Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Human interest in sleep is as old as humanity itself, but the specialty of sleep medicine is less than 50 years old. The characterization of sleep into periods based on electroencephalography (EEG) and the presence or absence of rapid eye movements (REMs) did not occur until the 1950s. Early developments in the field included the original description of sleep apnea and the development of all-night sleep studies incorporating EEG, respiratory, and cardiac measurements, known as polysomnography (PSG). More recently, additional research has elucidated the neurophysiologic mechanisms governing sleep and wakefulness. Anesthetic drugs modulate key components of sleep–wake neuronal circuits. This chapter will review basic sleep physiology, the differences between sleep and anesthesia states, functional neuroanatomy of sleep and arousal, and sleep-disordered breathing (SDB). The pathophysiology and perioperative management of obstructive sleep apnea (OSA) will be highlighted.
Sleep is defined as a state of decreased arousal that is actively generated by nuclei in the hypothalamus, brainstem, and basal forebrain. It is crucial for the maintenance of health. , Humans spend approximately one-third of their lives in sleep. Sleep is described to be under the control of two processes: a circadian clock (the circadian drive) that regulates the appropriate timing of sleep and wakefulness across the 24-hour day and a homeostatic process (the homoeostatic drive) that regulates sleep need and intensity according to the time spent awake or asleep. The daily drive to sleep is modulated by the hypothalamic suprachiasmatic nuclei that coordinate circadian (24-hour) rhythm. The perceived sensation of sleepiness is the result of a circadian drive, process C (people tend to get sleepy according to their accustomed sleep times during a 24-hour cycle), along with a homeostatic drive, process S (sleep deprivation leads to increasing sleepiness). These two sleep drives are additive, and temporal organization must be preserved to obtain a subjective experience of being refreshed and restful. For example, patients with chronic insomnia often have difficulties because the two sleep drives are not aligned with each other (e.g., frequent afternoon naps would delay sleep onset later in the night).
Normal sleep exhibits a dynamic architecture and is a nonhomogeneous state that can be divided into non–rapid eye movement (NREM) and rapid eye movement (REM) sleep. These two states cycle at an ultradian (less than 24 hours) rhythm of approximately 90- to 120-minute intervals, consolidated in bouts of 6 to 8 hours. ,
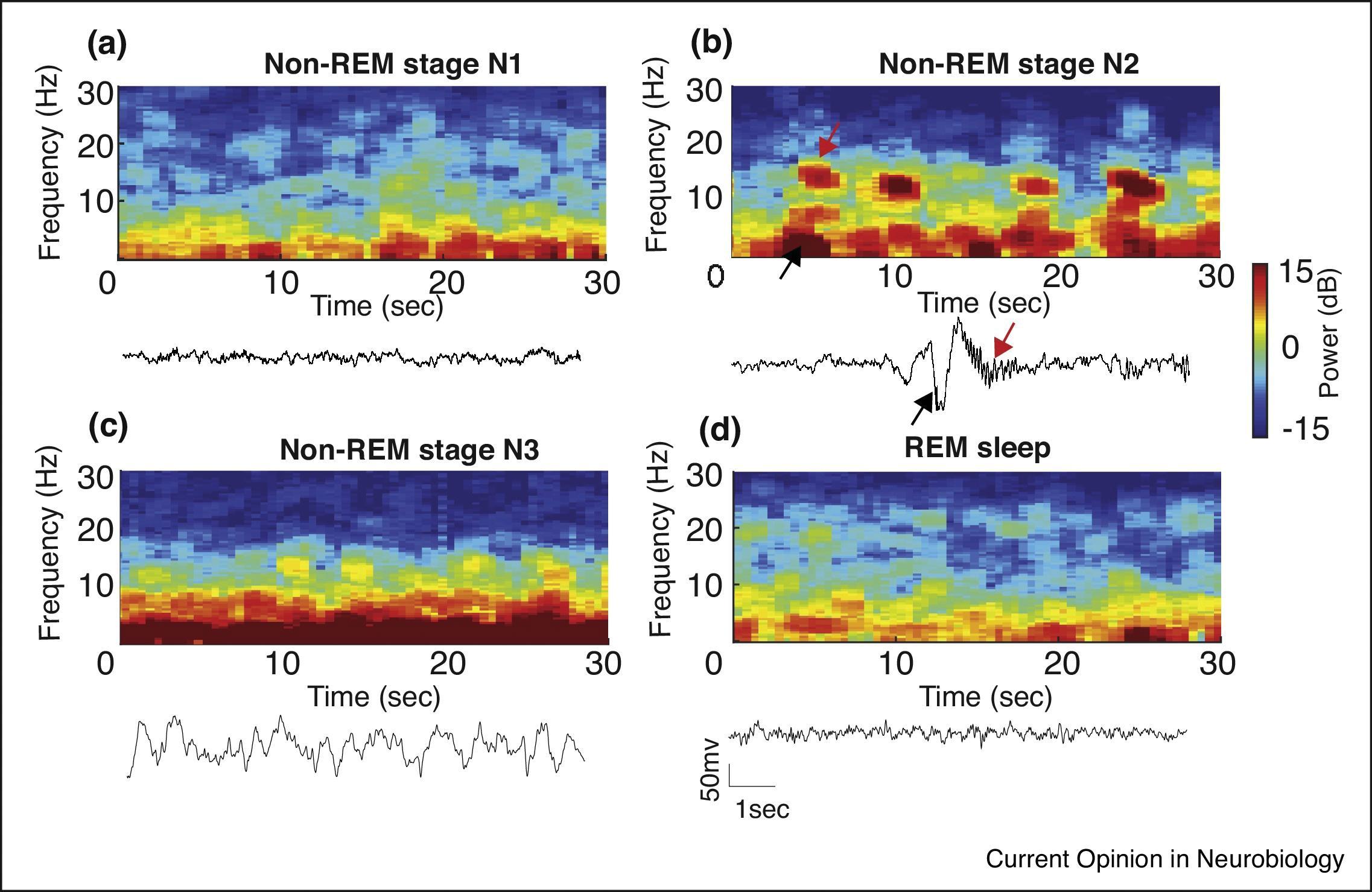
The American Academy of Sleep Medicine (AASM) has classified wakefulness and sleep into various stages based on characteristic EEG patterns. , Wakefulness , or Stage W, is characterized by beta activity with eyes open (low amplitude, 12–40 Hz) and alpha activity with eyes closed (low amplitude, 8–13 Hz). Non-REM sleep has three distinct stages, based on characteristic patterns on the EEG ( Fig. 48.1 ). Stage N1 sleep is characterized by attenuation of alpha activity during wakefulness to a low-amplitude, mixed-frequency signal (4–7 Hz) and vertex sharp waves (prominent sharp waves lasting <0.5 second and maximal over the central EEG region). Stage N2 sleep is characterized by the presence of K-complexes (well-delineated, negative, sharp waves followed by a positive deflection, lasting 0.5 second) and sleep spindles (high-frequency bursts of 11 to 16 Hz, with tapering ends, distinct from the background rhythm and lasting ≥0.5 second). Stage N3 sleep is characterized by the presence of higher-amplitude (75 microvolts), lower-frequency (0.5–2 Hz) rhythms, also known as delta waves, accompanied by waxing and waning muscle tone, decreased body temperature, and decreased heart rate. Stage R, or REM sleep, is characterized by REMs, dreaming, irregular breathing and heart rate, and skeletal-muscle hypotonia. In REM sleep the EEG shows active high-frequency, low-amplitude rhythms (see Fig. 48.1 ). This activated EEG pattern has given rise to descriptions of REM sleep as “active” or “paradoxical” sleep and to the NREM phase of sleep as “quiet” sleep. Cognitive changes and vivid dreaming are well known to occur during REM sleep.
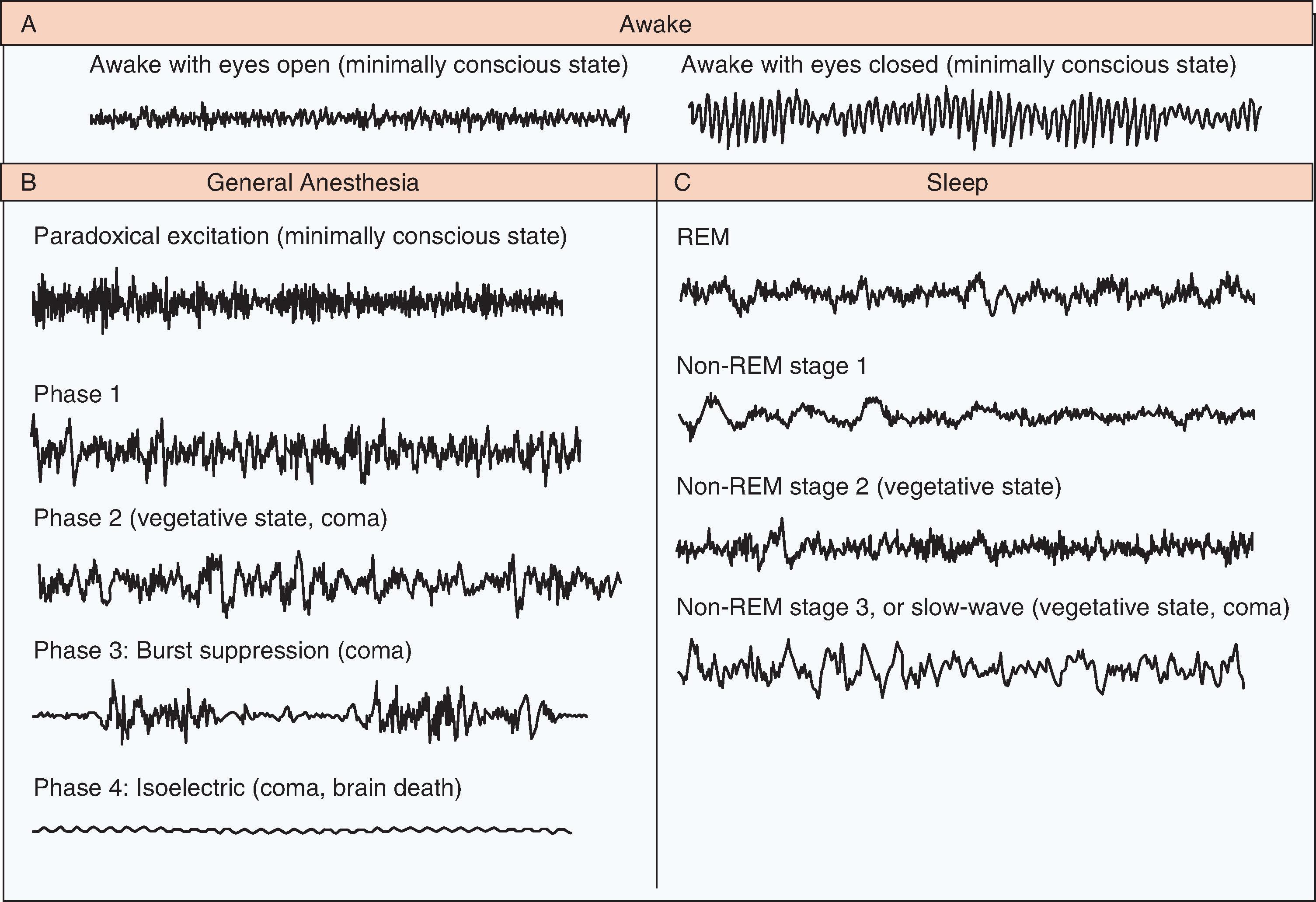
General anesthesia could be described as a reversible drug-induced coma. Nevertheless, anesthesia providers refer to it as “sleep” to avoid disquieting patients when referring to unconsciousness induced by anesthetic drugs. The EEG patterns of general anesthesia–induced consciousness are described in three periods (see Fig. 48.1 ).
Before induction, the patient has a normal, active EEG with prominent alpha activity (10 Hz) when the eyes are closed. Small doses of hypnotic agents acting on the γ-aminobutyric acid type A (GABA A ) receptors induce a state of sedation in which the patient is calm and easily arousable, with the eyes generally closed. This is followed by a brief period of paradoxical excitation, characterized by an increase in beta activity on the EEG (13–25 Hz).
During the maintenance period, four distinct phases have been described. Phase 1, a light state of general anesthesia, is characterized by a decrease in EEG beta activity (13–30 Hz) and an increase in EEG alpha activity (8–12 Hz) and delta activity (0–4 Hz). During phase 2, the intermediate state, beta activity decreases and alpha and delta activity increase, with so-called anteriorization—that is, an increase in alpha and delta activity in the anterior EEG leads relative to the posterior leads. The EEG in phase 2 resembles that seen in stage 3, non-REM (or slow wave) sleep. Phase 3 is a deeper state, in which the EEG is characterized by flat periods interspersed with periods of alpha and beta activity (burst suppression). As this state of general anesthesia deepens, the time between the periods of alpha activity lengthens, and the amplitudes of the alpha and beta activity decrease. Surgery is usually performed during phases 2 and 3. In phase 4 the most profound state of general anesthesia, the EEG is isoelectric (completely flat), indicated in conditions such as induced coma or neuroprotection during neurosurgery (also see Chapter 30).
During emergence from general anesthesia, the EEG patterns proceed in approximately reverse order from phases 2 and 3 of the maintenance period to an active EEG that is consistent with a fully awake state. Anesthetic drugs induce unconsciousness by altering neurotransmission at multiple sites in the cerebral cortex, brainstem, and thalamus. Recent advances in spectral EEG analysis have allowed spatiotemporal characterization of the effects of various intravenous and inhalational anesthetics ( Figs. 48.2A and B ).
Coma is characterized by a state of profound unresponsiveness, which could be drug-induced or a result of brain injury. EEG activity in comatose patients is variable and resembles the high-amplitude, low-frequency activity seen in patients under general anesthesia. The EEG patterns are also dependent on the severity and extent of brain suppression or injury (see Fig. 48.1 ). The EEG patterns during recovery from coma—coma, vegetative state, and minimally conscious state—resemble the patterns during general anesthesia, sleep, and the awake state.
It is important to understand the similarities and differences between sleep and anesthesia states. Sleep is a natural state of decreased arousal controlled by circadian and homeostatic drives. Anesthesia is a drug-induced state that is independent of these intrinsic rhythms. Sleep states are amenable to disruptive influences such as psychological and environmental factors. Anesthesia is immune to such influences. Sleep is a characteristically nonhomogeneous state with distinct stages, periodic arousals, and variable body postures, occurring in a cyclical pattern. Anesthesia is a more or less homogeneous state, the depth and duration of which are directly dependent on drug pharmacokinetics and pharmacodynamics. In the presence of significant sensory stimulation the sleep state becomes disrupted and the subject arouses. On the other hand, anesthesia results from suppression of arousals, rendering the subject insensate to bodily injury during surgery. Whereas sleep state reversal occurs spontaneously after restorative functions are completed, anesthesia state reversal requires voluntary stoppage of drug administration and effective drug elimination.
Common neurophysiologic mechanisms and neural pathways engaged in sleep are activated by anesthetic drugs. This knowledge has provided new insights into the mechanisms of sedation and anesthesia. , Sedative drug requirements appear to decrease with both sleep deprivation and circadian rhythm disruption. In an animal model propofol anesthesia, in the absence of surgical stimulation, has been observed to have sleep-like restorative properties after a period of sleep deprivation. , However, isoflurane anesthesia did not demonstrate this effect, suggesting differences in the neurophysiologic interface between sleep and anesthesia for inhaled anesthetics.
Anesthesia-induced loss of consciousness results from interactions of anesthetics with the neural circuits regulating sleep and wakefulness states. Ascending activation of the cerebral cortex by subcortical center activity is important in the maintenance of wakefulness. Deactivation of thalamus activity has been observed in imaging studies for both the sleep and anesthesia states, suggesting that thalamic and extrathalamic pathways are involved in sleep state modulation.
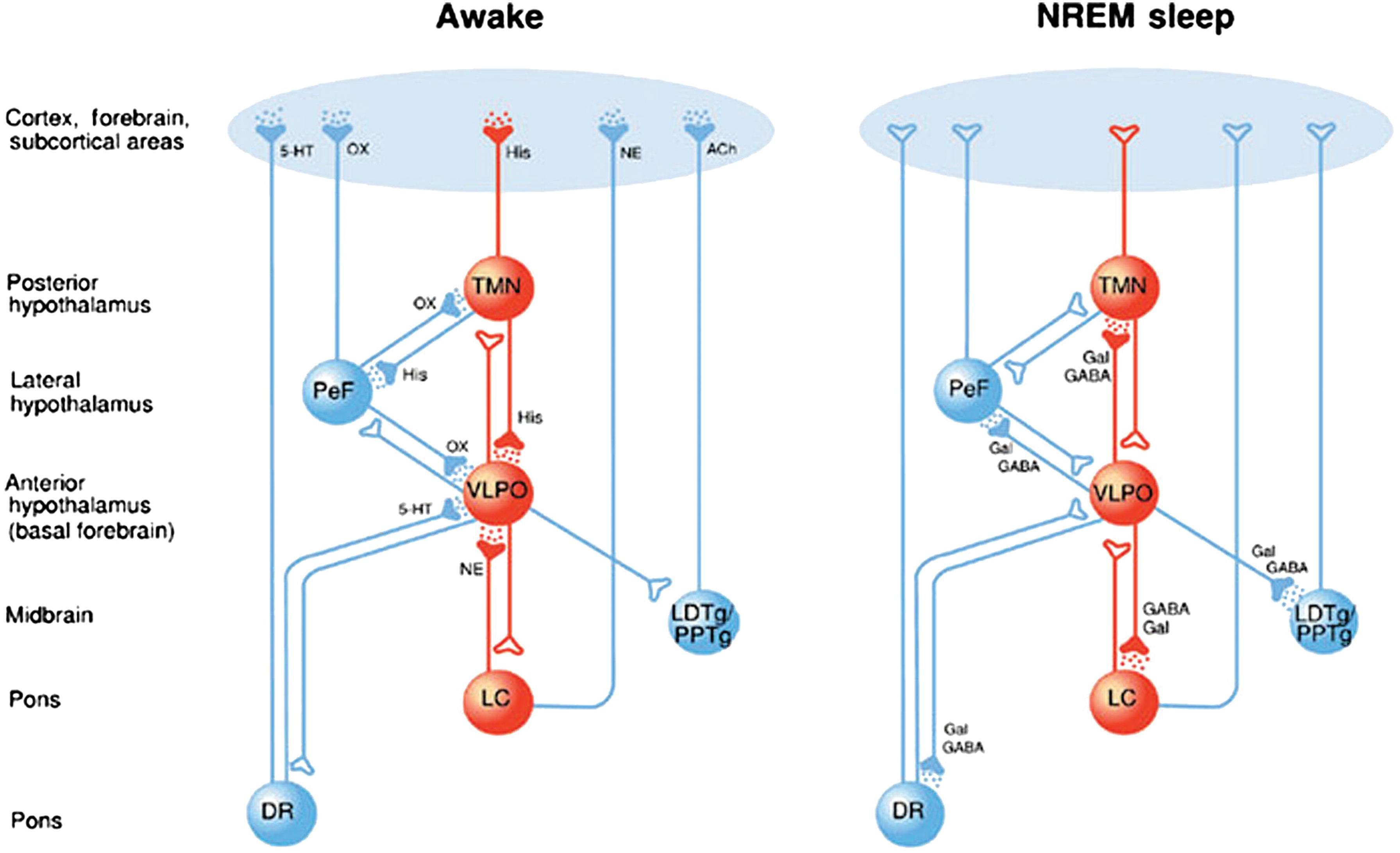
Sleep state modulation is regulated by two groups of neural centers: those that promote wakefulness and those that promote sleep , ( Fig. 48.3 ). The wakefulness-promoting centers are the locus coeruleus (LC), dorsal raphe (DR), and tuberomammillary nucleus (TMN). The sleep-promoting center is primarily the hypothalamic ventrolateral preoptic nucleus (VLPO). , The preoptic area of the hypothalamus contains neurons active in both sleep and thermoregulation. Discrete neurochemical mediators are involved in the sleep–wake transition (see Fig. 48.3 ). The mutual inhibition between VLPO and LC acts to produce switchlike, bistable states of wakefulness and sleep at a certain threshold. This effect is also seen with the use of anesthetic agents such as propofol and benzodiazepines acting on the same target receptors and neural pathways integral to sleep and wakefulness.
SDB is characterized by abnormalities of respiratory patterns during sleep. The abnormal patterns of breathing are broadly grouped into OSA disorders, central sleep apnea (CSA) disorders, sleep-related hypoventilation disorders, and sleep-related hypoxemia disorder. OSA disorders are characterized by complete or incomplete upper airway (UA) closure during sleep in the presence of respiratory effort during some portion of the event. CSA disorders are characterized by reduction (hypopnea) or cessation (apnea) of airflow caused by absent or reduced respiratory effort. Central apnea or hypopnea may occur in a cyclical, intermittent, or irregular (ataxic) fashion. We will primarily focus on OSA, as this is the most common entity encountered perioperatively.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here