Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Proper selection of the surgical approach requires thorough evaluation to meet the surgical goals, while attempting to minimize injury to adjacent neurovascular structures.
A multidisciplinary team is helpful in the management of skull base tumors, including an otolaryngologist, neurosurgeon, head and neck or neuroradiologist, neuropathologist, radiation oncologist, and medical oncologist.
The nasoseptal flap is a popular method of reconstruction that can provide reliable closure for large, complex defects.
If there is a CSF leak following a repair, consider conservative management using a lumbar drain if the leak is small and the repair is sound . In addition, recognize the potential need for early intervention for continued leaks due to risk of postoperative meningitis. Exercise caution with lumbar drains in some cases due to risk of tension pneumocephalus and active CSF leaks with inadequate repairs.
Clinically significant pneumocephalus should address closure of the skull base defect. More aggressive evacuation is sometimes necessary to prevent major neurologic compromise.
Endoscopic skull base surgery has a learning curve for both the otolaryngologist and neurosurgeon. It is best to begin with simple cases or those in which the endoscope is used as an adjunct to an open approach.
CN VI is the most medial nerve in the cavernous sinus and the most commonly injured or affected by sinus pathology.
CNs III, IV, V1, V2, VI, internal carotid artery, and venous channels are present in the cavernous sinus.
The Vidian nerve consists of parasympathetic and sympathetic fibers from the greater petrosal nerve and deep petrosal nerve. The canal lies medial to the foramen rotundum along the greater wing of the sphenoid sinus. The maxillary branch of the trigeminal (V2) nerve runs through the foramen rotundum.
CSF production is approximately 20 mL/hour.
The crista ethmoidalis is a reliable landmark for endoscopically locating the sphenopalatine artery.
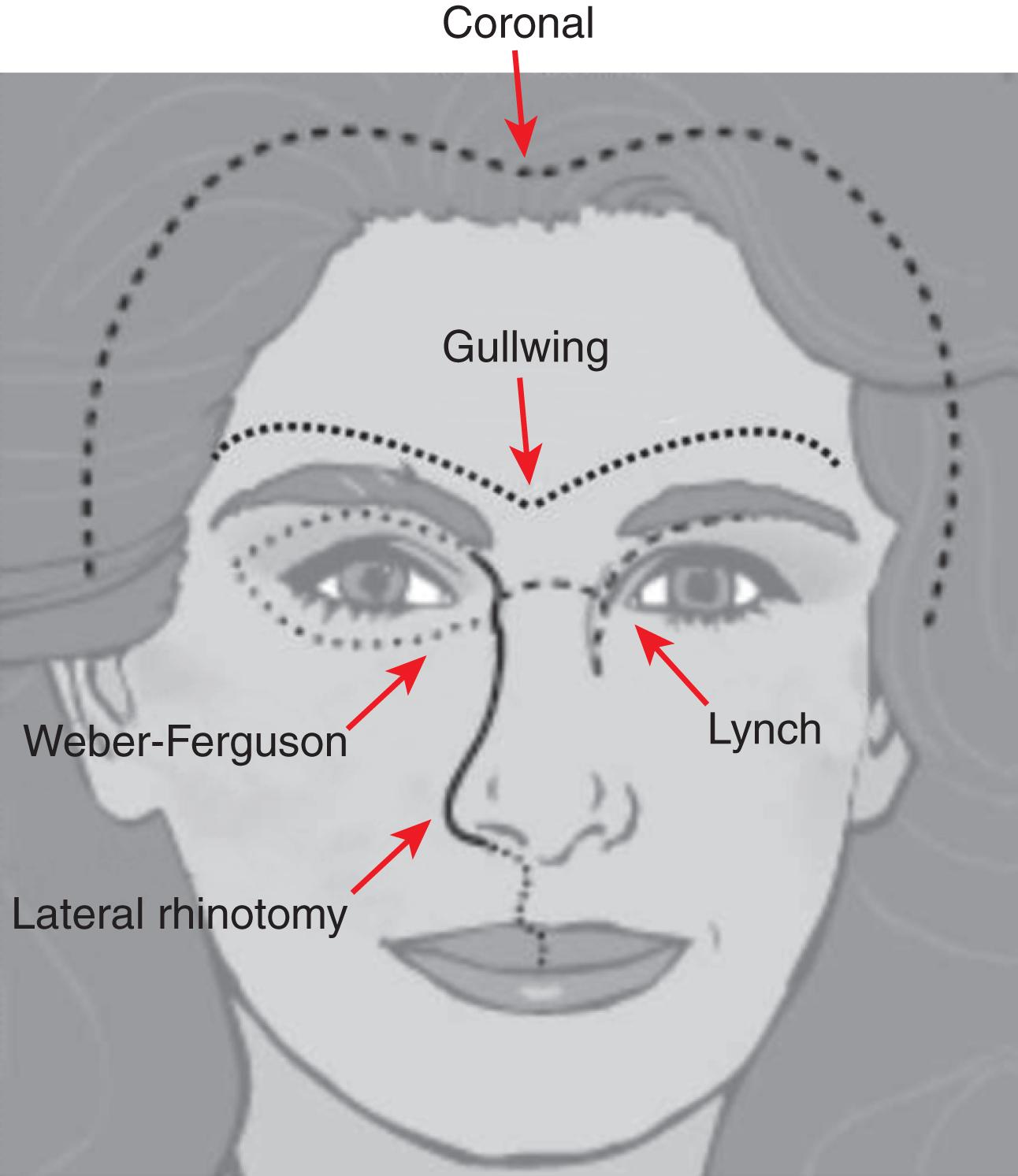
A Weber-Ferguson incision consists of a lip-splitting extension of a lateral rhinotomy and a subciliary incision extended to the lateral canthus.
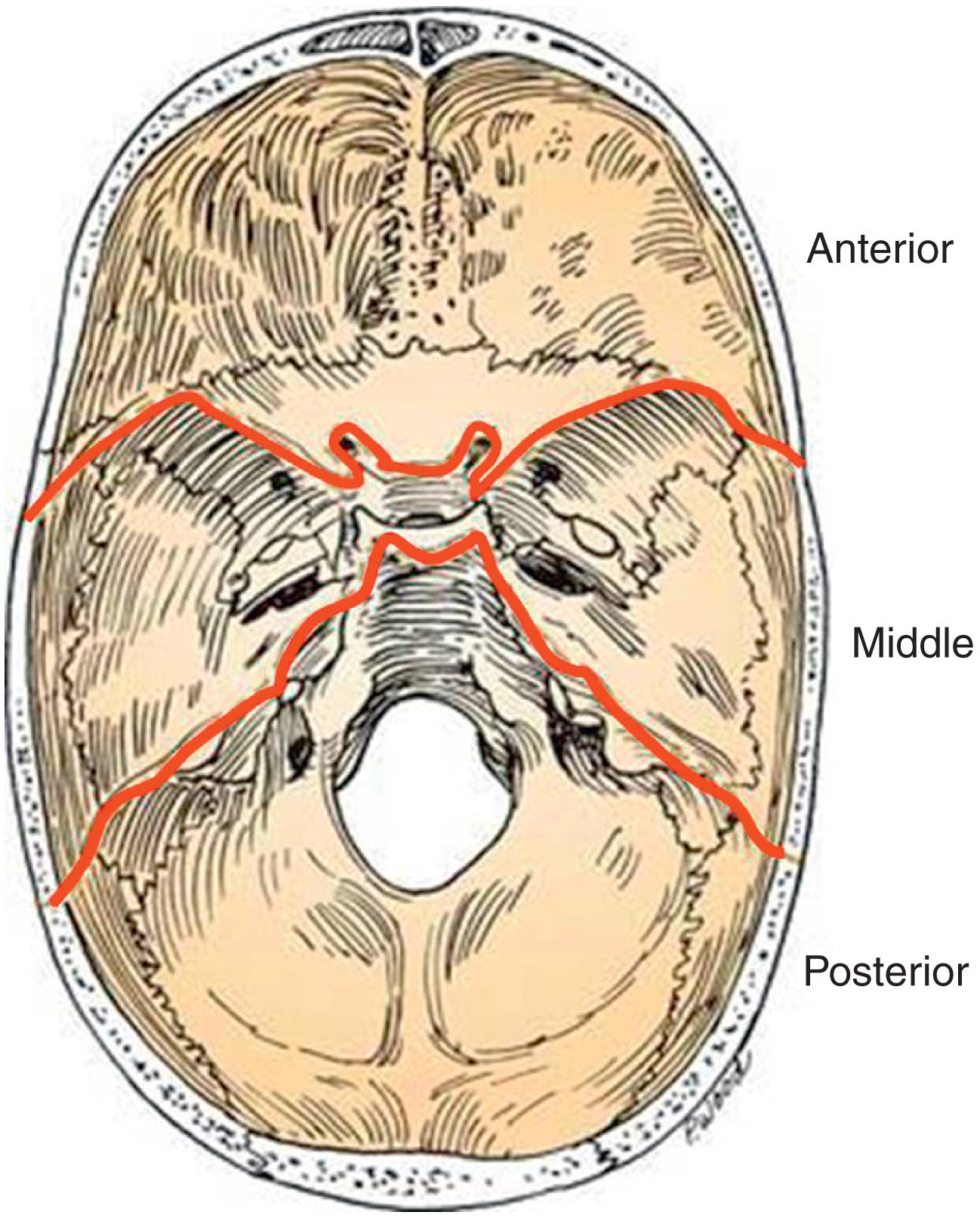
The skull base is separated into three compartments, or fossae: anterior, middle, and posterior. The anterior compartment extends from the frontal sinus to the anterior clinoid process and planum sphenoidale (sphenoid roof). The middle cranial fossa extends from the greater wing of the sphenoid to the clivus, including the sella turcica. The posterior fossa consists of the occiput and begins from the basal aspect of the occipital bone ( Fig. 20.1 ).
Benign sinonasal pathologies include inverted papilloma, juvenile nasal angiofibroma, fibrous dysplasia, and osteoma. Common intracranial skull base tumors include pituitary tumor, craniopharyngioma, and meningioma. Sinonasal malignancies include olfactory neuroblastoma (esthesioneuroblastoma), sinonasal undifferentiated carcinoma (SNUC), squamous cell carcinoma, adenocarcinoma, and melanoma.
The skull base can be accessed using open, endoscopic, microscopic, or combined open-endoscopic approaches. The goal of any approach is to provide the best visualization and exposure to surrounding neurovascular structures and the tumors.
See Table 20.1 .
| Anterior Skull Base |
| Subfrontal approach |
| Transfrontal sinus – osteoplastic flap |
| Frontotemporal – orbitozygomatic |
| Transmaxillary sinus |
| Transfacial |
| LeFort osteotomy |
| Facial translocation |
| Lateral infratemporal fossa approach Fisch type approaches Type A: Anterior transposition of CN VII Type B: Sigmoid to petrous tip Type C: Extended approach to include cavernous sinus |
| Middle Skull Base |
| In addition to the anterior skull base approaches: |
| Transoral |
| Transseptal |
| Palatal split |
| Mandibular split |
| Middle cranial fossa subtemporal |
| Posterior Skull Base |
| Transoral approach |
| Palatal split |
| Translabyrinthine approach |
| Retrosigmoid |
| Suboccipital |
Open approaches to the anterior fossa may use the coronal, lid crease, brow, gullwing, Lynch (medial orbital), lateral rhinotomy, Weber-Ferguson, or midface degloving incisions ( Fig. 20.2 ).
For surgical planning, basic laboratory studies including CBC, BMP, and coagulation studies should be performed, as well as imaging of the primary site (fine-cut CT and MRI). Other data may be obtained depending on tumor type (e.g., for pituitary tumor: ACTH, PRL, LH/FSH, GH, TRH) and location (arteriography). If indicated, a thorough evaluation for distant metastases should be performed.
Fine-cut CT scans (at least 1-mm section) should be performed for evaluation of bony anatomy of the skull base; the use of contrast may be helpful in defining the lesion and nearby vasculature. MRI with and without contrast is useful to delineate the lesion from surrounding structures, intracranial evaluation, help create a radiologic differential diagnosis, and evaluate for soft tissue invasion and perineural spread. CT, MR, or fused CT-MR images are helpful for intraoperative image guidance. PET scans can be used to rule out distant metastases.
Endoscopic skull base surgery is an extension of endoscopic sinus surgery. A rigid endoscope is used to improve visualization and illumination and may obviate the need for facial incisions such as a lateral rhinotomy. Recent reports have demonstrated that endoscopic resections of skull base tumors may yield similar oncologic results to traditional open approaches in appropriately selected cases.
Indications for endoscopic transnasal skull base surgery are increasing. The technique is utilized in pathology from the frontal sinus, entirety of the anterior skull base, much of the middle cranial fossa, and portions of the posterior fossa. ESBS performed through small incisions also allows minimally invasive lateral and neurosurgical approaches.
Compared to open surgery, the endoscopic approach allows for a more direct visualization with less manipulation of the surrounding soft tissues. This can allow for a more precise resection of the lesion due to better visualization for more posterior lesions and minimal manipulation of nearby neurovascular structures. Compared to the traditional microscopic view, endoscopes give a dynamic operative view with the added ability to see around corners using angled endoscopes. ESBS can avoid external incision, decrease hospital stays, and cause less postoperative pain.
Not all areas of the skull base can be visualized and safely instrumented via a transnasal endoscopic route. Malignancies involving the orbit, lateral frontal sinus, and facial skin are considered contraindications to endoscopic skull base surgery. As a general rule, the endoscopic approach should not compromise the ability to achieve appropriate oncologic resection, and crossing major neurovascular structures is not advised.
The primary modality of treatment for the majority of skull base malignancies is surgical resection. There are some exceptions to the rule (e.g., lymphoma and plasmacytoma). Adjuvant radiotherapy is considered when there is a high propensity for tumor recurrence, which includes presence of perineural invasion, high-grade tumor, and close or positive margins. Chemotherapy can be considered for induction therapy and metastatic disease or may be used as adjuvant therapy for chemosensitive histology. Radiation therapy may have a role in the management of certain benign skull base pathologies, such as meningioma, schwannoma, vascular tumors, and chordoma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here