Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Sleep is an active state of decreased responsiveness during which multiple vital functions occur to promote higher cognitive functioning and overall good health. During this physiologic state, the brain restores several metabolic processes, clears waste products, and furthers synaptic reorganization to promote alertness, memory, and learning. To achieve these goals, the brain cycles through discrete stages of sleep that allow progressive revitalization to support neuronal function. The non–rapid eye movement (non-REM) sleep stages appear to affect the glia and neurons in functions such as replenishing of energy stores and recycling of neurotransmitters. In addition, the glymphatic system clears protein waste products of brain metabolism, predominantly during slow wave sleep. Synapses are pruned and reinforced during the cycling of slow wave and rapid eye movement (REM) sleep. Conversely, the disruption of sleep is associated with a variety of health issues, as well as impaired performance and psychosocial interactions. Second in frequency only to pain, sleep-wake complaints lead one in three individuals to seek medical attention. Untreated sleep disorders and disruption may also exacerbate symptoms of other diseases by worsening a preexisting disorder or impairing the ability to cope with or counter the symptoms of the original disease. Thus, clinicians should recognize that such signs and symptoms may be related to dysfunctional sleep.
Complex humoral, neurochemical, and neuronal networks determine the sleep-wake state. Dynamic in organization, sleep is composed of REM and non-REM sleep (denoted as stage R). Non-REM is divided into three stages (N1, N2, and N3). Each stage has a distinct physiologic regulation, and each contributes to health and improved brain function.
Wakefulness involves activation of the monoaminergic neuronal groups, basal forebrain cholinergic neurons, and the brainstem reticular activating system ( E-Fig. 374-1 ). The reticular activating system promotes the relay of sensory information to the cerebral hemispheres, and the forebrain cholinergic and monoaminergic neurons promote attention of the hemispheric networks to sensory information.
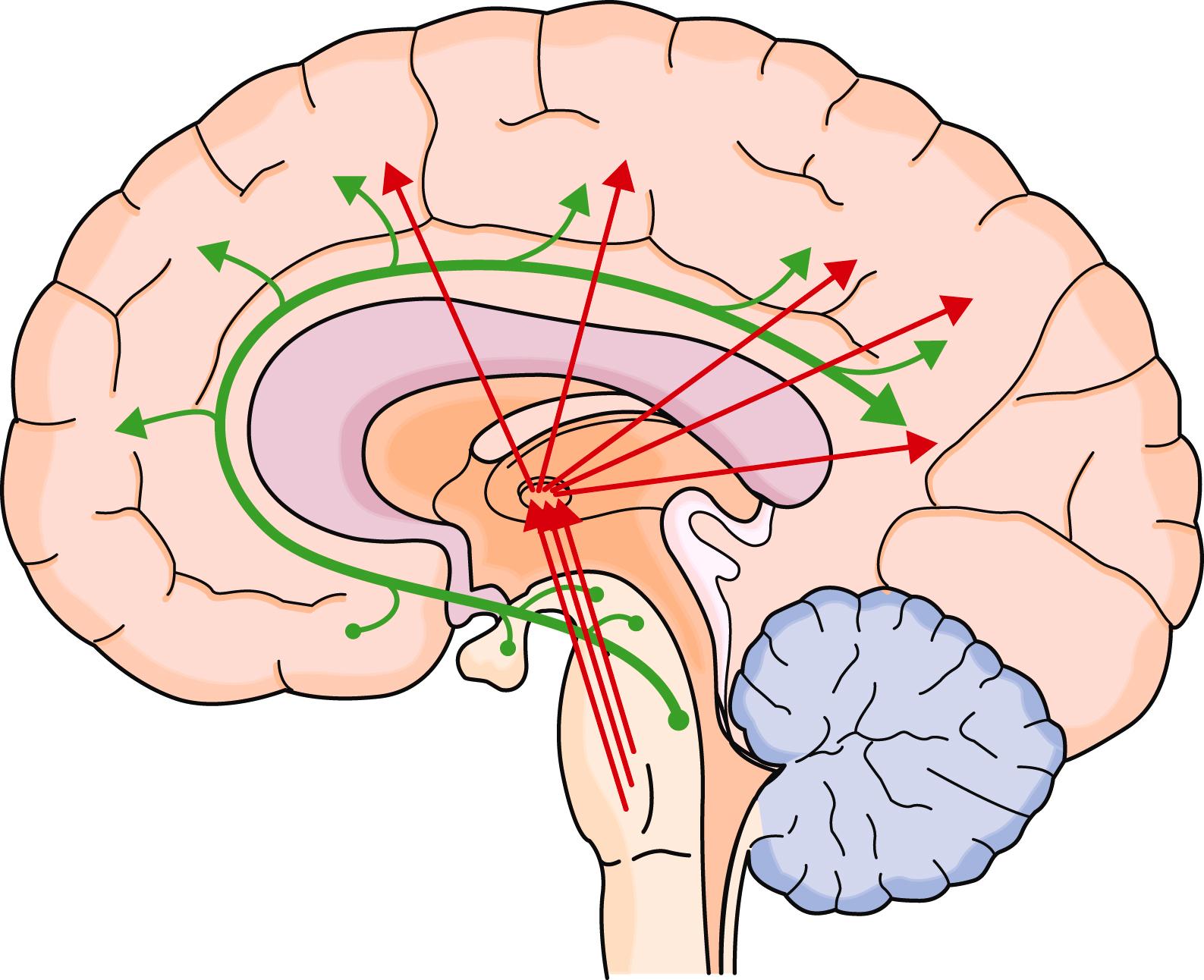
With the onset of sleep, information from two major drives (homeostatic, the chemicals that build during wakefulness; and circadian, the internal body clock) influence the ventral lateral preoptic nucleus to actively suppress the networks of wakefulness and allow the initiation of non-REM sleep ( E-Fig. 374-2 ). Non-REM sleep typically starts as stage N1, with slowing of the electroencephalogram (EEG), and slow eye movements ( Table 374-1 ). Stage N1, which is associated with the feeling of drowsiness, accounts for about 5% of nocturnal sleep time. Although minimal sensory processing can occur, memory is not typically stored. Blood pressure may decrease slightly, and breathing becomes more periodic. FLOAT NOT FOUND
| STAGE | EEG FINDINGS | EYE MOVEMENTS (EOG) | SUBMENTAL EMG | ASSOCIATED PHYSIOLOGY |
|---|---|---|---|---|
| Wakefulness (W) | More than 50% of an epoch has the posterior dominant rhythm present | Rapid eye movements to slow movements. Blinking may be present | Normal to high muscle tone | Memory registration, voluntary control over breathing |
| Stage N1 | Attenuation of the posterior dominant rhythm with mixed theta frequency low-amplitude background activity | Slow rolling eye movements | Variable but less than wake | Automatic behavior can occur; diminished cognitive processing, periodic breathing |
| Stage N2 | K-complexes and/or sleep spindles . Low-amplitude mixed-frequency EEG | No eye movements, but slow eye movements may persist | Variable amplitude, typically lower than W and higher than R | No memory, decreased arousal to stimuli, less response to elevated CO 2 and low oxygen |
| Stage N3 | Slow wave activity (0.5-2 Hz, >75 µV) for >20% of an epoch . Sleep spindles and K complexes may persist | No eye movements seen | Variable amplitude, typically lower than N2 and can be as low as R | No memory, rhythmic breathing pattern with less responsiveness to elevated CO 2 and low O 2 |
| Stage R (REM sleep) | Low-amplitude mixed-frequency EEG . Sawtooth waves | Rapid eye movements | Low muscle tone | Similar response to stimuli as light sleep, irregular breathing pattern, least response to elevated CO 2 and low O 2 |
∗ Sleep staging requirements. Boldfaced items are requirements for staging. Italicized items are nonrequired associated findings that may be present in that stage.
The hallmark of stage N2 sleep is an EEG with sleep spindles and K complexes. N2 typically represents about 50% of a night’s sleep for an adult. Although stage N2 is considered light sleep, it is associated with less responsiveness to stimuli and to changes in CO 2 and O 2 than stage N1. Stage N3 is characterized by slow waves (0.5 to 2.0 Hz, >75 µV) in the EEG. These slow waves are more prominent in brain areas that are more heavily used during the preceding awake period. In stage N3, which constitutes 20% of nocturnal sleep, the individual is difficult to arouse and has rhythmic breathing that is less responsive to elevated CO 2 and low O 2 than during stage N2. This stage is most associated with metabolic rebalancing of the neurons and glia, as well as the pruning of lesser used synapses.
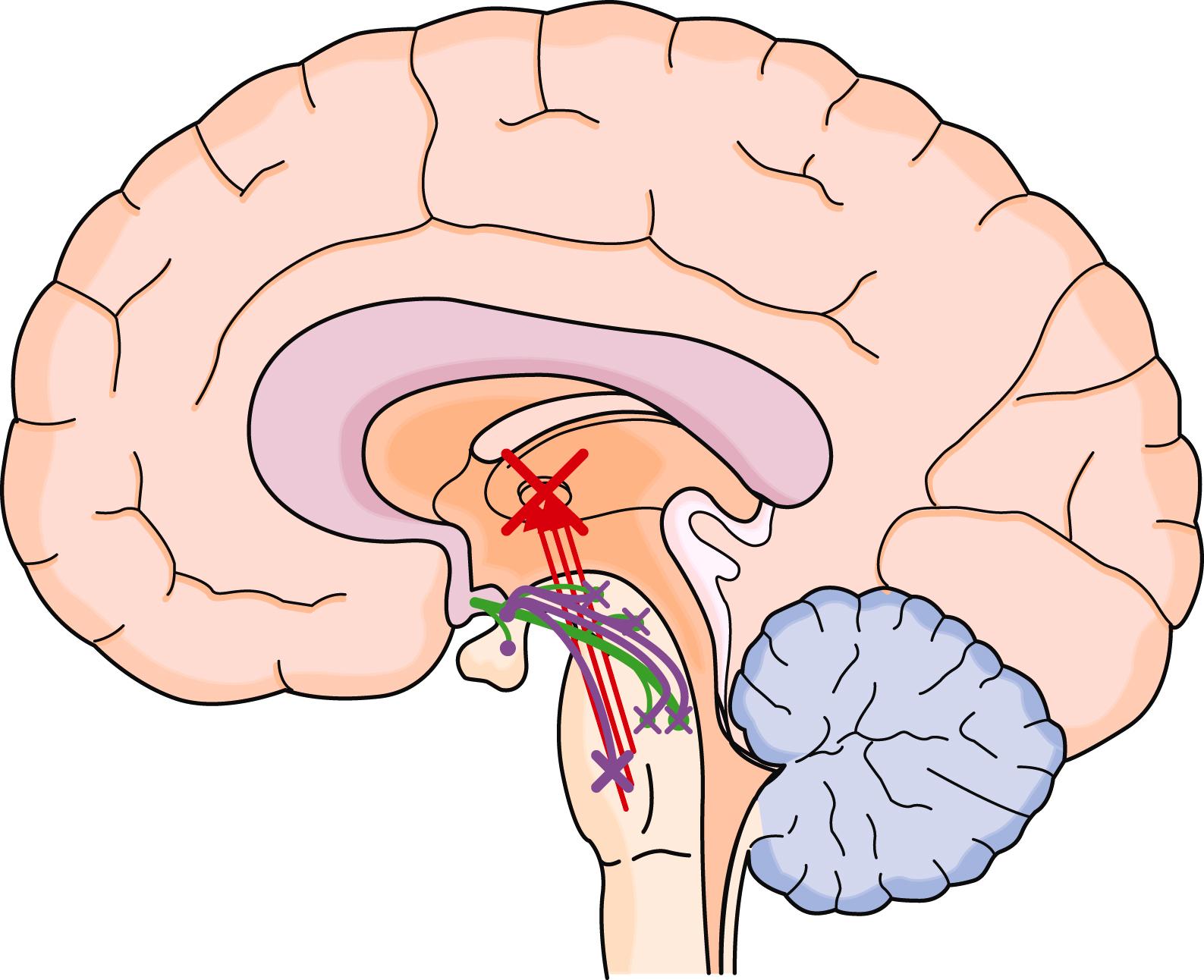
REM sleep (stage R) is generated by cholinergic neurons in the subcoeruleus nucleus in the brain stem, which then activate other neuronal groups to produce the associated rapid eye movements, active theta and alpha frequency EEG waveforms, skeletal muscle paralysis, and reduced temperature regulation. Most vivid dreaming occurs in stage R, but dreams can occur in other stages. Because most skeletal muscles, including accessory respiratory muscles, are paralyzed in stage R, ventilation is solely dependent on the diaphragm. This stage is associated with the least amount of responsiveness to low O 2 and elevated CO 2 . Stage R typically encompasses about 20% of the night’s sleep and is ended by activation of norepinephrine and serotonergic neurons ( E-Fig. 374-3 ). Age influences the distribution of sleep stages: REM sleep occupies 50% of sleep at birth and then gradually declines to 25% by age 3 years. Similarly, slow wave sleep is prominent in children and declines in men in their 30s and in women by age 40 to 50 years.
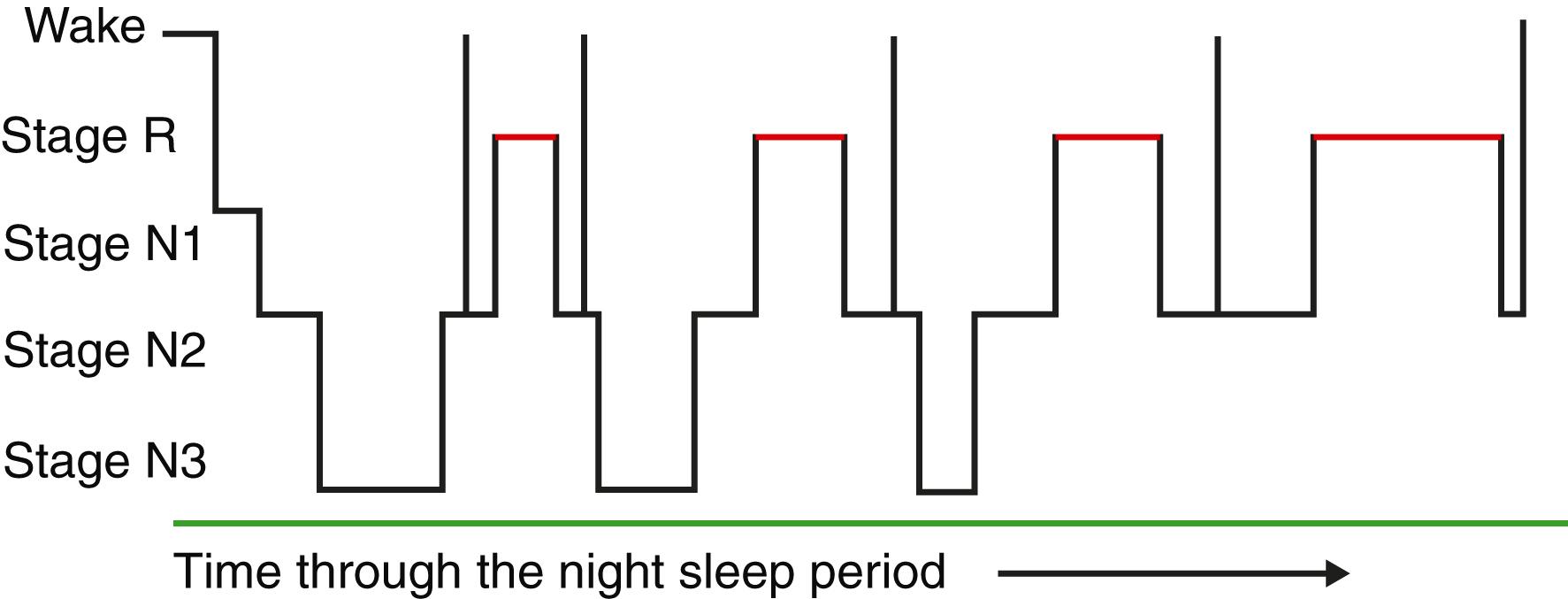
Sleep stages graphed through the night demonstrate the dynamic interplay of the various stages. As seen in an idealized hypnogram ( Fig. 374-1 ), sleep has repeating cycles of approximately 90 minutes. These cycles show a predominance of stage N3 in the first two cycles, and a gradual lengthening of the periods of stage R through the night. This cycling and progression of sleep stages appear to aid in the revitalization of the brain.
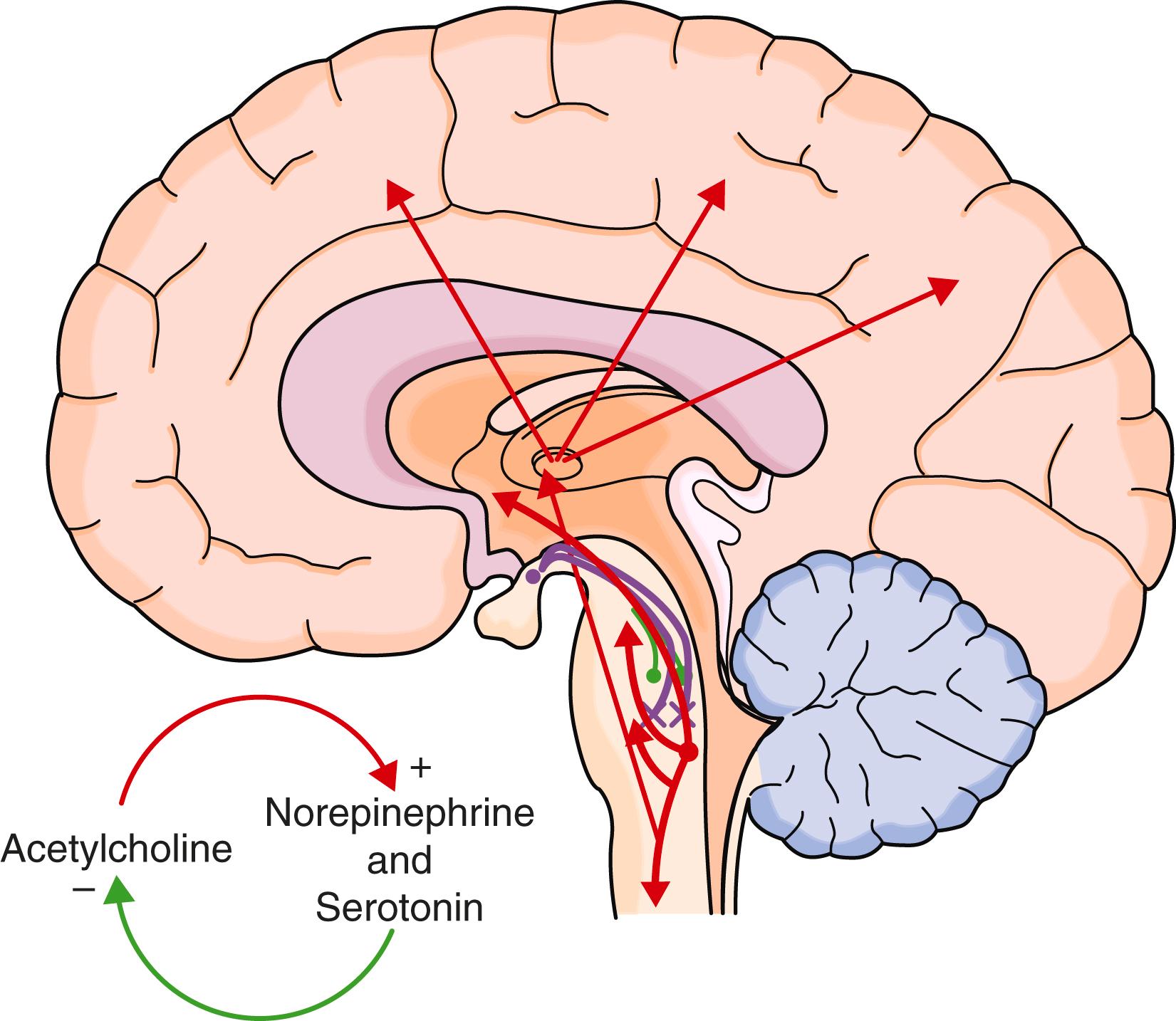
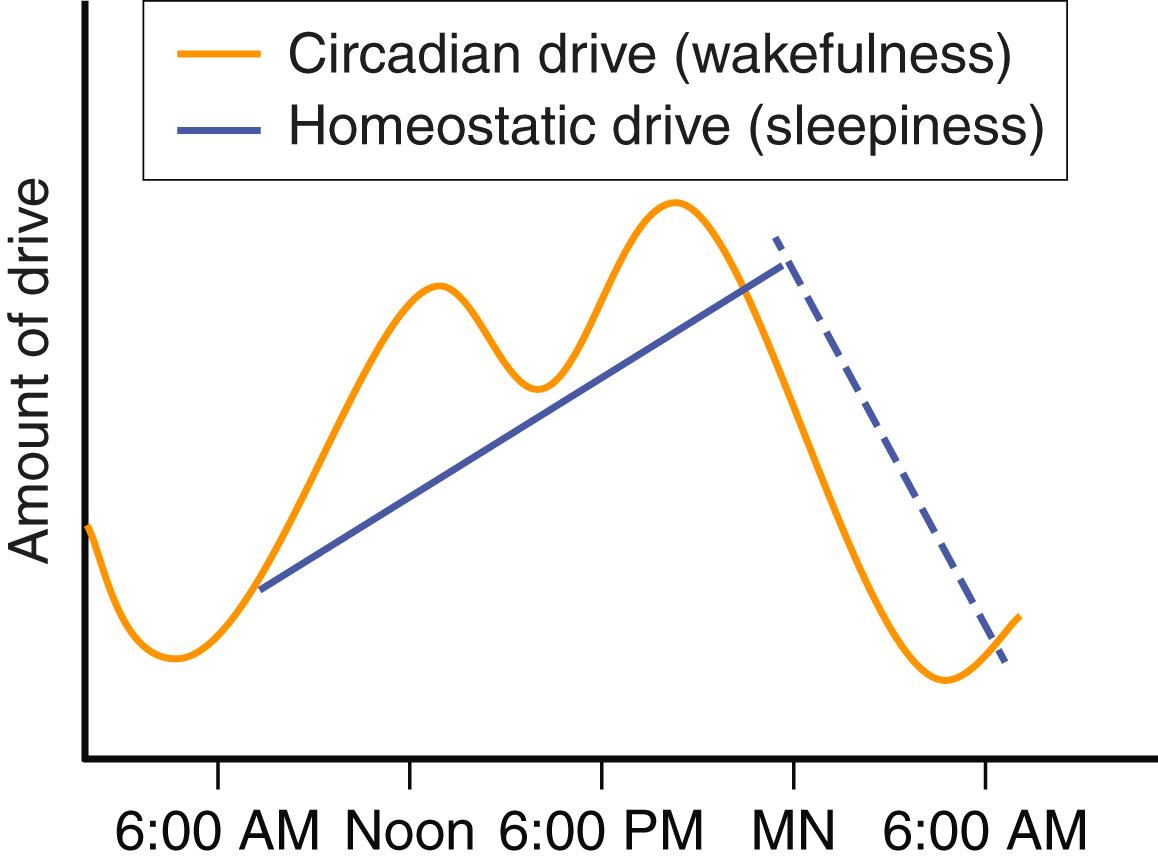
Many models that consider the array of neurochemical pathways that influence sleep can theoretically explain its physiologic regulation. The most accepted two-driver model uses the homeostatic and circadian drivers to explain sleep-wake state. Other issues such as psychological status also play a role. The homeostatic drive is the accumulation of substances that promote sleepiness and increase while the person is awake. Once asleep, these substances are metabolized. Mental and physical activities increase this drive by producing neuronal byproducts (e.g., adenosine), whereas caffeine blunts this drive by blocking adenosine. In contrast, the circadian rhythm drive promotes wakefulness and, through its predictable cycle, prepares the body for anticipated activities. The circadian rhythm is a naturally occurring rhythm that is slightly longer than 24 hours but is readjusted each day to maintain alignment with the natural day-night cycle. This cycle is driven by a genetic transcription-translation feedback loop that occurs in every cell of the body. These cells are synchronized by a master clock in the suprachiasmatic nucleus by complex chemical signaling, including through the hormone melatonin, which is released in response to darkness. The circadian rhythm is primarily adjusted by bright light and to a lesser extent by exercise, food, and social interactions. When the circadian rhythm is stronger than the homeostatic drive, the person is awake, and when the homeostatic drive is stronger than the circadian rhythm, the person is sleepy ( E-Fig. 374-4 ). This theoretical model helps explain aspects of sleep-wake regulation, such as the periods of postlunch sleepiness or evening wakefulness.
Most patients who seek medical help for sleep issues present with one of three complaints: (1) excessive sleepiness, (2) difficulty attaining or sustaining sleep, (3) or unusual events associated with sleep. Excessive sleepiness, which is the propensity to fall asleep, should be distinguished from fatigue or lack of energy. Insomnia, the difficulty initiating or maintaining sleep at night with resulting daytime sequelae, may be a clue to daytime issues, and nocturnal events may be a clue to possible neurologic issues. Other common presenting symptoms include morning headaches, lapses of attention, or diffuse muscle aches.
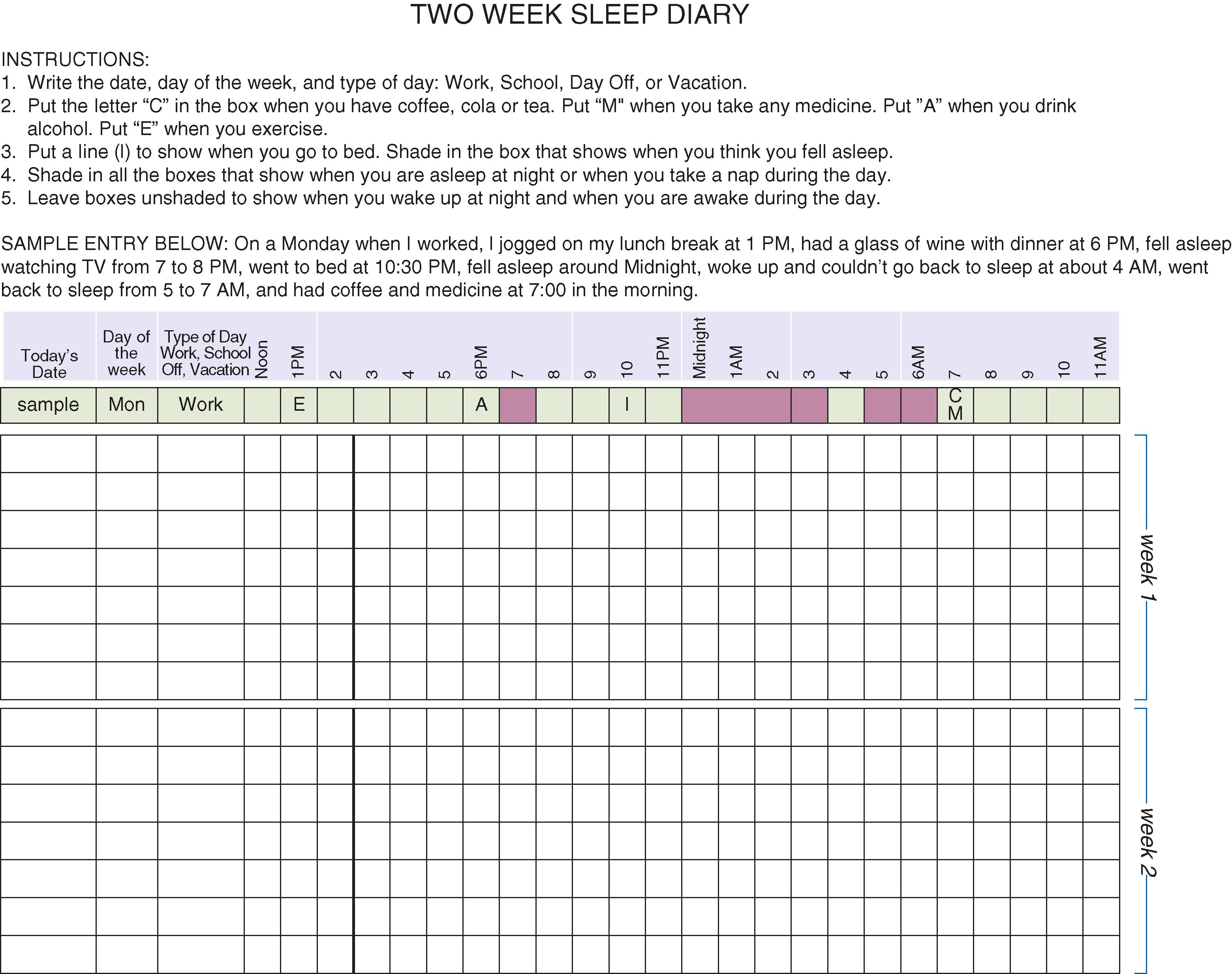
Both subjective information and objective tests are used to investigate sleep complaints. Questionnaires such as the Pittsburgh Sleep Quality Index provide a broad overview of sleep symptoms, including bedtime, wake time, activities, medications, and other substances that could influence sleep. Sleep diaries can also give a subjective report of the daily schedule ( Fig. 374-2 ).
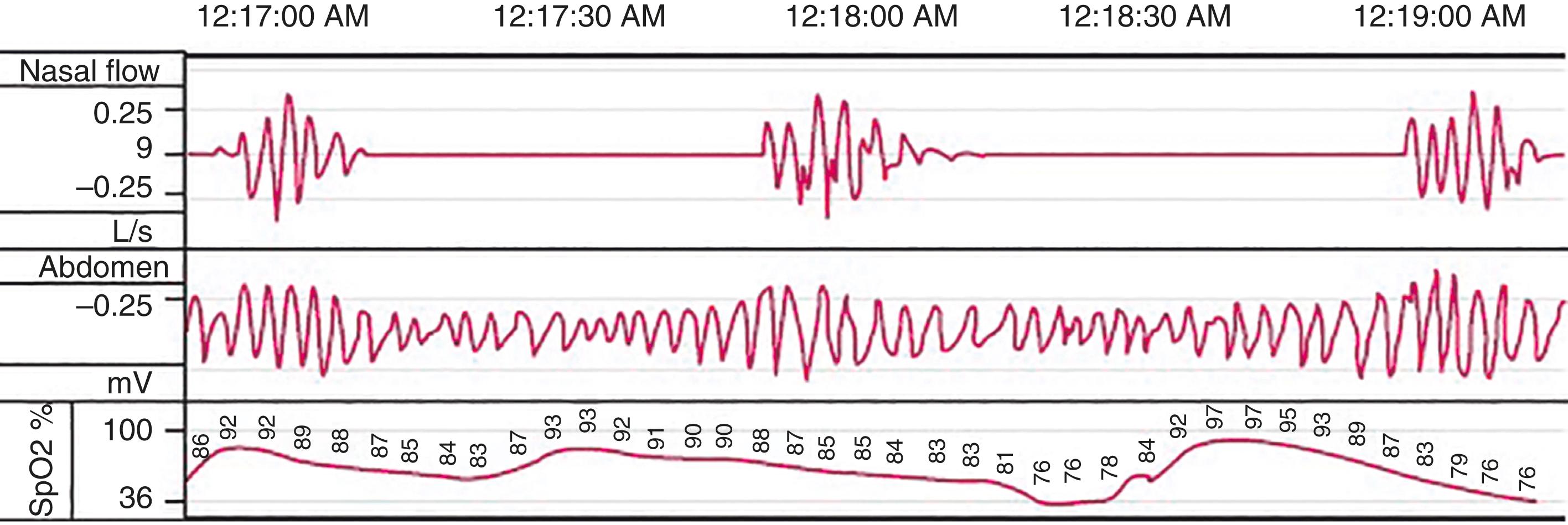
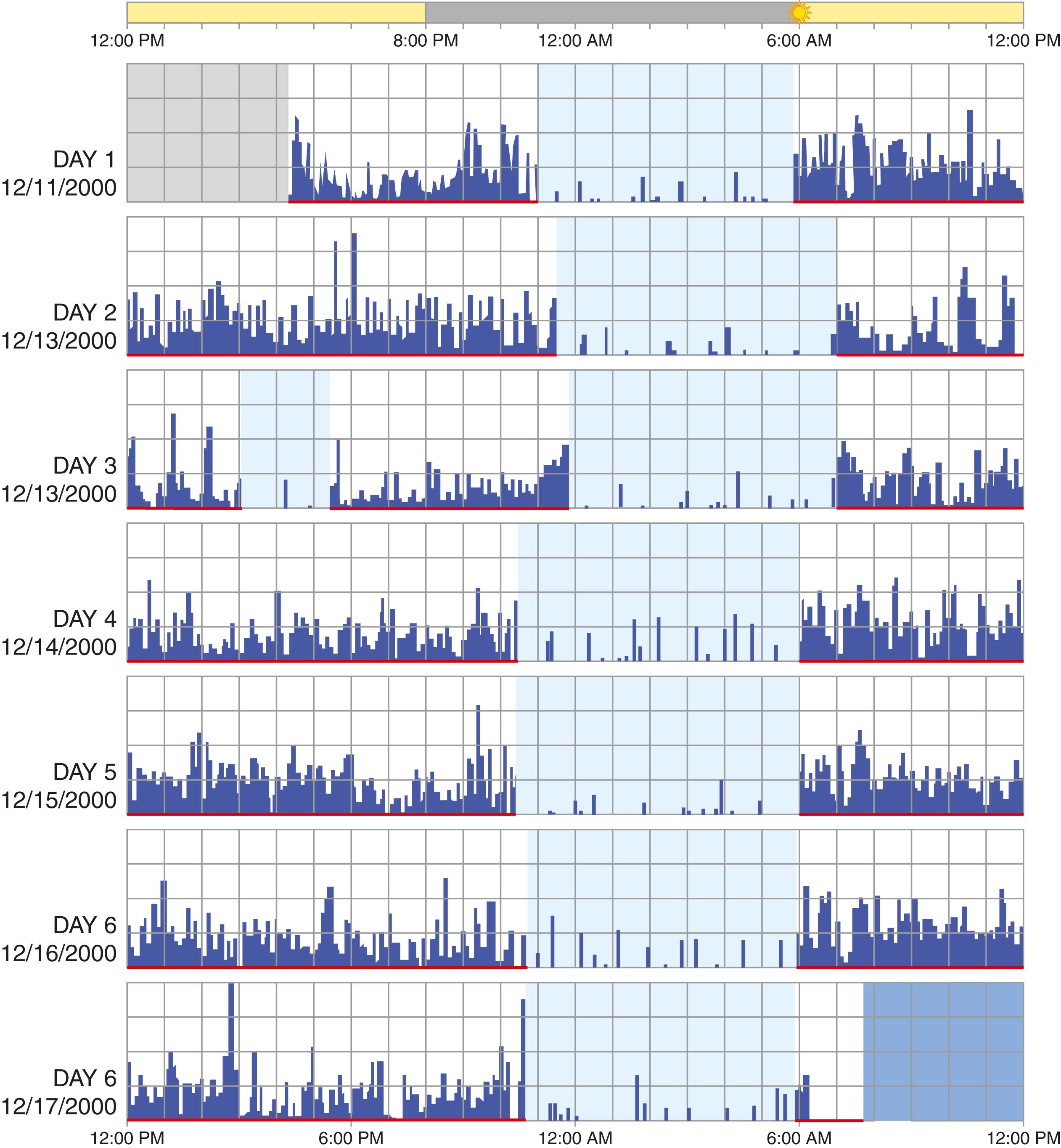
Objective testing of sleep includes polysomnography, actigraphy, multiple sleep latency testing, and maintenance of wakefulness testing. Polysomnography ( Fig. 374-3 ) assesses both sleep stage and associated physiology. Sleep stage is determined by three parameters: EEG, electro-oculogram, and submental electromyogram activity. Measures assessing associated physiology include respiratory function (airflow, effort, and gas exchange), limb muscle activity, and the electrocardiogram. Polysomnography, which assesses sleep-stage–associated physiology, is most useful for evaluating sleep disruption, including sleep apnea, excessive movements, and parasomnias ( Table 374-2 ). More limited overnight recordings may focus on strictly respiratory measurements. Actigraphy monitors movement, typically of a nondominant extremity, over 7 to 28 days to assess patterns of activity ( Fig. 374-4 ). When combined with a sleep diary, actigraphy estimates total sleep time and assesses the sleep-wake schedule. Many wearable and nonwearable consumer devices claim to provide an assessment of sleep, but these typically are much less accurate than medical grade devices and are not currently reliable as diagnostic tools.
| POLYSOMNOGRAPHY IS ROUTINELY INDICATED FOR: |
| Diagnosis of sleep-related breathing disorders, including suspected obstructive sleep apnea in patients with daytime sleepiness, unrefreshing sleep, or insomnia Patients with a sleep-related breathing disorder and heart, brain (stroke), neuromuscular, lung, or other major organ disease Positive airway pressure (PAP) titration in patients with sleep-related breathing disorders A preoperative clinical evaluation to evaluate for the presence of obstructive sleep apnea before upper airway surgery or oral appliance therapy Patients suspected of nocturnal movement disorders Patients suspected of narcolepsy or unexplained excessive daytime sleepiness may require polysomnography and multiple sleep latency test on the ensuing day Follow-up Polysomnography:
|
| POLYSOMNOGRAPHY IS NOT ROUTINELY INDICATED FOR: |
| Patients whose symptoms resolve with continuous positive airway pressure (CPAP) treatment Diagnosis of a sleep-related breathing disorder in a patient with chronic lung disease Diagnosis of typical, uncomplicated, and noninjurious parasomnias when the diagnosis is clearly delineated Patients with a seizure disorder who have no specific complaints consistent with a sleep disorder Diagnosis or treatment of restless legs syndrome, except where diagnostic uncertainty exists Establishing the diagnosis of depression Diagnosis of circadian rhythm sleep disorders |
Two daytime tests can help estimate the degree of physiologic sleepiness. The multiple sleep latency test quantifies objective sleepiness based on the time to onset of sleep across five daytime naps; it is validated for diagnosing narcolepsy when paired with a polysomnogram and clinical criteria. The maintenance of wakefulness test, which quantifies the propensity to stay awake across four 40-minute epochs, can provide objective evidence of a person’s ability to stay awake.
Sleepiness is normal just before a typical sleep period or after prolonged wakefulness. About 5 to 20% of adults have hypersomnia, in which sleepiness occurs in inappropriate settings and affects quality of life due to lapses of attention, decreased mood, or diminished cognitive abilities. After chronic sleep deprivation, the perception of sleepiness is reduced such that individuals become accustomed to their impairment and fail to recognize their degree of sleepiness.
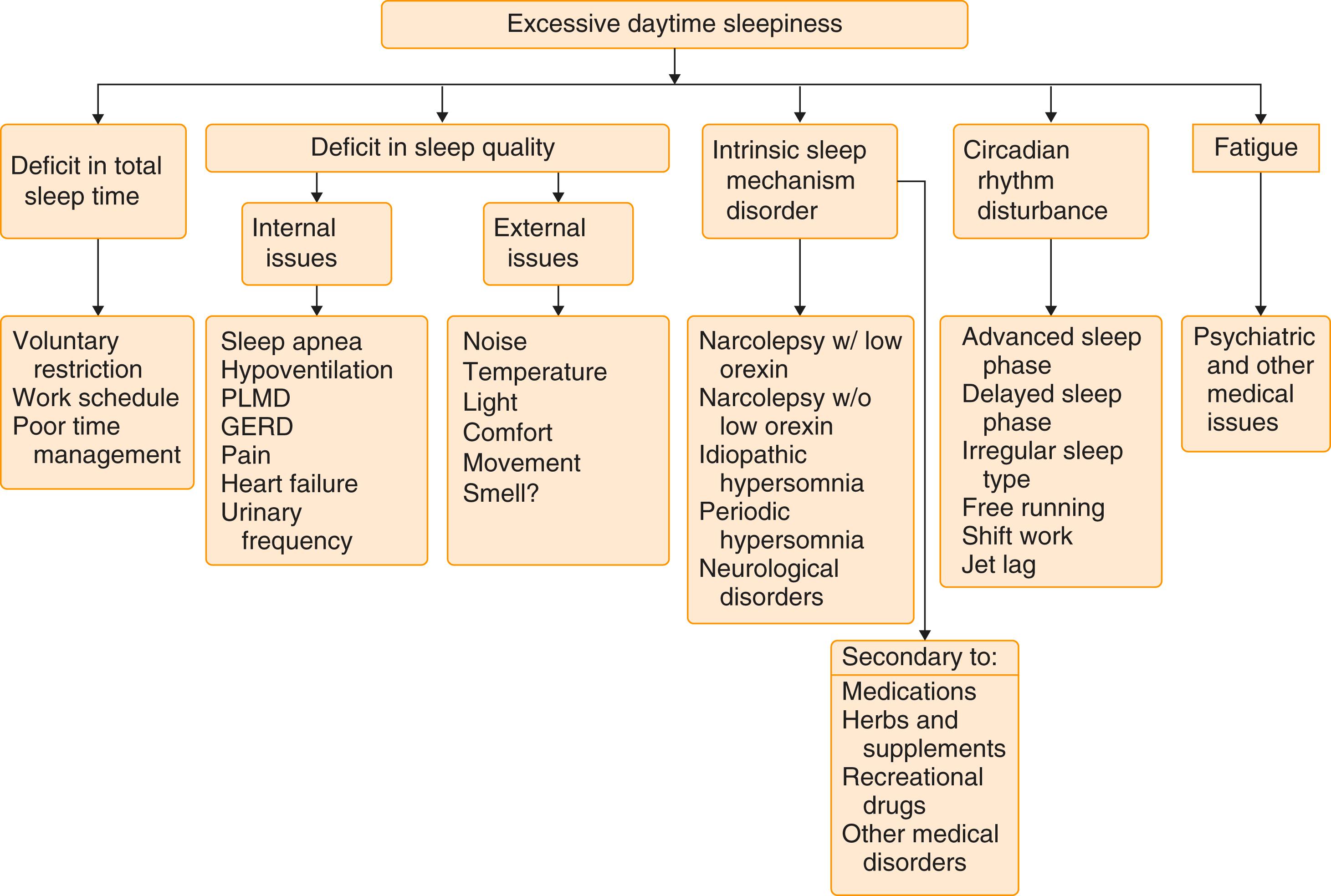
Clinicians should question hypersomnic patients for clues about sleep restriction, sleep disruption, neurologic issues, medication effect, or medical or psychiatric causes ( Fig. 374-5 ). Information regarding sleep habits, including week and weekend schedules, as well as environment, may disclose important contributing factors. Patients with sleep apnea, narcolepsy, excessive periodic limb movements, circadian rhythm disorders, and parasomnias may have excessive daytime sleepiness as their main complaint. A history of snoring, observed apnea, morning headaches, cataplexy, sleep paralysis, hypnogogic hallucinations, or altered sleep schedule suggests other specific sleep disorders. Excessive sleepiness can also result from many medical disorders and medications. Patients with heart ( Chapter 45 ), kidney ( Chapter 117 ), or liver failure ( Chapter 139 ), rheumatologic disease, or endocrinologic disorders such as hypothyroidism ( Chapter 207 ) and diabetes ( Chapter 210 ) may note sleepiness and fatigue. Neurologic disorders such as stroke ( Chapter 376 ), degenerative diseases ( Chapters 371 and 378 ), tumor ( Chapter 175 ), demyelinating disease ( Chapter 380 ), and head trauma ( Chapter 368 ) can cause excessive sleepiness.
Sleepiness can be quantified subjectively by questionnaires or by physiologic measures such as a multiple sleep latency test. The Epworth Sleepiness Scale quantifies sleepiness by asking the subject to rate on a scale of 0 to 3 (0, no chance; 3, high likelihood) the chance of dozing in eight situations ( Table 374-3 ). A score of 7 is considered average, whereas a score of 10 or more is consistent with subjective sleepiness. This score has a modest correlation with objective measures of sleepiness and better correlation with the severity of obstructive sleep apnea. Other objective tests, such as the multiple sleep latency test, give clues to the degree of sleepiness and possible sleep-onset REM, whereas the maintenance of wakefulness test gives a snapshot of the patient’s ability to stay awake.
| How likely are you to doze off or fall asleep in the following situations, in contrast to just feeling tired? This refers to your usual way of life in recent time. Even if you have not done some of these things recently, try to work out how they would have affected you. Use the following scale to choose the most appropriate number for each situation. | |
| 0 = would never doze 1 = slight chance of dozing 2 = moderate chance of dozing 3 = high chance of dozing |
|
| SITUATION | CHANCE OF DOZING |
| Sitting and reading Watching TV Sitting and inactive in a public place (theater or meeting) As a passenger in a car for an hour without a break Lying down to rest in the afternoon when circumstances permit Sitting and talking to someone Sitting quietly after lunch (without alcohol) In a car, while stopped for a few minutes in traffic Total |
_____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ |
Narcolepsy includes a tetrad of excessive sleepiness, cataplexy, sleep paralysis, and hypnogogic hallucinations. Narcolepsy has been divided into patients with low neurotransmitter hypocretin-1 (also known as orexin) or cataplexy (type 1), and patients without cataplexy or low hypocretin-1 (type 2).
Narcolepsy type 1 affects 1 in 2000 to 6000 individuals; 40 to 80% have the complete tetrad, and approximately 50% complain of sleep disruption. Over 90% of individuals in the United States with cataplexy have the HLA-DQB1∗0602 gene. Narcolepsy type 2 occurs in about 2 per 1000 individuals; approximately 40% have the HLA-DQB1∗0602 gene, and some exhibit intermediate cerebrospinal fluid (CSF) hypocretin-1 levels. Despite the connection to a gene, the risk to first-degree relatives is only 1 to 2%, or about a 10- to 50-fold increased risk compared with the general population.
Narcolepsy type 1 reflects the loss of hypocretin-producing neurons in the lateral hypothalamus. This neurotransmitter is important for stabilizing the sleep-wake state and for motor control. Thus the manifestations of the disease are related to frequent stage shifts and intrusion of fragments of REM sleep into wakefulness. An immune mechanism is postulated, potentially through a T-cell–mediated response and the loss of orexin-producing neurons. Narcolepsy type 2 appears to be a more diverse group of mechanisms that most likely do not share a common pathophysiology.
The tetrad of excessive sleepiness, cataplexy, hypnogogic hallucinations, and sleep paralysis is the major clinical manifestation. Cataplexy is abrupt loss of muscle tone triggered by strong emotional stimuli such as laughter, surprise, or anger. Patients are aware of their surroundings but lose muscle control, first in the face and neck, followed by the arms and then the trunk and legs. Hypnogogic (sleep-onset) and hypnopompic (sleep-offset) hallucinations are vivid and often frightening visual or auditory events. Sleep paralysis is an inability to move or speak, typically during the transition out of sleep when individuals have complete or partial awareness of their surroundings. Patients may describe a strong feeling of impending doom or having to escape imminent danger. Patients with narcolepsy often feel brief naps are refreshing. Frequently, their sleep is fragmented, with sleep intruding into daily activities and wakefulness interrupting nighttime sleep. Sleep paralysis and hypnogogic hallucinations can occur in normal individuals, especially after sleep deprivation, but cataplexy is virtually pathognomonic for narcolepsy.
The diagnosis of narcolepsy type 1 and type 2 is based on a mean sleep latency of less than 8 minutes and the presence of REM sleep on at least two of the five naps of a multiple sleep latency test. The multiple sleep latency test is predicated on the documentation of at least 6 hours of sleep before the study. The previous night’s polysomnography must also not show other sleep pathologies. Low CSF hypocretin level in the setting of excessive sleepiness can also confirm of the diagnosis of narcolepsy type 1.
Treatment of narcolepsy focuses on improving symptoms of excessive sleepiness, cataplexy, and REM sleep intrusion into wakefulness ( Table 374-4 ). Narcolepsy requires a three-pronged approach of improving the quality of sleep, prescribing stimulants, and controlling cataplexy. Nighttime sleep may be improved with sodium oxybate or the mixed salt oxybate (both at 4.5 to 9.0 g in divided nighttime doses or as the single-dose formulation), which improves daytime alertness and reduces cataplexy. , Planned naps may help some patients, and stimulants such as modafinil (100 to 600 mg/day), armodafinil (50 to 250 mg/day), methylphenidate (5 to 60 mg/day), and dextroamphetamine (5 to 60 mg/day) can improve but do not eliminate daytime sleepiness. Solriamfetol (a selective dopamine and norepinephrine inhibitor at 75 to 150 mg daily) and pitolisant (a central histamine H 3 receptor inverse agonist, starting at 8.9 mg and increasing to 17.8 to 35.6 mg daily) also promote wakefulness. To avoid further sleep disruption, patients should not use stimulants in the evening and night hours. Selective serotonin reuptake inhibitors (SSRIs) ( Chapter 362 , Table 362-5 ) and combined serotonin-norepinephrine reuptake inhibitors (SNRIs) ( Chapter 362 , Table 362-5 ) also reduce cataplexy, as well as sleep paralysis and hallucinations.
| MODALITY | STARTING DOSE | HIGHEST DAILY DOSE | DOSE AT |
|---|---|---|---|
| Scheduled naps | 10-15 minutes | 1-3 naps | Just before time needing to be awake |
| OVER-THE-COUNTER STIMULANT | |||
| Caffeine | 25 mg | 300 mg | am |
| WAKE PROMOTING | |||
| Modafinil | 100-200 mg | 400 mg | am and noon |
| Armodafinil | 50-150 mg | 250 mg | am |
| Methylphenidate | 5-10 mg | 120 mg | am and noon |
| Methylphenidate ER | 10-20 mg | 120 mg | am |
| Dextroamphetamine | 5-10 mg | 60 mg | am and noon |
| Combination dextroamphetamine/amphetamine | 5-10 mg | 60 mg | am and noon |
| Pitolisant | 8.9 mg | 35.6 mg | am |
| Solriamfetol | 37.5-75 mg | 150 mg | am |
| IMPROVE NIGHTTIME SLEEP, DAYTIME ALERTNESS, AND CATAPLEXY | |||
| Sodium oxybate | 2.25 g | 9 g | Bedtime and 4 hr into sleep |
| Calcium, magnesium, potassium, sodium oxybate | 2.25 g | 9 g | Bedtime and 4 hr into sleep |
| Once nightly sodium oxybate | 4.5 g | 9 g | Not currently FDA-approved |
| THERAPIES FOR CATAPLEXY (NOT FDA APPROVED) | |||
| Fluoxetine | 10-20 mg | 40 mg | am |
| Venlafaxine | 75 mg | 225 mg | am at lower dose or divided |
| Protriptyline | 5 mg | 30 mg | am at lower dose or divided |
Narcolepsy is a lifelong disorder. Patients who present in adolescence or young adulthood may progress to more severe symptoms, but the disorder does not affect longevity.
Idiopathic hypersomnia is a disorder in which hypersomnia cannot be explained by another disorder, is characterized by unrelenting hypersomnia, and is only minimally improved with therapy. Patients find that their symptoms persist despite sleep periods of longer than 10 to 12 hours. These patients have average sleep latencies of less than 8 minutes, typically do not display REM sleep, but may have stage N3 on their multiple sleep latency test studies. Periodic hypersomnia can also occur in in Kleine-Levin syndrome (a syndrome of periodic hypersomnia, hyperphagia, and hypersexuality) and in perimenstrual hypersomnia.
Treatment of sleepiness should focus on correcting the underlying cause. One early step is to extend the total sleep time for at least a 3-week period to see if sleep deprivation plays a role. Stimulants such as modafinil (100 to 400 mg) should be tried in individuals who are impaired by the symptoms and in whom other therapies have failed to correct the hypersomnia. In some patients, sodium oxybate can improve daytime symptoms. Some patients with Kleine-Levin syndrome respond to lithium.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here