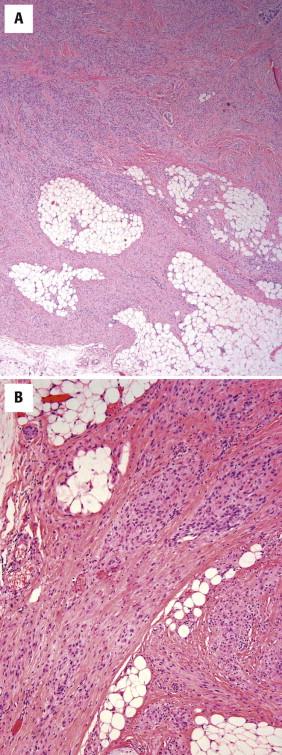
The histiocytic cells show strong CD68 expression. The spindled cells show a membranous pattern of SMA expression indicative of a myofibroblastic phenotype.
The differential diagnosis of PFHT includes giant cell tumor of soft tissue, reactive (granulomatous) inflammation, fibromatosis, and cellular neurothekeoma. The nodules of rounded cells and osteoclasts seen in PFHT are essentially indistinguishable from the tumor nodules of giant cell tumor of soft tissue, but the latter does not have the fascicular spindle cell component of PFHT. Features suggestive of PFHT include younger patient age and a plexiform growth pattern with minute nodules in contrast to the larger, coarser multinodular pattern of giant cell tumor of soft tissue. The presence of metaplastic bone or aneurysmal bone cyst–like changes is supportive of giant cell tumor of soft tissue.
PFHT can resemble a granulomatous process. However, the nodules in PFHT contain osteoclast-like rather than Langhans-type giant cells seen in granulomas. A predominantly fibroblastic growth pattern in a PFHT may be very similar to fibromatosis; however, the histiocytes and the plexiform growth pattern are not typical of the latter.
Cellular neurothekeoma is a superficial cutaneous tumor that, similar to PFHT, has a distinctly nodular growth pattern but typically not plexiform. In contrast to PFHT, most cellular neurothekeomas are superficial tumors confined to the dermis and composed of larger, more epithelioid cells with eosinophilic cytoplasm in contrast to small histiocytic cells and myofibroblasts of the former. Myxoid stromal changes, which are commonly found in association with cellular neurothekeoma, are rarely seen with PFHT. Clinically, cellular neurothekeomas have a predilection for the head and neck region. However, occasionally, it can be difficult to distinguish predominantly histiocytic PFHT from cellular neurothekeoma, particularly in partial biopsies. By immunohistochemistry, both tumors express CD10 and NKIC3. Cellular neurothekeomas express MiTF, but this marker is negative in PFHT.
Plexiform fibrohistiocytic tumors may recur locally, but have a very low risk of metastatic disease. Only rare cases of lymph node or pulmonary metastases have been reported. Wide excision is the treatment of choice. There is no role for chemotherapy or radiation therapy.
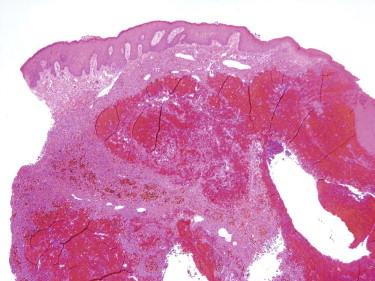
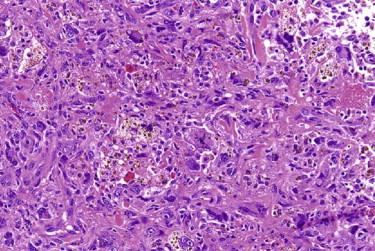
Angiomatoid fibrous histiocytoma (AFH; in the past also referred to as angiomatoid malignant fibrous histiocytoma [MFH]) typically occurs in children and young adults, but cases at both extremes of age have been described. AFH usually occurs as a solitary mass in the subcutis or deep dermis, most commonly in the extremities. Clinically, the lesion may resemble a hematoma, hemangioma, or a benign cyst. They may be associated with a variety of systemic manifestations, including fevers, weight loss, anemia, polyclonal gammopathy, and rarely a Castleman's disease–like lymphadenopathy. These systemic symptoms are attributed to cytokine production by the tumor because the systemic findings resolve shortly after the curative excision.
Histologically, AFH is characterized by the presence of a dense fibrous capsule and a surrounding chronic lymphocytic infiltrate ( Fig. 13-36, A ). This lymphocytic infiltrate is generally most prominent around the periphery of the tumor, and in some cases, there is germinal center formation. The blood-filled cystic spaces for which this tumor is named are present in most but not all cases. These spaces are lined by flattened tumor cells, not endothelium ( Fig. 13-36, B ). Cases without the large cystic spaces show at least some evidence of focal hemorrhage or hemosiderin deposits. The tumor cells may be either spindled, growing in a variety of patterns, including sheets, meningioma-like whorls, and short fascicles, or histiocytoid in appearance ( Fig. 13-36, C ). Although the tumor cells are typically cytologically bland, with uniform oval nuclei and vesicular chromatin, occasional cases may show striking pleomorphism, which does not appear to have clinical significance. The mitotic rate is typically low.
Usually an extremity mass in young patients
Systemic symptoms (e.g., fever, weight loss)
Dense fibrous capsule
Lymphoid aggregates with germinal centers at the periphery
Proliferation of histiocytic or spindled cells
Blood-filled pseudovascular spaces
Positive for desmin, CD68, EMA, CD99 in ~50% of cases
Negative for cytokeratins, CD31, CD34, FXIIIa, S100
Aneurysmal dermatofibroma
Malignant neoplasms metastatic to a lymph node
EMA, Epithelial membrane antigen; FXIIIa, factor XIIIA.
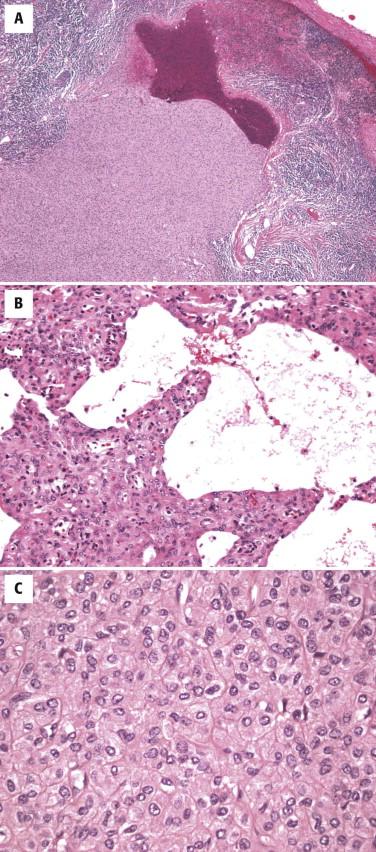
The cells of AFH have a unique immunophenotype, with expression of desmin, EMA, CD99, and CD68 in more than 60% of cases. Rare cases may show actin expression. They do not express S100, CD31, or CD34.
At the genetic level, AFHs are characterized by t(2;22) ( EWSR1-CREB1 ), t(12;22) ( EWSR1-ATF1 ), or t(12;16) ( FUS-ATF1 ). The t(2;22) appears to be the most common translocation. Detection of the gene rearrangement by fluorescence in situ hybridization (FISH) is a valuable adjunctive test in difficult cases, with the EWSR1 gene rearrangements being most common in this tumor. It should be noted that the t(12;22) translocation and resulting fusion protein are identical to those seen in clear cell sarcoma of tendons and aponeuroses.
The main differential diagnosis includes dermatofibroma with aneurysmal or hemosiderotic change (aneurysmal benign fibrous histiocytoma) and a malignant tumor metastatic to a lymph node. Vascular neoplasms can also be considered if the angiomatoid change is prominent. Unlike AFH, aneurysmal dermatofibroma shows features common to all dermatofibromas such as peripheral collagen trapping, overlying epidermal hyperplasia, a storiform growth pattern, and a more polymorphous cell population with siderophages and foamy macrophages.
The dense capsule and the surrounding lymphoid infiltrate seen in AFH may impart the appearance of a metastatic neoplasm in a lymph node. However, the pseudocapsule surrounding the lesional cells of AFH does not have subcapsular sinuses or afferent lymphatics that would indicate a true nodal architecture. AFH can easily be distinguished immunohistochemically from vascular neoplasms by lack of staining for vascular markers CD31, CD34, or ERG (ETS family-related gene).
Once thought to be a fully malignant sarcoma based on the initial description of this tumor, the lesion was originally designated angiomatoid malignant fibrous histiocytoma . Subsequent studies have shown a more indolent course with primarily a risk of local recurrence of approximately 10% to 15% and only a very low risk of metastasis, mostly to regional lymph nodes. Owing to this now recognized more indolent behavior, the most recent World Health Organization (WHO) classification no longer uses the term angiomatoid (malignant) fibrous histiocytoma . As with other tumors in this group, surgical excision remains the mainstay of therapy.
Giant cell tumor of soft tissue, also referred to as giant cell tumor of low malignant potential, typically manifests as multinodular masses in the skin or subcutis of middle-aged adults, although occasional cases may manifest as more deeply seated masses or in children.
There is involvement of the hypodermis and subcutis by a multinodular mass. The individual tumor nodules consist of a mixture of osteoclastic giant cells, bland mononuclear cells, and short fascicles of bland spindled cells ( Fig. 13-37 ). These appearances are similar to the giant cell tumor of bone. Metaplastic bone formation is common. Cystic changes reminiscent of an aneurysmal bone cyst can also be identified. Mitotic figures are usually easily identified, but atypical mitotic figures are not seen. Vascular involvement by the tumor is common and does not connote malignancy.
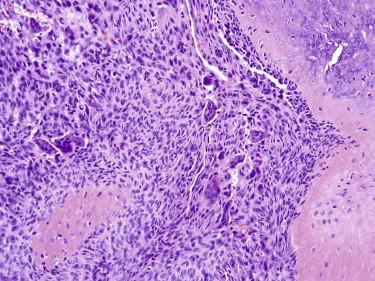
Sarcomas with giant cells differ from giant cell tumor of soft parts cytologically (greater degree of nuclear atypia and pleomorphism, atypical mitotic figures) and associated features such as malignant osteoid in osteosarcoma. With regard to benign tumors, giant cell tumor of soft tissue is frequently mistaken for tenosynovial giant cell tumor. Tenosynovial giant cell tumor can be distinguished by virtue of its usual location near joint spaces or bursae; prominent stromal hyalinization; and a different cellular composition, including small mononuclear cells, siderophages, foamy histiocytes, and lymphocytes. Metaplastic bone production and aneurysmal bone cyst–like changes are uncharacteristic of tenosynovial giant cell tumor. The histiocytic component of a PFHT can be similar to the tumor nodules of giant cell tumor of soft tissue. However, the former usually occurs in a younger patient population and has the fibromatosis-like spindle cell component not typical of giant cell tumor of soft tissue. The presence of metaplastic bone or aneurysmal bone cyst–like changes is not characteristic of PFHT.
Giant cell tumor of soft tissue has a tendency for local recurrence. Wide excision is the treatment of choice. Very rare cases of systemic metastasis have been reported.
Atypical fibroxanthoma typically presents as a papule or small nodule on the head and neck of elderly patients. The overlying epidermis may be ulcerated, and the clinical appearance can be confused with common cutaneous malignancies such as BCC or squamous cell carcinoma (SCC).
Papule or small nodule on sun-damaged skin of elderly patients
Often rapid growth
Often on face
Dermal tumor with a pushing border
Pleomorphic spindle or epithelioid cells
Multinucleated cells and foamy cells
Often ulcerated with epidermal collarette
Really no good positive markers
Negative for cytokeratins and S100
Pleomorphic sarcoma (looks like atypical fibroxanthoma but invades subcutis)
Melanoma (positive for S100)
Sarcomatoid squamous cell carcinoma (positive for 34BE12, MNF116, CK 5/6, p63)
Leiomyosarcoma (positive for desmin, SMA, H-caldesmon)
SMA, Smooth muscle actin.
Atypical fibroxanthoma is a dermal tumor composed of pleomorphic stellate to spindled and epithelioid cells with atypical hyperchromatic nuclei and amphophilic cytoplasm ( Fig. 13-38, A ). The tumor cells can have a random arrangement or a fascicular to storiform growth pattern. Mitotic figures, including atypical forms, are usually present. A clear cell variant exists ( Fig. 13-38, B ). There is typically a background of marked solar elastosis. Atypical fibroxanthoma is a nodular, relatively circumscribed tumor with a pushing margin. Chronic inflammatory infiltrate and an epidermoid collarette may be present at the periphery of the tumor. Infiltration of the subcutis, perineural or perivascular invasion and necrosis are not characteristic of an atypical fibroxanthoma.
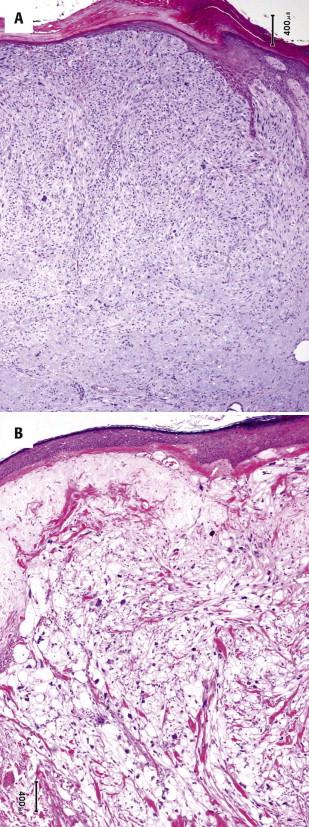
The tumor cells of atypical fibroxanthoma are positive for CD10 and CD68. Unfortunately, these stains are not discriminatory because they may be positive in other tumors in the differential diagnosis such as sarcomatoid SCC. Actins may also be expressed (usually only focally or weakly). Atypical fibroxanthoma is negative for cytokeratins, EMA, and desmin. Scattered S100–positive dendritic cells may be present, but the tumor cells are negative for S100.
Atypical fibroxanthoma is a diagnosis of exclusion. The differential diagnosis includes malignant melanoma, sarcomatoid SCC, and a variety of dermal pleomorphic sarcomas. Although atypical fibroxanthoma is often ulcerated, the overlying epidermis is otherwise intact, and identifying an in situ carcinoma or an intraepithelial melanocytic proliferation would help to exclude carcinoma and malignant melanoma. Melanoma can be identified by positive staining for S100 protein or other markers of melanocytic differentiation. If cytokeratin immunoreactivity is noted, especially in the setting of associated actinic keratosis, a tumor, which otherwise could pass as an atypical fibroxanthoma, is best classified as poorly differentiated (spindled cell or sarcomatoid) SCC. Although broadly reactive antibodies such as to pan-cytokeratins AE1/AE3 identify most cases of spindled cell SCC, antibodies directed against high-molecular-weight cytokeratin such as 34βE12, CK5/6, or MNF116 are a better first-line choice because many spindled cell SCCs express only high-molecular-weight cytokeratins.
Atypical fibroxanthoma also should be differentiated from superficial pleomorphic undifferentiated sarcoma (formerly known as MFH) and cutaneous leiomyosarcoma. On cytologic grounds alone, atypical fibroxanthoma is identical to a pleomorphic sarcoma, and this distinction is strictly based on anatomic level of invasion. The term atypical fibroxanthoma should be restricted to tumors limited to the dermis and with a pushing margin, which often requires examination of a completely excised specimen. We apply the term pleomorphic sarcoma to cytologically similar tumors that extend into the subcutis or primarily present in deep soft tissue with extension superficially into the dermis because these tumors have a high risk for local recurrence and some risk for metastasis. The presence of tumor necrosis, infiltration of the subcutis, and perivascular or perineural invasion is not compatible with the diagnosis of an atypical fibroxanthoma, and such lesions should be classified as pleomorphic sarcoma. Although atypical fibroxanthoma may conceptually be viewed as a small dermal sarcoma, given its benign behavior, if strictly defined, the term atypical fibroxanthoma seems preferable to avoid overtreatment as for a high-grade sarcoma. Immunostains for desmin and H-caldesmon help to distinguish leiomyosarcoma from atypical fibroxanthoma, which is typically negative for these markers.
Owing to its small size and superficial location, most atypical fibroxanthomas behave in a benign fashion and are cured by simple complete excision. Mohs surgery is an option. Occasionally, they may recur locally. Metastases are rare and usually develop only after repeated local recurrences or from tumors erroneously reported as atypical fibroxanthoma, which extended into the subcutis and should have been reported as sarcoma. Complete excision with negative margins is requisite treatment.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here