Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Basic Principles
Anatomy
Physiologic Considerations
Technical Considerations
Post-Operative Care
From Neligan PC, et al: Plastic Surgery, 3rd edition (Saunders 2012)
Hair follicles, sweat glands, and dermal nerves can often be transferred within thick, split-thickness skin grafts (STSGs) and full-thickness skin grafts ( Figure 87-1-1 ). Thin STSGs will not allow the transfer of hair or other adnexal glands, as the regenerating bulb is not harvested. Hair regrowth can occur in STSGs but, due to the shallow depth of harvest, is rather unlikely. Full-thickness and composite grafts will show hair regrowth 2–3 months after grafting.
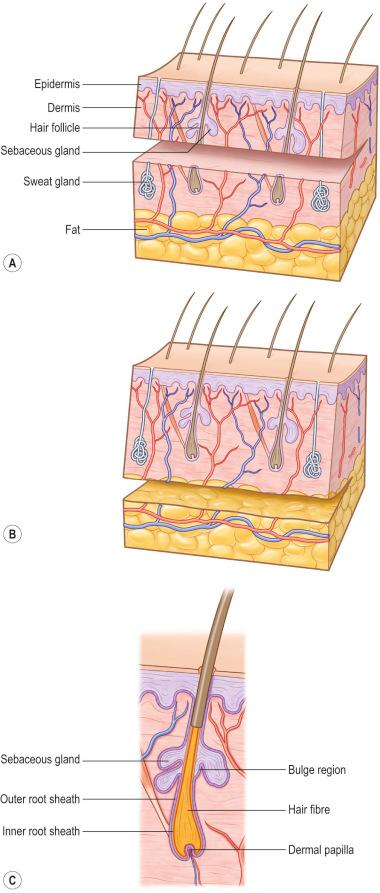
It is still unclear how nerves regrow into the skin graft. Studies demonstrated that recipient nerves use the basal lamina infrastructure of degenerated blood vessels and Schwann cells of donor nerves to grow. Although histological images reveal similar neural structures between healthy skin and integrated skin grafts, patients report abnormal sensation, including hypersensitivity and pain, up to 1 year. Usually patients regain sensitivity of the grafted area after 1 year, but the result is not completely normal.
Neural reconnection to sweat glands will reactivate their function up to 3 months after grafting. For this reason, moisturizing of the skin graft is advised for at least 3 months to avoid dryness.
Full-thickness skin grafts include skin appendages that can survive and be functional at the recipient side, while STSGs do not contain the deep structure skin appendages and remain without glandular function or hair growth.
Skin wounds that extend into the deep dermis heal through the mechanisms of scarring and wound contraction. For large wounds this process leaves the body at risk of infection and when it occurs around joints, this can lead to significant scar contractures that affect functionality. For example, in anterior neck wounds, the contractile processes can be so strong that, over time, the chin can be fixed to the chest, often with a thick scar. Also, wounds that are left open for months to years can degenerate into skin cancer (Marjolin's ulcer). For these reasons, methods that rapidly facilitate wound coverage or resurfacing are desired.
Skin grafting is still the gold standard to cover large areas of skin loss. The concept is to take skin from an area where the donor site will heal with minimal scarring and transplant it to an area of need. As skin grafting always leaves some sort of scar, donor site considerations are important when balancing the needs of the recipient site for a given skin graft.
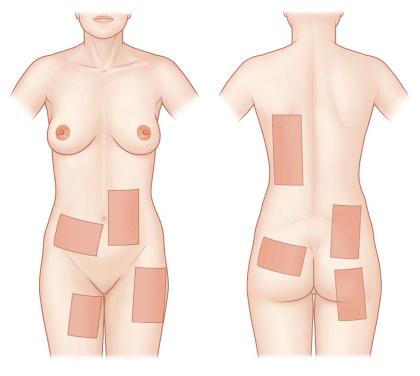
Skin grafts can be of different origin ( Table 87-1-1 ), from different anatomical sites ( Figure 87-1-2 ), and can be harvested in different thicknesses ( Table 87-1-2 ). Depending on the histological level of the graft the skin graft type is classified by thin and thick STSGs, full-thickness skin grafts, and composite grafts ( Figure 87-1-1 ). Skin grafts are further classified according to their thickness into thin (0.15–0.3 mm, Thiersch–Ollier), intermediate (0.3–0.45 mm, Blair–Brown), and thick (0.45–0.6 mm, Padgett). Skin grafts thicker than 0.6 mm usually correspond to full-thickness skin grafts and are called Wolfe–Krause grafts.
| Graft Type | Graft Origin: Donor and Recipient of: |
|---|---|
| Autograft | Same subject |
| Homograft | |
| Isograft |
|
| Allograft |
|
| Hetero- or xenograft | Different species |
| Indications | Advantages | Disadvantages | |
|---|---|---|---|
| Thin STSG |
|
|
|
| Thick STSG |
Same indications as thin STSG |
|
Slower donor site re-epithelialization |
| FTSG |
|
|
|
The thickness of the dermal layer classifies the STSG as either thin or thick. An STSG consists of epidermis and a variable amount of superficial to profound (papillary) dermis. As the dermis is responsible for the viscoelastic property of the skin, it is crucial for stability of the future skin. The amount of dermis grafted is key to the outcome: body areas with high mechanical friction are ideally grafted with thicker dermal layers. If donor sites do not allow this, skin quality can be improved with a combination of skin and dermal substitute grafts.
Thin STSGs include the epidermis and a thin layer of the dermis ( Figure 87-1-1 ). STSGs are commonly taken from the lateral thighs and trunk ( Figure 87-1-2 ). They do not include the full length of appendages and are therefore unlikely to grow hair or to develop full sweat gland function. The main advantage of thin STSGs is the reduced morbidity of the donor site and the possibility of performing multiple harvests from the same donor area about 2 weeks after the previous harvest. Although thinner grafts allow for more frequent reharvest, they result in additional wound contraction. The clinician must weigh the advantages and disadvantages of these conflicting goals when deciding the thickness of the graft.
Thick STSGs include more dermis with a greater number of full hair follicles and glandular structures ( Figure 87-1-1 ). These grafts will likely develop some hair growth and sweat gland function about 2–3 months after grafting. Thick STSGs are commonly selected to cover areas of high mechanical friction, such as joints, plantar soles, and the palm. Since hair regrowth is common in thick STSGs, the donor site should be carefully chosen to avoid unpleasant hair growth. The sensitivity and function of sweat glands are often better in thick than in thin STSGs. Because of decreased nutrient diffusion, thick grafts require a better recipient wound than thin grafts during the revascularization process. Therefore, thick grafts should be avoided in unhealthy wound beds such as in chronic ulcers. The donor site usually heals with more obvious scarring and discoloration but less graft contraction.
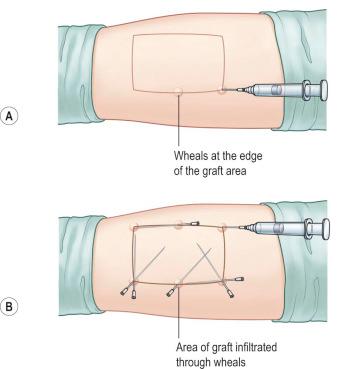
To reduce bleeding during skin harvest some surgeons prefer to infiltrate the donor site area primarily with epinephrine diluted in saline subcutaneously (diluted technique: Figure 87-1-3 ). If small to medium-sized areas of skin are needed and a dermatome is not available, surgeons can skillfully take skin grafts with a surgical knife or with the oscillating Gulian knife ( Figure 87-1-4 ). The disadvantage of skin grafts taken manually is the difficulty of achieving uniform depth that can result in aesthetically unpleasant donor and recipient site skin pattern. To increase the uniformity and expedite the harvest of skin grafts, a number of electrical or air-powered dermatomes have been designed to take uniform small to large skin grafts. Powered dermatomes have adjustable guards to set the graft thickness. There is a fair amount of variability in thickness depending on how hard the operator pushes on the dermatome. Drum dermatomes are precision instruments that can take large graft areas reliably ( Figure 87-1-5 ). They require placement of adhesive on the skin and an oscillatory movement by the operator.
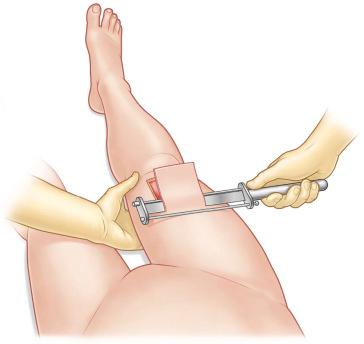
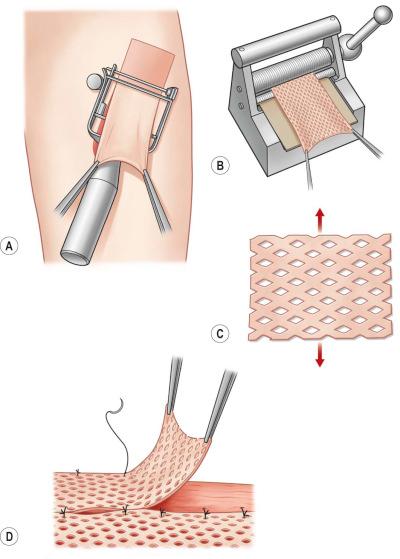
When possible, harvesting the skin graft first and covering the donor site will avoid contamination from the wound. When the excisional preparation of the recipient site needs to be performed first, a separate instrument set-up for the donor site can be considered. The size of the graft needed should be accurately measured prior to harvest. The graft thickness can be adjusted by a lever near the end of the dermatome between 0.1 and 1.0 mm. The surgeon presses the dermatome in 45° to the skin surface on to the tissue and moves the device from distal to proximal with uniform pressure and speed ( Figure 87-1-5 ). A second assistant can pick up the skin graft with two anatomical forceps during the harvest process to avoid damage of the graft. If the desired length is taken, the dermatome will cut the edge when elevating it while running the motor. Keeping the graft moist with saline-impregnated gauze is of vital importance if not immediately grafted.
Larger skin grafts should be incised with an 11 knife multiple times to allow wound fluid drainage and prevent collections between the skin graft and the wound bed.
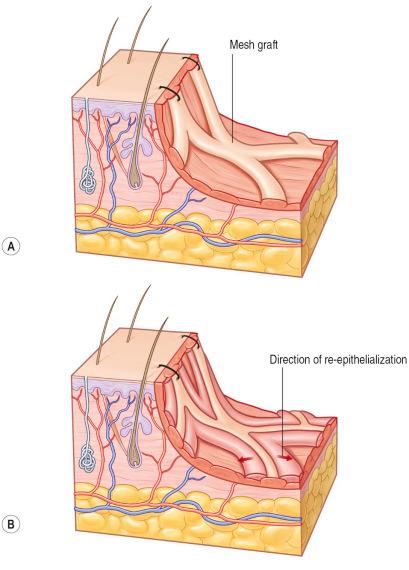
STSGs can be enlarged up to six times their original size. Enlargement of the graft can vary from just a few manually applied perforations with an 11 blade to a systematic enlargement with a hand-powered meshing device (mesher) that applies multiple slits at regular intervals ( Figure 87-1-5 ). Meshed grafts are often used following large burns when the wound area exceeds available healthy donor sites. Meshed skin grafts are also very helpful to cover irregular geometric surfaces such as around joints as they minimize folds in the graft. Development of contractures should be taken into consideration in functional areas. If meshing is not necessary, the graft should be perforated multiple times to allow fluid removal under the graft (pie-crusting) that can minimize the risk of hematoma, seroma, or infection under the graft. Using different mesh templates, from 1 : 1 to 1 : 9, can regulate the extent of the enlargement of meshed grafts. The most commonly used mesh ratio is 1 : 1.5 in smaller wounds, while a mesh ratio of 1 : 3 and 1 : 6 is often needed to cover large burns.
Meshing devices are manual and come with different plastic templates where the skin graft is placed on upside down. The graft should be taken with anatomical forceps to avoid mechanical damage. The mesh gaps will be subsequently filled by keratinocytes from the skin stripes ( Figure 87-1-6 ). This process takes longer with higher mesh expansion ratios. Since grafted cells excrete growth factors, underlying granulation tissue will be stimulated until full re-epithelialization occurs. Aesthetically unpleasant hypergranulation in the open areas of the mesh are therefore more often seen in large mesh ratios. Meshed skin grafts leave unsightly long-term results that need to be considered when selecting this technique. Very thin meshed skin grafts used in combination with dermal substitutes or keratinocyte cultures are other strategies to mitigate the result ( Figure 87-1-6 ).
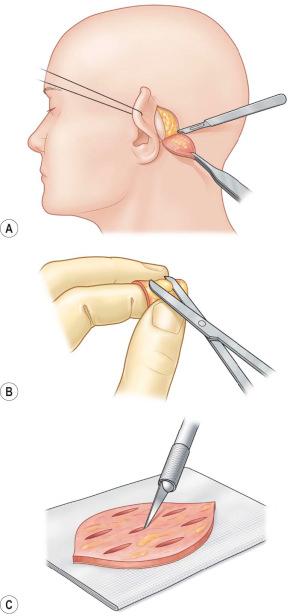
Full-thickness skin and composite grafts are limited in availability but show excellent function and sensitivity after engraftment. Full-thickness grafts should be considered in the reconstruction of aesthetically dominant (face) or functionally important areas (hand). Full-thickness grafts from the retroauricular region and above the eyebrows are an excellent choice to maintain tissue quality and color of the surrounding skin in the face ( Figure 87-1-7 ). If needed, foreskin can also be used and in adults retroauricular skin is helpful in face reconstruction. Also, excess skin from the upper eyelid and submental area can be taken into consideration for full-thickness reconstruction if the patient is comfortable with an aesthetic-like intervention.
In hand reconstruction, elbow crease and wrist fold grafts have been described but should be avoided in cultures where these donor site scars may result in stigmatization of the patient as they can be associated with suicide. Hypothenar skin is useful for glabrous reconstruction but can leave a painful scar that can cause an unpleasant sensation if the hand is positioned on a table in a relaxed position. Therefore, full-thickness skin graft from the hypothenar area should be harvested elliptical with the main axis and slightly more dorsal in relation to the glabrous-skin border ( Figure 87-1-7 ).
Full-thickness skin grafts are taken in an area where loose surrounding skin is available to achieve primary closure. Skin grafts can be designed elliptical and excised with a knife. Harvesting should be carried out trying not to elevate the underlying tissue. Most of the full-thickness grafts need defatting and this can be easily performed by spreading the graft over the index finger and trimming the fat tissue tangentially to the skin. Defatting of the graft will encourage graft take ( Figure 87-1-8 ).
Composite grafts include a layer of subcutaneous fat tissue under the dermal and epidermal layer. The donor sites are principally the same as the full-thickness donor sites. Since fat tissue is less vascularized and more vulnerable to ischemia, optimal revascularization is needed in order to achieve graft survival. Composite grafts can be used in children as they show a remarkable capacity to revascularize thicker grafts. Some surgeons use composite grafts to reconstruct the nasal tip, the alar, and the columella in cleft lip patients.
Over time, the appearance of skin grafts tends to improve in both color and texture. Nevertheless, skin grafts rarely have the aesthetic appearance of normal skin and patients should be advised about the likely final appearance of the graft.
Once the autologous skin is grafted on to the wound site the revascularization depends on multiple factors. One of the most important factors to achieve stable taking is the immobility of the graft during the revascularization process. An open technique requires labor-intensive monitoring on the graft and any fluid that is formed beneath the graft is rolled out with a cotton-tipped applicator. More often, skin grafts are fixed through a series of sutures and overlying compressive dressing materials (bolster: Figure 87-1-9 ). Scattered sutures through the graft on to the wound bed can additionally immobilize larger skin grafts. If large and multiple skin grafts are placed as frequently needed in burn victims, fixation can be performed with staplers to shorten operation time. Staples are painful when removed and may be overgrown by skin, especially in large skin grafts. Vacuum-assisted pressure devices can be used with a protective interface of petroleum gauze or a silicone sheet to permit continuous compression on to the graft and fluid removal. The suction dressing is especially useful if fast mobilization is desired in patients with wounds in joint regions or the lower extremities ( Figure 87-1-9 ). Compression to stabilize the graft on to the wound bed should be performed until 5–10 days when the graft is usually stable and wound areas are completely closed.
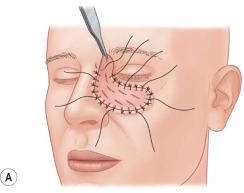
Splints can be used as adjuncts if the risk of wound contraction is high, such as in chin scar release or in joint and web space release of the hand. These splints or casts should be worn up to several months after grafting, in the beginning 24 hours per day, later during the night, to avoid the loss of mobility. Physiotherapy and scar massage are also important elements for obtaining better results with skin grafts.
Negative-pressure wound-healing devices also make very effective bolsters, especially on largely grafted surfaces and chronic ulcers.
Fibrin glue can also be helpful to assist skin graft fixation. In this case, some surgeons spray fibrin glue on to the skin graft dermis just before placing it on to the wound site. The fibrin network may even act as a provisory extracellular matrix under the graft.
The first dressing change should occur once the skin graft is revascularized and has a stable physical connection to the wound bed. Early dressing change around the third day after grafting may allow predicting the “take rate” of the graft but risks secondary graft loss through shear forces that disturb nascent vessel connections. More commonly the dressings are taken off for the first time 5–10 days after grafting.
Before planning a skin graft, several factors have to be taken into consideration to achieve optimal tissue cover at the wound site. The wound bed quality has to be optimized for a successful “take” of the graft and the skin color, thickness, and mechanical resistance of the donor site area should ideally match the recipient site skin quality. Wound conditions often found in chronic or insufficiently debrided burn wounds and characterized by low wound bed vascularization or high bacterial loads will not allow skin grafts to be taken.
The successful engraftment of skin grafts highly depends on the quality of the wound bed. Revascularization particularly depends on a vital recipient wound bed. A good quantity of blood vessels near the surface is critical in order to support graft viability. Irradiated, ischemic and scar tissue, bone, and tendon do not ordinarily have sufficient blood supply to allow the skin graft to take. If highly vascularized peritendon and periosteum are still intact, skin grafts can be performed. In chronic wounds re-epithelialization occurs at the wound margins that can grow into the tissue and may inhibit the lateral reconnections of the graft. Therefore, wound margins should be sharply excised with a blade before grafting.
Experimental data suggest that the bacterial level must be brought down below the critical level of 10 5 bacteria per gram of tissue to allow a skin graft to take. The practical problem with quantitative bacterial cultures is that it takes about 48 hours to obtain the result, long after the decision to graft is typically made. As a result, this methodology seems most commonly applied to research studies. Surgeons often take a fairly aggressive approach to making sure all necrotic tissue has been debrided and that the wound is clinically “clean” prior to grafting.
Wound debris or necrotic tissue physically inhibits and chemically slows ingrowth of blood vessels into the skin graft. Wounds that were left open for several days contain high bacterial loads and therefore need to be extensively debrided before skin grafting. Preparation of the recipient site commonly occurs by sharp excision, but can also be performed using a variety of other debridement techniques, including laser, water jet, and ultrasound as well as standard dressing changes. Surgeons have learned over time that a “granulating” wound has a high likelihood of taking a skin graft. Active bleeding of the wound bed will likely lead to blood collection between the graft and the wound bed, inhibiting graft take. Accurate homeostasis can be performed with bipolar cautery and larger blood vessels can be ligated with fine resorbable sutures.
If tendons or bones are exposed without peritendon or periosteum, the surrounding soft tissue can often be adapted to cover critical structures before skin grafting. Small areas of tendons and bones can either be prepared by vacuum-assisted closure therapy to grow granulation tissue from the sides or dermal substitutes can be used to cover functional structures.
Skin grafts can often provide functional and aesthetic skin reconstruction. Consideration needs to be given to the size of graft needed, the degree of wound contraction expected, the color and texture of the skin required, and the need for adnexal glands. The amount of wound contraction expected is inversely related to the amount of dermis in the skin graft.
Full-thickness grafts, those that take the entire epidermis and dermis, maximally restrain contractile forces and give excellent cosmetic results. Full-thickness skin grafts are frequently used for nipple–areola reconstruction, syndactyly release, or ectropion release. Full-thickness grafts are in short supply and can be augmented by tissue expansion prior to harvesting full-thickness skin grafts.
Very thin grafts such as epidermal grafts result in a donor site that heals quickly with minimal contraction but provide little constraint to wound contraction. The surgeon can use this as an advantage if wound contraction is desired. For example, on large scalp wounds or abdominal wounds, wound contraction may be desired to keep the skin graft as small as possible while pulling the wound edges together over time. Secondary excision of the contracted skin graft and primary wound closure can be performed in a second stage to achieve best functional and aesthetic results.
As the skin thickness varies throughout the body, a variety of skin thicknesses are available for use. For example, full-thickness upper eyelid skin is very thin but provides good resistance to wound contraction. Thicker skin is available in the trunk and leg region, where thick STSGs can be taken, leaving enough adnexal structures and dermal thickness to minimize donor site scarring.
Skin color is determined by a complicated integration of skin texture, melanin pigmentation, and blood flow. In general, replacement of tissue from a similar or adjacent site will give the best color match. In the face, this is often the most critical aesthetic area and choosing donor sites such as supraclavicular, posterior auricular, upper eyelid or scalp skin grafts can often lead to an excellent color match. For darker areas of the skin, such as the nipple–areola complex, grafts from the contralateral areola or genitalia have been used. More commonly today, skin grafts in this area can be tattooed with vegetable dyes to give an excellent color match.
Skin texture is most commonly an issue when dealing with glabrous skin (palms and soles of feet). In this case, placing a nonglabrous skin graft to cover these areas can result in a very unnatural look, often with significant color match differences. Glabrous skin grafts are obviously in short supply but can be harvested from the hypothenar eminence. Skin adnexal glands are difficult to replace but can sometimes be successfully transferred with full-thickness grafts.
The obligatory scarring or discoloration associated with donor sites must be considered when taking a skin graft. For large surface area burns, the surgeon must use whatever donor site is available to close the wound; in contrast, for smaller grafts, a thorough discussion of the donor site possibilities with the potential risks and benefits allows for the best skin match with the least iatrogenic damages.
Common donor sites include the thigh, trunk, and buttocks, regions frequently covered by clothing (see Figure 87-1-2 ).
When defects of the face need to be covered, full-thickness skin grafts are often preferred. The donor area for a graft on the face should be preferably chosen among scalp, neck, and supraclavicular area to obtain the best color match. Important aspects are the thickness, color, and texture of the graft, which should closely match those of the recipient site.
Eyelid skin, which is thin with a few glandular structures, is generally best replaced by eyelid skin. Thick, highly glandular nasal skin can be replaced by skin from the nasolabial folds, supraclavicular area, or anterior auricular area.
In trauma, avulsed (degloved) skin can often be prepared by extensive defatting and regrafted primarily or stored and used later.
Full-thickness donor sites in the head and neck are pre- and postauricular regions (the first is generally thicker), nasolabial crease, supraclavicular region, eyelids, and neck.
Other common regions include the inguinal crease that is often used to cover large defects. In this case it is important to harvest laterally and away from potentially hair-bearing regions of the pubis. This area generally heals well and is hidden ( Figure 87-1-7 ).
In nipple–areola reconstruction after mastectomy, a full-thickness graft from the contralateral region is often taken in combination with a full-thickness graft from the groin.
Donor sites from full-thickness skin grafts are generally closed primarily. Particular attention should be taken when supraclavicular donor sites are used, as this region is prone to develop hypertrophic scars. Sometimes large donor sites that cannot heal primarily are used for covering extensive defects, for example, in the face or joints. In this case, an STSG can be used to cover the donor site.
Topical gauze soaked with diluted epinephrine solution is useful to stop bleeding at the donor site and can be left until the operation is ended, adding an analgesic effect. The donor site of an STSG generally heals (re-epithelializes) in 7–21 days depending on the size and depth of the graft taken and the age of the patient. A myriad of donor site dressings are available with multiple studies on a variety of products. Traditionally, fine-meshed gauze, often impregnated with a petroleum-based product, is placed over the wound and fixed in place. Cotton gauze is placed over this and removed a day or two after the operation. The wound heals under this dressing and the dressing spontaneously comes off when healed. The advantages of this type of dressing system are its simplicity, low cost, and minimal wound care requirement. The work of Winters in the early 1960s showed that a moist wound heals faster. As a consequence many have advocated a simple semiocclusive adhesive, semipermeable polyurethane dressing, although it promotes serum accumulation between the wound and the dressing that should be evacuated by a drain or a syringe. This process can be labor-intensive for a busy inpatient service or difficult to manage on an outpatient basis. In addition, when an infection develops, it can rapidly spread to the entire donor site area with this dressing.
A large number of other dressings, including silver-based, absorptive, and biological, have been studied. In most of the cases the donor site heals spontaneously, thus simple impregnated gauze is still the gold standard. When some complications occur, the harvest site can deepen and become a full-thickness defect, mainly due to infections in the elderly, infants, or critically ill patients. As a consequence, excision and grafting of the donor site may be required.
Skin grafts can be stored on a moist gauze at 4°C for up to 2 weeks, although the viability decreases over time. Experimentally, storage can be extended using cell culture media. For degloving injuries, the skin can be defatted acutely and reapplied as a full-thickness graft.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here