Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Efficient absorption of nutrients and maintenance of orderly aboral movement of chyme and indigestible residues are the most important goals of small intestinal motor and sensory function. Small intestinal motility is also critically important in preventing SIBO (see Chapter 105 ). This is achieved by the net aboral flow of luminal contents during both the fed and fasting states, probably with the assistance of the gatekeeper function of the ileocecal junction, which prevents backflow of cecal contents.
Optimal progression of luminal contents allows mixing of digested food with intestinal secretions, and contact of the luminal contents with the epithelium. This contact is important for the absorption and sensing of nutrients within the lumen. Both absorption and mucosal sensing of nutrients exert feedback control on gastric and small intestinal motor function, an interplay that optimizes the rate at which additional nutrients are presented to the absorptive epithelium, and minimizes the amount of nutrients lost to the colon. Thus, while the net movement of luminal contents along the small intestine is antegrade, retrograde flow also occurs in normal physiological situations over short distances. Retrograde movement of small intestinal contents can also occur over long distances, when a unique pattern of a strong zone of phasic small intestinal contractions travels in an oral direction over a large portion of the small intestine. These contractions deliver luminal contents back to the stomach for ejection into the esophagus during emesis. This coordinated motor pattern underscores the versatile modulation of small intestinal motility according to precise physiological needs.
The motor function of the small intestine depends directly on smooth muscle in the intestinal wall, which contains the basic control mechanisms that initiate contractions and regulate their frequency. Overlying these basic control mechanisms are the enteric nervous system (ENS) and the autonomic nervous system (ANS). In addition, a number of hormones modulate the frequency and patterning of small intestinal contractions. Each of these factors plays a role in the motility of the small intestine in health, and specific damage to each of these components in certain diseases has helped define their discrete roles.
In adult humans, the small intestine is approximately 3 to 7 m long and extends from the duodenal side of the pylorus to the ileocecal valve. It is divided into 3 regions based on structural and functional considerations: duodenum at the oral end, followed by jejunum, and ending with the ileum. These regions exhibit similar motor characteristics, despite some structural and functional differences. Physiologic sphincters, namely the pylorus and ileocecal valve, have distinctly different motor patterns, permitting them to act as controllers of flow between the antrum and duodenum and between the ileum and colon, respectively. The motor function of the pylorus and stomach are discussed in Chapter 50 , motility of the ileocecal region is discussed in Chapter 100 , and general anatomy of the small intestine is discussed in Chapter 98 . The duodenum is a fixed, largely retroperitoneal structure located in the upper abdomen, and the distal ileum generally is anchored in the right iliac fossa by its attachments to the cecum. The small intestine is mobile within the peritoneal cavity outside of these regions.
The wall of the small intestine comprises mucosa (which consists of the epithelium and lamina propria), submucosa, muscularis, and serosa ( Fig. 99.1 ). The muscularis is composed of inner circular and outer longitudinal layers of smooth muscle that are present in continuity along the entire length of small intestine. Contractions within these layers are responsible for gross small intestinal motility. Another much thinner muscular layer, the muscularis mucosae, is present between the mucosa and submucosa and plays a role in mucosal or villous motility. The muscularis mucosae does not contribute to gross motility and is not considered further in this chapter.
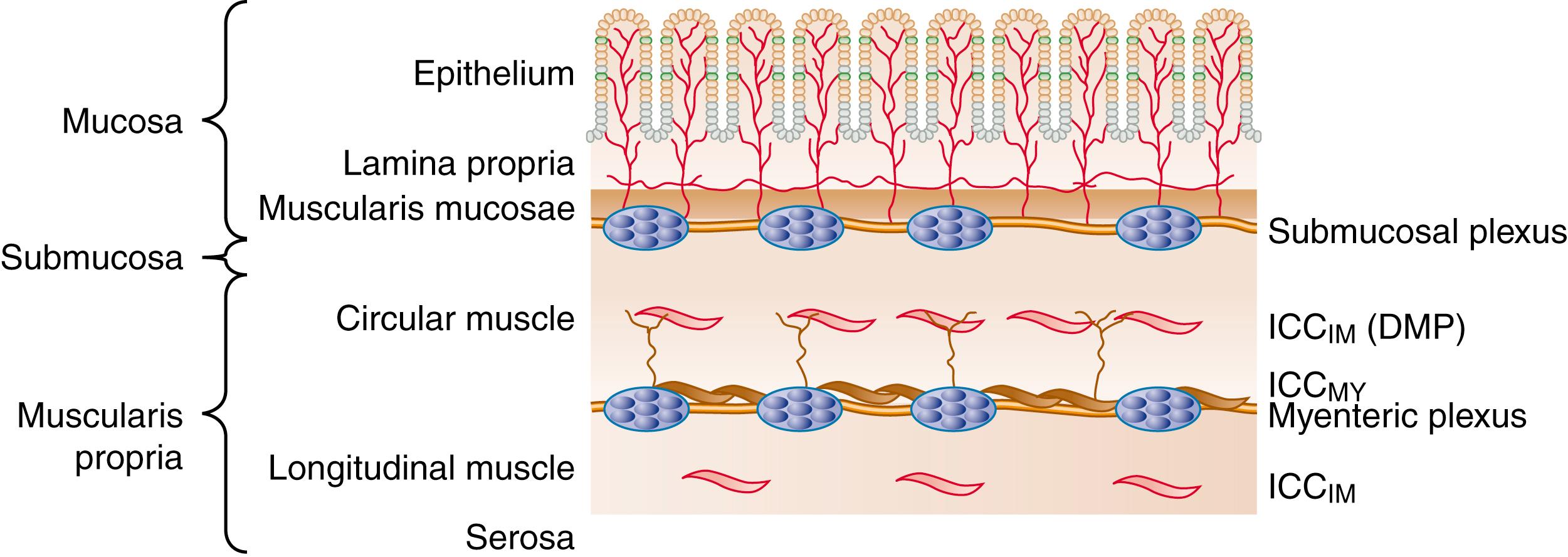
Smooth muscle cells within each muscle layer form a syncytium. Myocytes communicate electrically with each other through physically specialized areas of cell-to-cell contact called gap junctions , which are visible by electron microscopy. This intimate contact between adjacent myocytes gives low-resistance electrical contact or coupling among them, enabling them to be excited as a unit. Mechanical connections among myocytes in each layer enable them to function as a contractile unit. Mechanical connections are provided by intermediate junctions at the cellular level, and by the dense extracellular stroma of collagen filaments between bundles of smooth muscle cells at the tissue level. Smooth muscle cell bodies are arranged in parallel within each layer, such that the circular muscle layer encircles the lumen, and the longitudinal layer extends axially along the small intestine. Cell bodies in each layer may be controlled independently, and therefore luminal diameter can decrease (circular contraction) and small intestinal length can shorten (longitudinal contraction), alone or in combination.
The myocytes themselves are spindle-shaped cells that derive their contractile properties from specialized cytoplasmic filaments (actin and myosin) and from the attachment of these filaments to cytoskeletal elements. Electron microscopy reveals condensations of electron-dense, amorphous material around the inner aspect of the cell membrane (dense bands) and throughout the cytoplasm (dense bodies). The contractile filaments are arranged in a similar manner to that in skeletal muscle and insert onto the dense bands and bodies approximately in parallel with the long axis of the cell. Thus, cell shortening results when the contractile filaments are activated to slide over each other. Most of the Ca 2+ required for activating the contractile apparatus enters the cells via L-type Ca 2+ channels ( Fig. 99.2 ). Ca 2+ entry also can be supplemented to a varying extent by release of Ca 2+ from the sarcoplasmic reticulum membrane via IP3 receptor-operated Ca 2+ channels. IP3 is generated by phospholipase C, which in turn is activated by G proteins coupled to receptors for excitatory transmitters (G protein-coupled receptors).
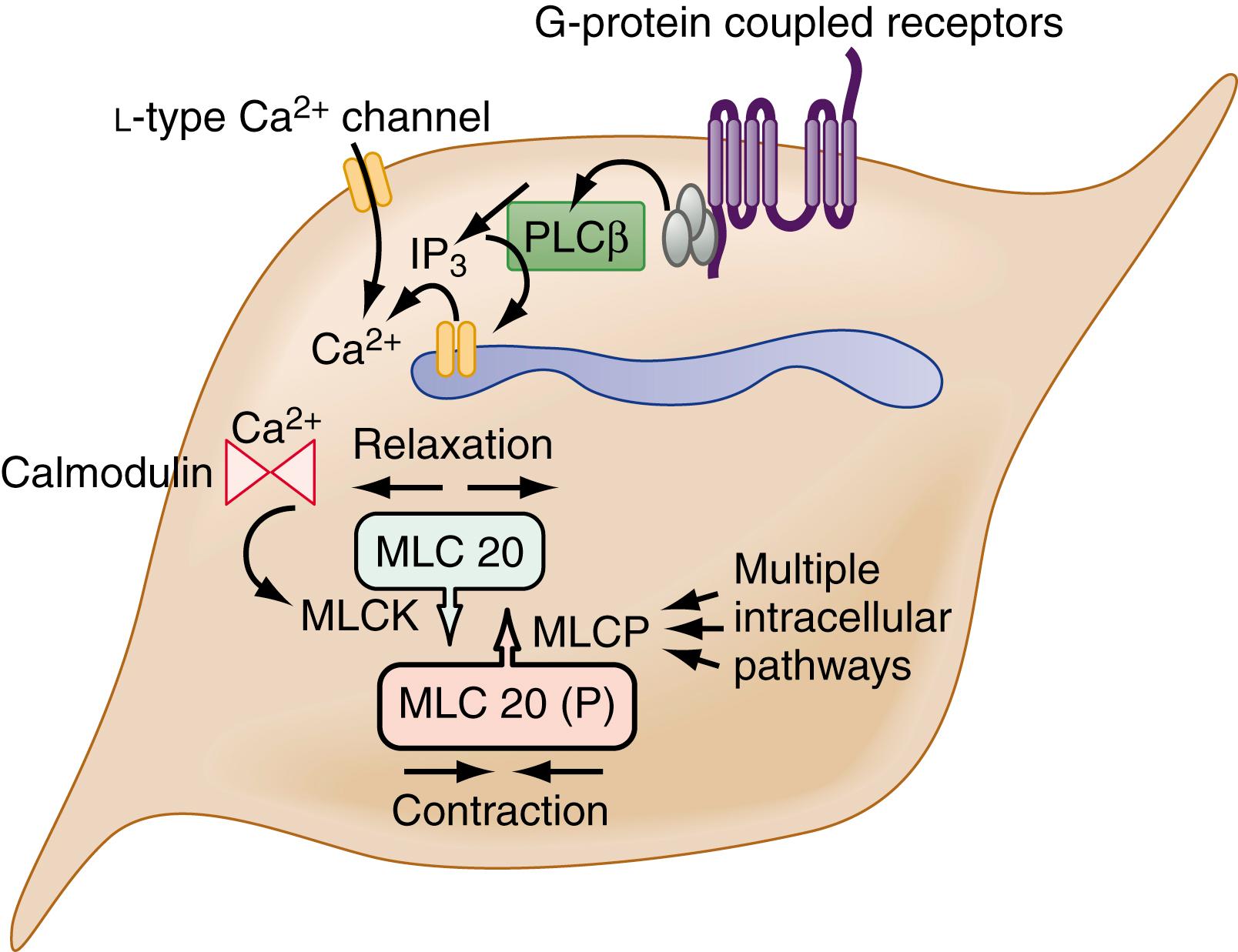
The increased cytoplasmic Ca 2+ binds to the Ca 2+ binding protein calmodulin, enabling it to activate myosin light chain kinase, which phosphorylates the 20-kd light chain of myosin (MLC20). Phosphorylation of MLC20 facilitates actin binding to myosin and initiates cross-bridge cycling and development of mechanical force. Phosphorylation of MLC20 is reduced by MLC phosphatase. De-phosphorylation of MLC20 reduces cross-bridge cycling and leads to muscle relaxation. The de-phosphorylation process is under a complex system of hierarchical control, which is important in setting the gain of smooth muscle contractility.
Interstitial cells of Cajal (ICC) are specialized cells within the smooth muscle layer that are vital for normal small intestinal motor function. ICC are pleomorphic mesenchymal cells that form an interconnecting network via long, tapering cytoplasmic processes. ICC lie in close proximity to both nerve axons and myocytes, with which they form electrical gap junctions. ICC serve 2 roles in the control of small intestinal motility: First, they act as pacemakers and generate the electrical slow wave that determines the basic rhythmicity of small intestinal contractions. Second, they transduce both inhibitory and excitatory neural signals to the myocytes and therefore can vary the myocyte membrane potential and, in turn, contractile activity. This transduction occurs because ICC are interposed functionally between nerve terminals and the smooth muscle that the nerves supply. The neuroeffector junctions of the small intestine are more complex than simple contacts between nerve terminals and smooth muscle cells; instead, they are contacts between enteric nerve terminals and ICC, and from there with myocytes by means of electrical gap junctions. Thus, effective neurotransmission results from the activation of specific sets of receptors on ICC, rather than by direct action on smooth muscle cells.
At least 3 separate functional groups of ICC exist. They are the myenteric ICC (ICC MY ), the intramuscular ICC (ICC IM ), and the ICC in the deep muscular plexus.
ICC MY are the pacemaker cells in the small intestine that trigger the generation of slow waves in the smooth muscle. ICC MY cells form a dense, electrically coupled network within the intermuscular space at the level of the myenteric plexus between the circular and longitudinal muscle layers. These cells possess a specialized mechanism that uses their oxidative metabolism to generate an inward (pacemaker) current resulting from the flow of cations through nonselective cation channels in the plasma membrane. A primary pacemaker initiates slow waves. This depolarization from the primary event then entrains the spontaneous activity of other ICC within the network. This sequence results in a propagation-like phenomenon by which slow waves spread, without decrement, through the ICC network by means of gap junctions. A specialized type of ICC MY lines the septa between circular muscle bundles; these cells form a crucial conduction pathway for spreading excitation deep into the muscle bundles of the human jejunum, which is necessary for the motor patterns that underlie mixing.
ICC IM , the second main population of ICC, are distributed within the muscle layers. ICC IM are innervated preferentially by intrinsic enteric motor neurons. The ICC in the deep muscular plexus are concentrated at the inner surface of the circular muscle layer at the region of the deep muscular plexus; they also receive preferential innervation and may be a specialized type of ICC IM in the small intestine.
Both inhibitory and excitatory enteric motor nerve terminals selectively target ICC IM . Their responses are transduced in turn to smooth muscle cells through gap junctions. Inputs from enteric excitatory motor neurons are mediated by muscarinic acetylcholine receptors (M 2 and M 3 ) and NK1 substance P-receptors that result in increased inward currents, thereby causing depolarization. When depolarization reaches the level of the smooth muscle, it increases the opening of L-type Ca 2+ channels during slow waves, resulting in greater Ca 2+ entry and more forceful phasic contractions. Inputs from inhibitory enteric motor neurons are mediated by neurotransmitters including nitric oxide (NO) and vasoactive intestinal polypeptide (VIP), which activate both receptor and nonreceptor mechanisms in ICC IM . The result of these inputs is an increased opening of K + channels that, in turn, has a stabilizing effect on membrane potential, reduces Ca 2+ channel opening, and results in less forceful contractions of the smooth muscle. Therefore, the mechanical response of small intestinal muscle to the ongoing slow wave activity depends strongly upon regulation of its excitability by the ENS via ICC IM .
ICC in general play broadly similar roles in the small intestine and colon (for colon, see Chapter 100 , Fig. 100.2 ). Absence or inactivity of ICC has been implicated in a number of clinical disorders that manifest as disturbed intestinal motility (see Chapter 124 ). Recent focus has also centered on involvement of “fibroblast-like cells,” which have similar anatomic distributions as ICC, but represent a discrete population of cells. These fibroblast-like cells can be identified by staining with antibodies for platelet-derived growth factor receptor α (PDGFRα) , which demonstrates that they are closely associated with both ICC and nerve varicosities . Myenteric and intramuscular PDGFRα - immunopositive cells express small-conductance Ca 2+ activated K + (SK3) channels, which are a potential mediator of purinergic enteric inhibition. Because PDGFRα-immunopositive cells also form gap junctions with smooth muscle cells, fibroblast-like cells are also likely participants in motor neurotransmission and thus may contribute to the integrated motor responses of smooth muscle and possibly the frequency of phasic activity, such as peristalsis and segmentation.
The small intestine is richly innervated with both intrinsic and extrinsic neurons. Intrinsic neurons have their cell bodies located within the wall of the small intestine and constitute the ENS. Most of these intrinsic enteric neurons have their peripheral terminals within the intestinal wall. However, a separate class of neurons termed intestinofugal neurons have cell bodies within the myenteric plexus but have projections from the intestinal wall to prevertebral ganglia (PVG) via extrinsic nerve trunks. Intestinofugal neurons sense and receive information regarding mechanical distension of the intestine and transmit this information to postganglionic sympathetic neurons in the PVG. Overall, these various types of intrinsic neurons greatly outnumber the neurons of the extrinsic supply which have their cell bodies outside the intestinal wall, but projections that end within the intestinal wall. Extrinsic neurons can be classified anatomically according to the location of their cell bodies (cranial or spinal ganglia) and the route along which their projections travel. Extrinsic motor neurons belong to the ANS and connect the CNS with the ENS and, from there, the small intestinal smooth muscle through the ICC. Furthermore, some extrinsic motor neurons terminate directly in the muscle layers. Extrinsic sensory neurons from the small intestine do not belong to either the ANS or ENS and are classified as vagal or spinal in origin, depending on whether they follow vagal or spinal nerve pathways toward the CNS ( Fig. 99.3 ).
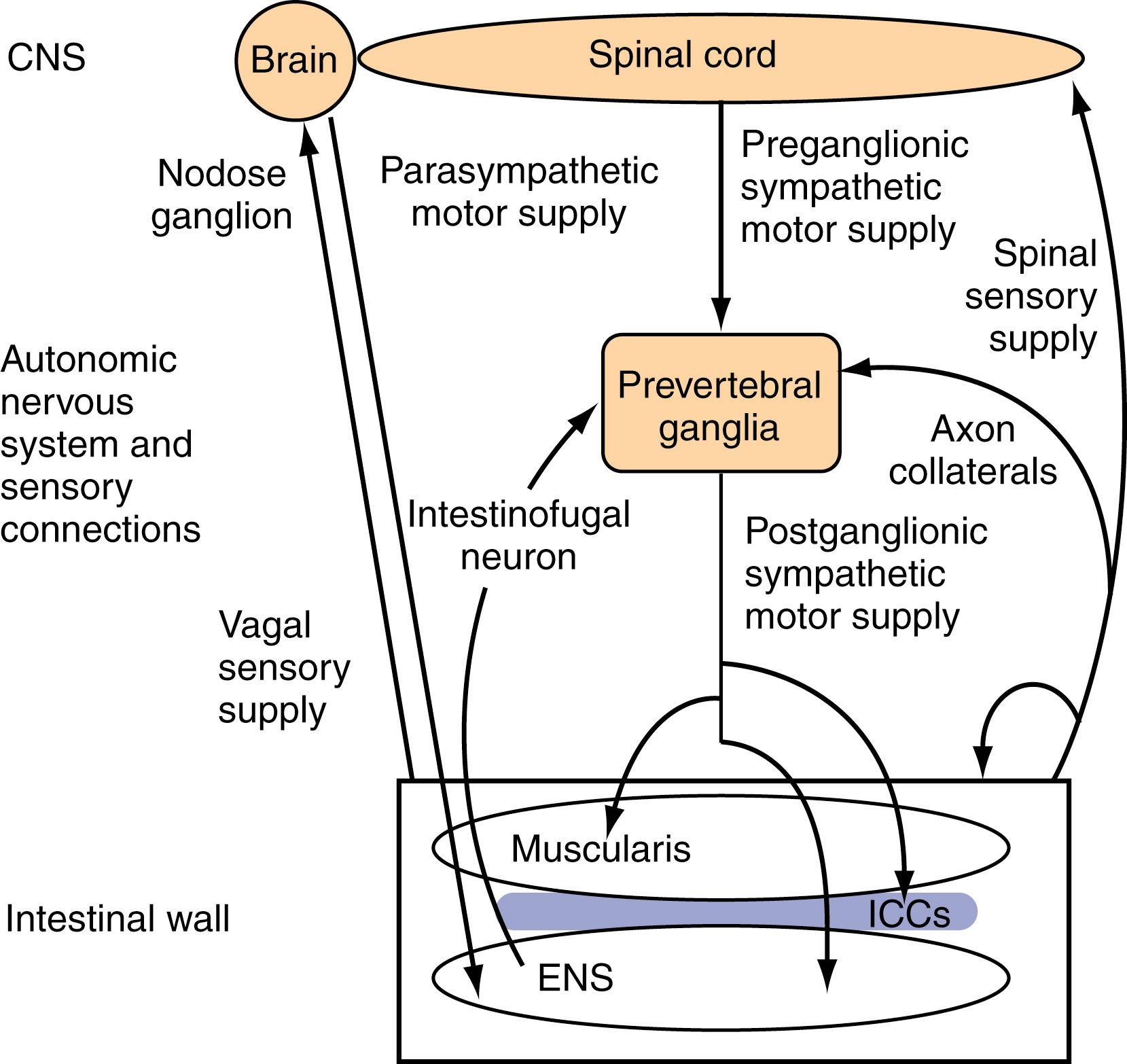
Neurons that supply the intestine are designated either afferent or efferent, depending on the direction in which they conduct information. Information is conducted centrally by afferent neurons and peripherally by efferent neurons. Thus, for the neural supply, the term afferent is used to describe pathways conducting information that is detected in the intestine and transmitted to the CNS. It should be noted that in most texts, the term afferent is interchangeable with sensory. However, most sensory information from the small intestine is not perceived at a conscious level. Examples of perceived sensory information include bloating, discomfort, and pain. The terms efferent and motor regarding neural supply are used to describe pathways that conduct signals toward the “effector,” in this case the small intestinal smooth muscle. Although the importance of motor innervation for motility is self-evident, the pivotal role of afferent function in determining motor responses has been less well appreciated. The importance of the extrinsic afferent innervation in sensory signaling is emphasized by the observation that at least 80% of vagal fibers are afferent rather than efferent.
ENS elements of the small intestine can be subdivided into 3 major functional groups: primary sensory (afferent) neurons, motor (efferent) neurons, and interneurons. The latter 2 groups can be further subdivided into excitatory and inhibitory motor neurons and ascending and descending interneurons, respectively. Together these 3 groups form complex networks that are responsible for coordinating intestinal motility. Within this network, specific ascending and descending circuits are synchronously activated in response to luminal contents. Contractions result from activation of ascending pathways, with ascending interneurons synapsing onto excitatory motor neurons, whereas relaxations result from descending interneuron activation of inhibitory motor neurons. Other categories of neurons, including secretomotor and vasomotor neurons and motor neurons to endocrine cells, are recognized but not considered further in this chapter. Additional distinct subgroups of enteric neurons are now well characterized both structurally and functionally and are reviewed in detail elsewhere.
The cell bodies of ENS neurons reside in ganglia in 2 main intramural plexuses, one in the submucosa (Meissner submucosal plexus) and the other between the circular and longitudinal muscle layers (Auerbach myenteric plexus). A deep plexus (Schabadasch plexus) exists within the circular muscle but does not contain ganglia. The ganglia in the submucosal and myenteric plexuses are connected by interganglionic fascicles. These fascicles are composed predominantly of the axons of motor neurons and interneurons because sensory nerve processes do not often extend for any distance outside the ganglia.
The myenteric plexus consists of ganglia that are spaced at regular intervals and connected by a network of interganglionic fascicles; this major network is known as the primary plexus . Within this main structure, smaller branches of nerve bundles arise from the primary plexus and form the secondary plexus, and still smaller branches form the tertiary plexus. The submucosal plexus has 2 layers, one close to the mucosa and another nearer to the circular muscle layer. These 2 layers are connected by interganglionic fascicles. The submucosal plexus does not have a hierarchy of subordinate plexuses.
The primary afferent neurons of the ENS are characterized morphologically as Dogiel type II neurons (neurons with numerous processes). Intrinsic primary afferent neurons (IPANs) that respond to mucosal chemical stimuli have their cell bodies in the myenteric plexus, and they project axons toward the mucosa. The myenteric plexus also contains the cell bodies of intrinsic afferent neurons that discharge in response to mechanical stimulation of the muscle layer induced by muscle activity or stretch. Intrinsic afferent neurons that respond to mechanical stimulation of the mucosa are also thought to exist based on enteric reflexes seen in extrinsically denervated preparations. Current evidence suggests that the cell bodies of these afferents reside in the submucosal ganglia; however, this is yet to be definitively described in the intestine. Intrinsic sensory neurons synapse in the intramural plexuses with intrinsic motor neurons and interneurons, which they excite mainly by the release of acetylcholine (ACh) and substance P.
It is currently thought that approximately 20% of all myenteric neurons in the ileum are activated by mechanical distortion of the myenteric ganglia, which occurs regularly during contraction or motility events. This indicates that IPANs are not the only class of mechanically sensitive enteric neuron. These ileal myenteric neurons display rapidly adapting responses to mechanical stimulation, whereas colonic neurons display slowly adapting responses, suggesting that these neurons can directly encode dynamic changes in force in response to phasic or tonic contractions. These findings also suggest that the ENS behaves as a global sensorimotor network rather than as separate components, allowing for processing of sensory, integrative, and motor functions.
The axons of the intrinsic motor neurons that supply small intestinal smooth muscle exit the intramural ganglia and enter either the circular or the longitudinal muscle layer where they pass in close proximity to both the myocytes and ICC. No specific neuromuscular junctions are present in small intestinal smooth muscle, unlike skeletal muscle, although multiple varicosities along the motor axons probably represent specialized areas of neurotransmission. The motor axons discharge along their length, potentially activating large numbers of myocytes through the ICC but possibly also directly activating them. The lack of exclusive specific neuromuscular junctions, the electrical gap junctions among myocytes, and the overlap of innervation of myocytes from more than 1 motor axon strongly suggest that functionally discrete motor units in the intestinal smooth muscle do not exist, in contrast to skeletal muscle. The ENS motor supply itself is both inhibitory and excitatory, with intrinsic inhibitory and excitatory motor neurons generally containing both fast and slow neurotransmitters. The predominant excitatory transmitters are ACh (fast) and substance P (slow), and the predominant inhibitory transmitters are NO fast), VIP (slow), ATP (fast), and β-nicotinamide adenine dinucleotide (fast).
Interneurons connect ENS neurons of the same class or of different classes with one another. They permit local communication within limited lengths of intestinal wall. They are implicated in simple local responses by means of release of ACh or NO, respectively, depending on their oral or aboral direction of projection. Evidence also suggests the presence of connections within the intestinal wall along greater distances, but these neural pathways are not well defined. These longer connections may be provided anatomically by the ENS or by connections between the ENS and ANS. Interneurons that have an additional sensory role have been identified, responding directly to mechanical changes in muscle length rather than muscle tone or tension.
Intestinofugal neurons are a special type of interneuron that may be important for controlling local reflexes. These neurons have cell bodies within the myenteric plexus, receive input from several local enteric neurons, and project to the PVG where they synapse with sympathetic motor neurons (see Fig. 99.3 ).
The various subtypes of ENS neurons have the potential to be modulated by enteric glia, a unique class of peripheral glial cells within the GI tract. Until recently, enteric glia were thought to be “silent” in the intestinal wall. Major populations of enteric glia are found in the myenteric and submucosal plexuses of the ENS and within the lamina propria of the mucosa and the circular muscle. Enteric glia are similar to astrocytes of the CNS in that they detect and integrate neural activity. Enteric glia are activated by synaptic stimulation, which suggests they play an active role in synaptic transmission and actively modulate physiologic intestinal processes. Recent studies have demonstrated that enteric glial populations display substantial heterogeneity, adding considerable complexity to an already intricate regulatory system.
The small intestine is innervated by both vagal and spinal extrinsic afferent nerves. The anatomy of small intestinal vagal afferent innervation is relatively straightforward with afferent endings in the intestinal wall and cell bodies within the nodose and jugular ganglia, which deliver input directly to the brainstem. Spinal afferent fibers travel along perivascular nerves to the PVG, where neurons give off axon collaterals that synapse on postganglionic sympathetic motor neurons. These fibers then pass into the thoracic dorsal ganglia and enter the spinal cord through dorsal roots, synapsing mainly on neurons of the superficial laminae of the spinal gray matter. These neurons in turn can send projections to numerous areas of the brain involved in sensation and pain control. Spinal afferent neurons can also give off axon collaterals closer to the intestinal wall that synapse on components of the ENS, blood vessels, smooth muscle, or secretory elements ( Fig. 99.4 ). In general, vagal afferents have lower mechanical activation thresholds and display saturated responses at higher intensities, whereas spinal afferents are also activated at higher thresholds. The different stimulus response profiles of vagal and splanchnic mechanoreceptors are generally interpreted as evidence that vagal afferents regulate fullness and satiety, whereas spinal afferents mediate discomfort, bloating, and pain.
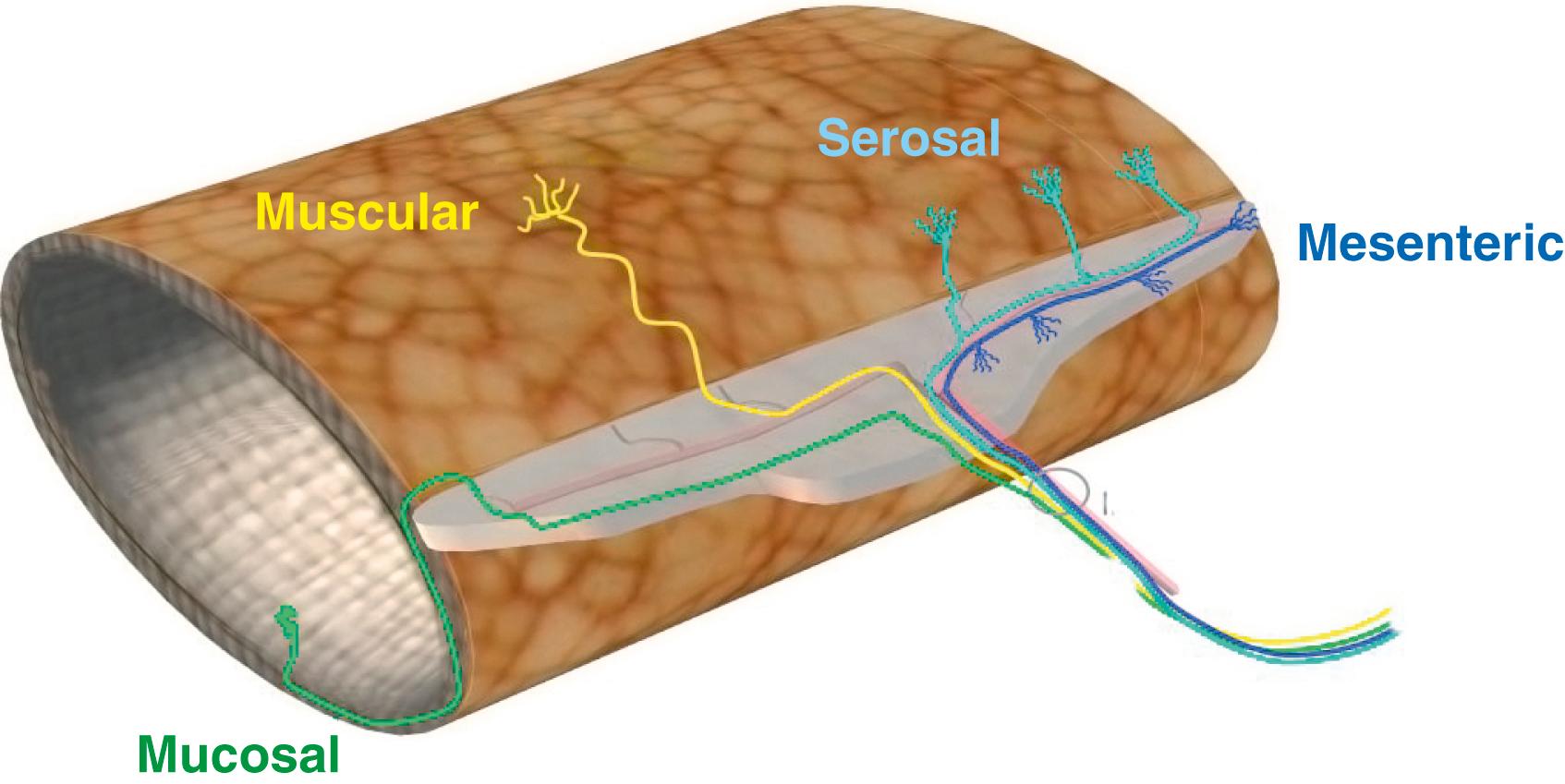
Classification of extrinsic afferents continues to evolve, and they are currently best described according to mechanical activation thresholds, proposed anatomical location, or by a mix of anatomy and function. Endings with low mechanical activation thresholds are observed in vagal pathways and spinal afferent pathways. These afferents are continuously active as they respond to fine tactile mucosal stimulation that saturates at high intensities, but do not desensitize. They do not respond to intestinal distension or contractions. They can be termed “mucosal” because they are thought to reside in the lamina propria where they are positioned to detect substances that are absorbed across the mucosal epithelium or are released from epithelial and subepithelial cells including enterochromaffin and immune cells. Activation of these afferents is unlikely to reach conscious perception under normal physiological conditions but may allow discrimination between gaseous and solid matter. Endings that have a wide dynamic range are also termed “muscular” or “tension” afferents and respond to circular stretch but not mucosal stimulation. These afferents form endings either within the muscle layers or within the myenteric plexus. Wide dynamic range afferents are activated by mechanical stimuli that are within the physiological range encountered during digestion. Responses to increasing stretch intensity generally do not appear to plateau, suggesting these afferents are likely to be involved in urge and bloating, and potentially in the modulation of pain. Muscular afferents show maintained responses to distention of the small intestine and signal each contractile event, giving rise to the term in-series tension receptors. Nerve tracing studies have identified vagal afferent terminals in the longitudinal and circular muscle layers that are described as intramuscular arrays (IMAs), which consist of several long (up to a few millimeters) rather straight axons running parallel to the respective muscle layer connected by oblique or right-angled short connecting branches. IMAs were proposed to be the in-series tension receptor endings, possibly responding to both passive stretch and active contraction of the muscle, although direct evidence for this proposal is currently lacking. Vagal afferent terminals that surround the myenteric plexus throughout the GI tract have been described as intraganglionic laminar endings (IGLEs). These are in intimate contact with the connective tissue capsule and enteric glial cells that surround the myenteric ganglia, and they have been hypothesized to detect mechanical shearing forces between the orthogonal muscle layers. Evidence for such a mechanosensory function of IGLEs has been elaborated by mapping the receptive field of vagal afferent endings in the esophagus, stomach, and large intestine, and showing morphologically that individual hot spots of mechanosensitivity correspond with single IGLEs. Functional evidence exists for muscular afferents in both the vagal and the spinal innervation, but the anatomic appearance of spinal distention-sensitive afferents in the small intestine remains to be determined. Its structure and the receptors and channels it expresses, however, are likely to be distinct from those of vagal afferents, owing to spinal muscular afferents displaying higher activation thresholds to distention. There are also afferents that respond to both low intensity mucosal stimulation and circular stretch. These afferents are termed “muscular/mucosal” or “tension mucosal” in the colon and stomach, respectively, but their presence in the small intestine is yet to be definitively shown.
Vagal afferent fibers have been shown to penetrate the circular muscle layer and submucosa to form networks of multiply branching axons within the lamina propria of both crypts and villi in the rat duodenum and jejunum. Their terminals are in close contact with, but do not seem to penetrate, the basal lamina and thus are in an ideal position to detect substances including absorbed nutrients and mediators that are released from epithelial cells and other structures within the lamina propria. Afferents with high thresholds to mechanical stimuli are observed in the deep muscular/serosal layers and in mesenteric connections, and have also been termed “serosal” or “mesenteric” afferents. These afferents are activated by intense distortion of the intestine and its attachments, and do not respond to low intensity mucosal stroking or circular stretch. Therefore, these afferents are inactive under normal physiological conditions and are thought to constitute primarily a pain-sensing afferent class. Importantly, each of these afferent classes is also activated by chemical mediators and expresses many algesic and analgesic channels and receptors. These types of responses may occur in the presence or absence of effects on the mechanosensitivity of the afferent endings, suggesting that the chemosensitivity of afferent endings is regulated independently of mechanosensitivity. These observations hint at the potential responsiveness to circulating or locally released factors like cytokines, proteases, and other immune mediators including histamine and bradykinin.
The extrinsic efferent pathways to the small intestine are supplied by the parasympathetic and sympathetic divisions of the ANS. The small intestinal parasympathetic supply is cranial and cholinergic, whereas the sympathetic supply is spinal (thoracic) and adrenergic. These 2 motor pathways are not entirely separate, however, because postganglionic sympathetic fibers arising from cervical ganglia are sometimes found within the vagus nerve.
The parasympathetic motor neurons of the small intestine have cell bodies within the dorsal motor nuclei of the vagi in the medulla oblongata. Their axons extend through the vagi to the intestinal intramural plexuses, where they synapse with motor neurons of the ENS. The sympathetic motor supply is more complex. Primary motor neurons within the intermediolateral horn of the thoracic spinal cord synapse with second-order neurons in the PVG. These then synapse with ENS motor neurons within the intestinal intramural plexuses, directly with smooth muscle, or possibly with ICC.
Both excitatory and inhibitory extrinsic motor outputs to the small intestine have been identified. Excitatory outputs depolarize and inhibitory outputs hyperpolarize smooth muscle, thereby facilitating and impeding the development of contractions, respectively. In general, the sympathetic motor supply is inhibitory to the ENS, and this ENS inhibition leads to decreased smooth muscle activity, with the opposite effect seen in sphincter regions. Direct sympathetic inhibitory and excitatory outputs to smooth muscle also exist. The parasympathetic motor output to the ENS is more diffuse, each primary motor neuron supplying a large area. Excitatory parasympathetic motor output occurs to either inhibitory or excitatory ENS motor neurons, through which parasympathetic efferents can selectively inhibit or excite smooth muscle. Interestingly, efferent and afferent innervation has also been shown to modulate immune and motor responses in the intestine, although the precise mechanisms underlying these effects remain contentious.
Centrally, the sensory and motor supplies to the small intestine are closely interrelated; the vagal sensory input and the parasympathetic motor output are closely located, as are the spinal sensory input and the sympathetic motor output. Both the vagal parasympathetic and the spinal sympathetic supplies have widespread connections to many other areas throughout the CNS that are implicated in feeding, arousal, mood, and other reflex behavior. The proximity of the CNS areas involved in small intestinal regulation, and their interconnections, makes it likely that the vagal parasympathetic and the spinal sympathetic control mechanisms are interconnected and might function less independently than has previously been thought.
Parasympathetic primary motor neurons are located bilaterally in the dorsal motor nuclei of the vagus in the medulla, which lie close to and receive substantial input from neurons of the nuclei tracti solitarii (NTS). The NTS are the sites of terminals of vagal afferent fibers that enter through the tracti solitarii and have cell bodies in the nodose ganglia. Each NTS also has extensive connections to other CNS regions, and several of these regions have input to the dorsal motor nuclei of the vagus, thereby influencing vagal motor output to the intestine.
The central connections of the spinal and sympathetic supply to the intestine are less well described. The spinal sensory neurons enter the spinal cord where they synapse ipsilaterally on second-order sensory neurons and provide direct feedback to sympathetic preganglionic motor neurons through axon collaterals. The second-order sensory neurons then ascend the spinal cord either contralaterally or ipsilaterally, after which they terminate in numerous areas, including the raphe nuclei and periaqueductal gray matter in the brainstem and the thalamus. The thalamus has extensive ramifications throughout the CNS. The central influence on sympathetic motor output to the small intestine is complex and not well understood, but stress and arousal level play a role. These influences have their output through the brainstem and descending tracts to the sympathetic preganglionic motor neurons in the intermediolateral horn of the spinal cord, which send their axons to the PVG, whereupon they synapse with sympathetic postganglionic adrenergic nerves.
GI hormones are discussed in detail in Chapter 4 , but it is important to emphasize here their vital role in modulating small intestinal motor and sensory function. GI hormones relevant to small intestinal function can act in either a humoral or paracrine fashion on both enteric neurons and myocytes and have also been shown to modulate colonic extrinsic sensory afferent function. These hormones are generally released in response to the presence (or in some cases anticipation) of enteral nutrition. The best known of these hormones include CCK, somatostatin, VIP, glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide, ghrelin, and motilin. Most of the hormones released in response to the presence of food in the lumen lead to slowing of small intestinal transit, signals of satiety, and increased mixing or segmenting contractions (see later).
The involvement of integrated hierarchical levels of control of the neuromuscular apparatus of the small intestine are nicely illustrated by 2 important processes: peristalsis and the interdigestive motor complex (IDMC). However, it must be noted that gaps remain in our understanding of how the structure and function of individual components of this apparatus cooperate to produce known motility patterns, because evidence for contributions to specific mechanisms is often circumstantial.
Peristalsis is the fundamental integrated motility pattern of the small intestine. It consists of progression of contractile activity that is usually in an aboral direction, can be coordinated entirely within the ENS and circular and longitudinal muscle layers, and may be initiated in response to a number of mechanical and chemical stimuli in the lumen. Peristalsis has both sensory and motor aspects.
The populations of IPANs described earlier probably are responsible for detection of luminal stimuli, either directly or following release of mediators from mucosal enteroendocrine cells. Their activation results in transmitter release onto neighboring interneurons and motor neurons, the activity of which is coordinated subsequently as a network to provide activation of circular and longitudinal muscles on one side of the bolus (usually the oral side) and synchronous inhibition of muscle on the other side (usually the aboral side). This networked activity normally travels aborally, but the mechanism of propagation is not yet understood; it might result from patterns of activity in interneurons that can project over distances of several millimeters and thus mediate a general descending excitation. The precise interactions of transmitters and mediators in the normal function of peristalsis are still not well defined. However, exogenous activation of several pre- and postsynaptic mechanisms is known to affect peristalsis, and some of these mechanisms may also be activated endogenously. Of particular interest are serotoninergic mechanisms, which have been shown to be involved in initiation of peristalsis and modulation of transmission between subclasses of enteric neurons. Peristalsis need not necessarily be antegrade; reverse peristalsis does occur in the small intestine generating emesis, for example in conditions of luminal toxicity, although the mechanisms underlying this phenomenon remain unknown.
The IDMC serves to demonstrate the extraordinary integrative capacity of the ENS; other aspects of the IDMC are described later in this chapter. The IDMC is a complex series of periods of variable contractile activity that have distinct phases with different contractile amplitudes, propagation, and regularity. This integrated pattern slowly sweeps down the small intestine in the fasted state, before recurring at regular intervals. Several candidate hormones have been proposed to initiate the IDMC, including motilin, ghrelin, and xenin; serotonin and somatostatin can potentially inhibit the IDMC in the antrum while stimulating it in the duodenum. However, the switch between quiescent and active phases and their orderly migration along the bowel are functions of the ENS, an autonomy that is demonstrated by occurrence of the IDMC in extrinsically denervated or auto-transplanted intestine. The ENS therefore is capable of controlling large segments of the small intestine independent of extrinsic input, probably by virtue of its extensive inter-neuronal connections and constant sensory feedback.
Although the ENS has this regulatory capacity, normal function of the small intestine is modulated by ANS efferent output, which in turn is influenced by locally or centrally processed information gathered from primary spinal or vagal afferents. Synapses outside the CNS in the PVG are capable of subserving inhibitory intestino-intestinal reflexes that are potentially important in the minute-to-minute regulation of motility. A number of hormones, acting in either an endocrine or paracrine manner, also influence neuromuscular function in the small intestine. Little direct information is available, however, on the precise contribution of each extrinsic pathway to motor function of the small intestine in humans. Vagal reflexes generally are thought to make an important contribution to the integration of major homeostatic functions like motility, secretion, blood flow, and control of food and water intake. Sympathetic reflexes are thought to be concerned primarily with inhibition of motility and other functions in response to noxious stimuli, rather than in digestive small intestinal functions.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here