Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The deleterious effects of smoking tobacco have been established for decades and largely began with the 1964 Surgeon General's Report, which linked smoking to the development of bronchogenic carcinoma. Since then, smoking tobacco has been causally linked to diseases of nearly every organ system in the body. Specific pulmonary diseases linked to smoking include bronchogenic carcinoma, chronic bronchitis, and chronic obstructive pulmonary disease, as well as a variety of other interstitial lung diseases.
Usually, patients with a history of smoking present with acute or chronic dyspnea related to their smoking-induced cardiopulmonary disease. Clinical signs and symptoms, along with the physical examination, can often help ascertain whether the cause is cardiac or pulmonary; however, this distinction is not always apparent. In these cases, the use of imaging can help establish the cause, as well as any related complications.
Imaging is frequently used in patients with confirmed or suspected smoking-related lung diseases, first to establish or confirm the diagnosis and second to evaluate the extent of disease. The most common first-line imaging examination in the evaluation of acute or chronic dyspnea is chest radiography, largely due to its low cost, low radiation exposure, and wide availability. There are a number of smoking-related diseases that can be identified on a chest radiograph; however, a negative radiograph does not exclude diffuse disease. Depending on the interpretation of the radiograph and the symptoms of the patient, proceeding to CT of the chest without IV contrast, using both thin section acquisition and a high-resolution filter, is frequently appropriate (commonly referred to as high-resolution CT [HRCT]). As part of the HRCT examination, expiratory CT imaging is useful for the evaluation of the small airway component of obstruction. CT of the chest with IV contrast is often not indicated unless there is suspected mediastinal or hilar adenopathy or if there is suspicion of a pulmonary embolism as a compounding factor in the patient's dyspnea.
Chronic obstructive pulmonary disease (COPD) is defined by the Global Initiative for Chronic Lung Disease as “a disease state characterized by airflow limitation that is not fully reversible” and is validated by decreased airflow at forced expiration. This is secondary to a combination of airway disease, referred to as obstructive bronchitis , and lung tissue parenchymal destruction, known as emphysema.
The major site of resistance in COPD is the small airways (airways <2 mm in internal diameter), although the entire airway is typically involved. Small airways vary from large airways in that they lack cartilage and contain a greater percentage of smooth muscle. Small airways are generations 8 to 14 of airway branching. The airflow reduction in the small airways is thought to be secondary to narrowing of the small airways from inflammation, fibrosis, and mucus plugging. The most significant contributor to airflow obstruction is mucous metaplasia.
Small airway inflammation following exposure to cigarette smoke precedes tissue destruction, fibrosis, and clinically detectable airflow obstruction. On the cellular level, this is thought to be secondary to migration of the CD8 + T lymphocytes inducing apoptosis and necrosis of epithelial and endothelial cells, which ultimately leads to the parenchymal destruction seen in COPD.
Currently, the diagnosis and assessment of severity of COPD predominantly involve pulmonary function tests (PFTs). PFTs are sometimes limited in their assessment because they are difficult to interpret in the presence of concomitant lung pathology. Additionally, they primarily provide an assessment of global lung function and are limited in determining any information regarding regional heterogeneity or quantification of emphysema versus small airway disease. Imaging with HRCT can provide additional diagnostic information and is substantially more sensitive than spirometry for detecting emphysema.
Classification of COPD falls into three primary categories—emphysema-predominant COPD, airway-predominant COPD (bronchitis and bronchiolitis), and mixed. The CT-based classification is based on the morphologic appearance of pathologic changes relating to airflow limitation—that is, the presence or absence of emphysema, bronchial wall thickening, and evidence of bronchiolitis. This categorization is somewhat limited given that the relative contributions of emphysema and airway disease vary among patients, as well as their correlation with symptomatology. The CT appearance can vary dramatically among patients with a similar degree of airflow limitation.
Emphysema is defined histologically as the abnormal permanent enlargement of the air spaces distal to the respiratory bronchioles, with destruction of the septal walls. On CT, this presents with regions of low density (i.e., decreased CT attenuation) surrounded by normal lung parenchyma. Depending on the distribution of these low-density changes relative to the secondary pulmonary lobule, the pattern of emphysema can be characterized as centrilobular, panlobular (panacinar), or distal acinar (paraseptal). A fourth type of emphysema is sometimes described but refers to emphysema associated with scarring, without a distinct relationship to the secondary pulmonary lobule (cicatricial).
Centrilobular emphysema is manifested by central destruction of the secondary pulmonary lobular parenchyma, which surrounds the pulmonary artery. Centrilobular emphysema is the most common type of smoking-related emphysema and, like many inhalational injuries, is upper lung predominant.
Uniform destruction of the secondary pulmonary lobule is seen in panlobular emphysema. Panlobular emphysema appears as a generalized decrease in CT attenuation; however, it is predominantly in the lower lobes. Pulmonary vessels in the affected areas are typically reduced in caliber. Panlobular emphysema is most characteristically seen with alpha-1-antitrypsin deficiency, although it can also been seen in severe, end-stage, smoking-related emphysema and other rare causes, such as IV methylphenidate (Ritalin) abuse or hypocomplementemic urticarial vasculitis syndrome.
Distal acinar (paraseptal) emphysema is a subtype of panlobular emphysema, which is localized to the parenchyma adjacent to the visceral pleural surface. It is usually in the upper lobes and is frequently seen in association with centrilobular emphysema in smokers.
The association between emphysema and airflow limitation is explained by a decrease in elastic recoil of the airway. Normal airways have a certain degree of elastic recoil that maintains airway patency, which is lost in patients with COPD because of the destruction of the lung parenchyma. Combined with the loss of elastic recoil of the airways, the hyperinflation characteristic of emphysema compresses and further obstructs the small airways.
Evaluation of COPD by a standard posteroanterior and lateral chest radiographs is easily obtained, inexpensive, and involves minimal radiation. Typical findings for detection of emphysema on chest radiographs include the following ( Fig. 18.1 ):
Increased radiolucency of lung fields
Flattening of the diaphragms
Pruning of the peripheral vasculature
Increased retrosternal airspace
Widening of the intercostal spaces
Narrowed and more vertical cardiac silhouette
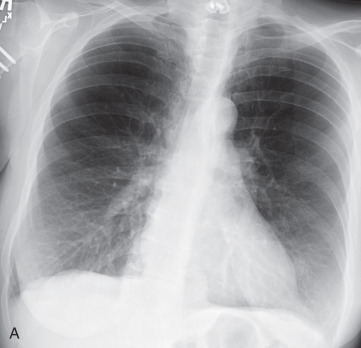
Historically, the application of these criteria has had mixed results in correlation with the histopathologic diagnosis of emphysema. However, a recent study has demonstrated that both experienced and inexperienced readers are able to identify moderate and severe emphysema with over 90% sensitivity, so chest radiographs do retain some clinical utility in the diagnosis and quantification of emphysema. Nevertheless, it is generally agreed that the accuracy of chest radiographs for detecting early emphysema is limited.
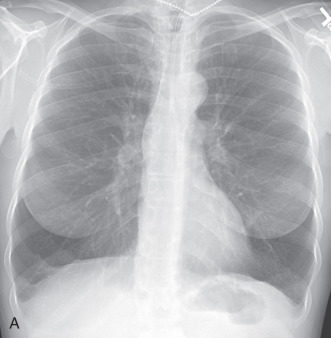
Additionally, distinguishing the types of emphysema on chest radiographs is limited. Radiographs can highlight the craniocaudal distribution of emphysema, with centrilobular emphysema related to smoking typically being upper lung predominant and panlobular emphysema being lower lung predominant ( Fig. 18.2 ; also see Fig. 18.1 ). The detection of paraseptal emphysema is generally limited to identifying larger peripheral blebs and bullae.
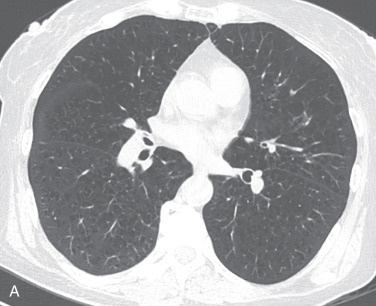
In contrast to radiographs, the evaluation of emphysema by CT is standard ( Fig. 18.3 ). The detection and quantification of emphysema on CT can be performed through both visual pattern analysis and, more objectively, by lung density measurements. Visual detection and quantification involve a global and regional qualitative assessment and a subjective grading of severity. This type of visual assessment has been shown in multiple studies to correlate with histopathology, lung function, and response to therapeutic intervention.
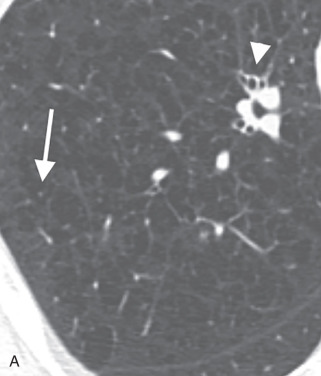
Centrilobular emphysema is characterized on HRCT by multiple small, round to polygonal areas of low attenuation ( Fig. 18.4 ). These are frequently situated near the center of the secondary pulmonary lobule, with the centrilobular artery at the center. Most of these areas should have no discernible wall, although thin walls can sometimes be seen related to fibrosis or adjacent septal interstitium. As the disease progresses, the emphysema will become more confluent, with bullae formation and involvement of the entire secondary pulmonary lobule ( Fig. 18.5 ). Despite the mimic appearance of this advanced centrilobular emphysema with panlobular emphysema, mentioning panlobular emphysema in a radiology report should not occur unless one specifically wants a referring clinician to consider alpha-1-antitrypsin deficiency; this is because some equate the two entities with each other.
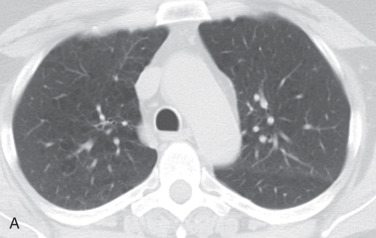
Panlobular emphysema results from a much more uniform destruction of the secondary pulmonary lobule, resulting in a more diffuse low attenuation of the lung parenchyma and hyperinflation (see Fig. 18.3 ). Early cases may be missed entirely without close evaluation and a high degree of suspicion. Note that bronchiectasis is common in cases of alpha-1-antitrypsin deficiency, likely related to repeat infections, at least in part.
Paraseptal emphysema on CT often has visible walls corresponding with interlobular septa due to the subpleural location (see Fig. 18.3 ). Bullae are areas of paraseptal emphysema larger than 1 cm in diameter, which can be seen in isolation or in association with other causes of emphysema.
More recent studies have attempted to quantify emphysema using CT densimetry parameters, such as relative low-attenuation area and the percentile of the frequency attenuation distribution. Low-attenuation regions of −950 Hounsfield units or less on thin section CT correlate well with PFTs. The anatomic distribution of low-attenuation regions should also be considered when performing quantitative analysis of these regions on CT because they may help clarify the pathophysiology.
Of note, the distribution of emphysema with regard to the lobes is important to recognize and comment on because of surgical options for treatment. Lung volume reduction surgery (LVRS) is a treatment option for patients with end-stage emphysema and preserved exercise tolerance. In these patients, CT is a critical preoperative step in determining surgical candidacy—specifically, diagnosing the emphysema as nonhomogeneous (upper lung predominant) is paramount ( Fig. 18.6 ). In effect, this means that the emphysema needs to be most severe in the area of lung to be resected (the upper lobes). If the lungs are affected more diffusely, then surgical resection is of no benefit.
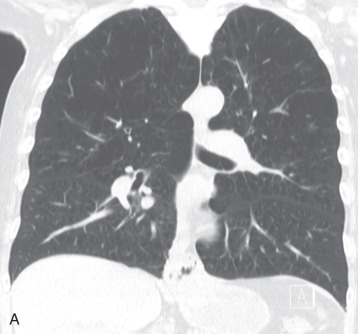
Langerhans cell histiocytosis (LCH) is a disorder of abnormal proliferation and infiltration of Langerhans cells into various organs. Pulmonary involvement has been historically known as histiocytosis X and associated with systemic disorders of histiocytes, such as eosinophilic granuloma, Letterer-Siwe disease, and Hand-Schüller-Christian disease. Pulmonary involvement in these LCH disorders is not common but, when it occurs, it tends to present in children, is not associated with a smoking history, and follows a more aggressive clinical course, with a worse prognosis. Today, pulmonary LCH (PLCH) is recognized as a variant of LCH that almost solely affects adult smokers.
PLCH is thought to be an uncontrolled immune reaction to a presenting antigen, commonly associated with smoking, although there have been reports of PLCH developing in response to other pulmonary irritants. Langerhans cells are almost exclusively located within the subendothelium of the tracheobronchial tree. The cells are a primary immune defense against inhaled antigens. Once activated by an antigen, they stimulate an immune response and increase lymphocyte production.
Characteristic features of PLCH on chest imaging are a combination of nodules that cavitate and evolve to cysts over time ( Fig. 18.7 ). There is a predominant distribution in the mid and upper lung zones, with sparing of the lung bases and general preservation of lung volumes. Nodules and some cystic lesions may be occasionally identified on chest radiographs but are best appreciated on HRCT.
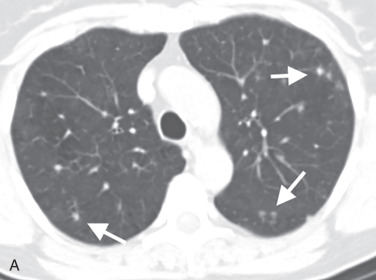
In the acute or early phase of the disease, small nodules predominate (see Fig. 18.7A ). Histologically, the nodules are composed of immune cells and fibroblasts that form a granuloma. Because these granulomas form in the subendothelium of the bronchi, the nodules will appear centrilobular, peribronchial, or bronchiolar in location on HRCT. These nodules tend to have irregular, poorly defined borders, although occasionally they may appear well defined and smooth. The nodules are generally small (<5 mm), but may be up to 1 cm.
With disease progression, the inflammation from the continued immune response results in destruction of the bronchiolar walls and the normal adjacent lung parenchyma, causing bronchiolar dilation. This results in cavitation of the nodules and the appearance of irregularly shaped cysts on HRCT and as a reticulonodular pattern on a chest radiograph (see Fig. 18.7B ). At this point in the disease, it is common to see a combination of cysts and nodules, which is considered diagnostic for PLCH. Over time, the cystic appearance dominates, with regression of nodular lesions. The cysts may even appear confluent and bullous-like.
End-stage disease is characterized by bizarre and irregularly shaped cysts, with a mild degree of fibrotic architectural distortion, which may cause further dilation of the bronchial tree (see Fig. 18.7C ). The combination of fibrosis and cystic changes often counteracts each other, resulting in little change in the lung volumes, which are normal or slightly increased. With advanced disease, patients may develop pulmonary artery hypertension, leading to enlarged pulmonary arteries on imaging.
The appearance of both cysts and centrilobular nodules in the upper and mid lung zones is considered nearly pathognomonic. When imaging findings are limited only to nodules or only to cysts, the differential diagnosis may include other lung diseases (see below). However, the sparing of the posterior costophrenic angles is highly characteristic of PLCH and can be useful as a distinguishing feature ( Fig. 18.8 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here