Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We would like to acknowledge and thank Dr. Eric T. Jones for his contributions to the previous versions of this chapter.
Consideration of growth potential is the major difference in treating injuries in children as compared with adults. Pediatric skeletal trauma can result in enhanced or diminished growth. Future growth is usually helpful because some angular and length deformities can correct themselves as the child grows. Loss of growth potential can be one of the more difficult problems to treat. Adult bone is dynamic; it is constantly involved in bone turnover and remodeling in response to aging and changes in stress on the skeleton. The pediatric skeleton not only remodels in response to alterations in stress but also grows in length and width and changes shape, alignment, and rotation as it matures. Understanding growth potential and the changing forces after skeletal trauma in children are important in determining the appropriate treatment for injured bones.
The following are the most common clinical questions in caring for children with fractures: (1) is the physis injured with an accompanying risk of growth disturbance, and (2) is the length and alignment of the fracture acceptable or unacceptable (i.e., will it improve with growth enough that function and cosmesis will not be adversely affected)? If the answer is no, a reduction is indicated. The response to these two questions requires knowledge of normal growth mechanisms and studies of fractures in children (the science), whereas applying this knowledge to an individual patient and making decisions about how to care for the fracture require an assessment of multiple factors related to the child and the fracture (the art).
Principles of fracture treatment are the same for all ages—the goal is to achieve restoration of normal length, alignment, rotation, and the anatomic reduction of articular surfaces. In children, attempting to preserve normal growth potential is also critical; thus, assessment of the integrity and alignment of the physis is important. Although some angulation is acceptable when treating fractures in children, it is best to keep the amount of angulation as small as possible by closed fracture treatment methods, regardless of the patient’s age. On the other hand, multiple attempts at anatomic reduction in a child, particularly in fractures involving the physis, may cause harm and should be avoided. The small amount of angulation associated with torus or so-called buckle fractures in children is almost always acceptable. Marked bowing that causes clinical deformity, which can be seen in plastic deformation and “greenstick” fractures in the forearm, should usually be corrected.
Bone healing in children is generally rapid, primarily because of the thickened, extremely osteogenic periosteum. The age of the patient directly affects the rate of healing of any fracture: the younger the child, the more rapidly the fracture heals. The periosteum thins as the child grows older and has less osteogenic capability. Injuries to the growth plate heal more rapidly than shaft fractures do. Physeal injuries, in almost all parts of the body, heal in approximately 3 weeks.
Treatment of trauma to the pediatric skeleton is generally straightforward. Dislocations and ligamentous injuries are uncommon in children in comparison with adults because the physis and bones in children are usually weaker mechanical links in the system and thus more susceptible to injury. Ligamentous injuries may occur, especially in older children, as physiologic physeodeses begin to occur, resulting in more secure attachments of the epiphyseal and metaphyseal regions. Most injuries, though, are simple fracture patterns caused by low-velocity trauma such as falls. In most cases, closed reduction followed by a short period of immobilization restores normal function to a pediatric extremity. However, a number of pitfalls can make treatment of pediatric fractures, particularly fractures of the growth plate, difficult and demanding.
In infants, skeletal trauma may be related to the birthing process or may be the only sign of child abuse because young children are at higher risk for abuse. The presenting sign may be deformity, swelling, or lack of movement in an extremity. Caregivers should be questioned about the circumstances of the injury, and a lack of a plausible mechanism of injury should prompt an evaluation for nonaccidental trauma. Radiographs of an infant can be difficult to obtain and interpret, especially those of bones in the elbow and hip region, which may require comparison views. Anteroposterior and lateral views, including the joints above and below the injured area, constitute a minimal radiographic evaluation. Usually, routine radiographs coupled with a good physical examination can establish the diagnosis. Arthrograms, ultrasonography, or magnetic resonance imaging (MRI) can be useful as a diagnostic aid when radiographs are confusing. Additionally, a skeletal survey can be used in the young patient because unsuspected fractures may be present up to 20% of the time.
Children with multiple trauma or head injuries or both can have occult axial fractures and physeal injuries that may not be suspected or may be difficult to diagnose, even with a good physical examination. This is more commonly seen in patients with a lower Glasgow Coma Scale and higher Injury Severity Score. In these children, historically, a bone scan assisted in diagnosing fractures unidentified by routine screening radiographs ; however, they can be difficult to obtain in multiply injured children. More recently, radiographic skeletal surveys and multiplanar imaging with computed tomography or MRI are favored for identifying occult injuries.
Fractures through the growth plate in children can be difficult to interpret if the fracture is not displaced. A thorough physical examination can usually identify this type of injury; the sign is swelling and maximal tenderness occurring over the injured physis, which occurs most commonly at the distal end of the radius or fibula. Palpation at or distal to the tip of the lateral malleolus usually identifies a ligamentous injury; swelling and tenderness at the growth plate may suggest a fracture undetected by radiographs. However, studies evaluating children with lateral ankle pain after injury but normal radiographs were not found to frequently have a physeal fracture by ultrasound or MRI. Another recent MRI study challenges the perception that distal fibular physeal injuries are common after twisting ankle injuries in skeletally immature patients. Often, a small metaphyseal fragment on the radiograph suggests physeal injury. Repeated radiographs in 1 to 2 weeks can confirm the physeal injury because the healing physis appears wider, and periosteal reaction may be seen.
Each age group has typical injury mechanisms and common fractures. Most infants and newborns (≤12 months of age) sustain fractures by having someone else injure them. When children are older, walking and running, accidental injuries are more common. Children most commonly fracture the forearm, usually the distal end of the radius. Clavicle fractures are common in infancy and in the preschool age group, but their incidence decreases with increasing age. Elbow hyperextension in early and midchildhood predisposes children in these age groups to supracondylar humerus fractures. Forearm fractures, although common in young children, show a progressive increase into the teenage years.
Most injuries occur when the child falls. Severe, high-energy injuries are less common in children and are frequently caused by automobiles, lawn mowers, or motorcycles/all-terrain vehicles. As a child approaches the midteens, injuries are much like those of an adult. The age at which the growth plates close varies greatly and depends on hereditary factors and hormonal variation. Skeletal age is an important factor in the consideration of injuries in children, in that the closer the child is to the end of growth, the less prominent the role of the growth plate in treatment of the injury and the less the remodeling potential. Healing capacity is inversely related to age.
Finally, child abuse must be considered in all children’s injuries, and, as noted earlier, it should especially be considered when treating very young children with fractures. Care must be taken to ensure that the child is checked for signs of abuse on the initial assessment and for possible subsequent injuries during follow-up. Repeating a skeletal survey at 2 to 3 weeks is strongly recommended in young patients to increase diagnostic yield in patients with suspected abuse injuries. Parents or guardians of children who are not brought back for follow-up appointments for fractures should be contacted and asked to schedule a return visit.
Embryonic bone forms through either membranous or endochondral ossification. In the former, mesenchymal cells proliferate to form membranes primarily in the region in which flat bones are fabricated. Endochondral ossification is bony replacement of a cartilage model and is the mode of formation of long bones.
Membranous bone formation increases the diameter of long bones and is responsible for the creation of flat bones such as the scapula, skull, and, in part, the clavicle and pelvis. Flat bones are formed as mesenchymal cells condense into sheets that eventually differentiate into osteoblasts. Surface cells become the periosteum. Primary bone is remodeled and transformed into cancellous bone, to which the periosteum adds a compact cortical bone cover. This type of growth is independent of a cartilage model.
As endochondral ossification lengthens bones, proliferation of bone occurs beneath the periosteum through membranous bone formation, thus enlarging the diameter of the diaphysis in long bones. This type of bone formation is also apparent in subperiosteal infection and after bone injury when periosteal bone forms around a fracture hematoma ( Fig. 1.1 ). The osteogenic periosteum of children contributes to rapid healing because the callus and periosteal new bone increase the diameter of the bone and provide early biomechanical strength.
Endochondral ossification requires the presence of a cartilage anlage. Early in gestation, mesenchymal cells aggregate to form models of the future long bones. A cartilage model develops, and the peripheral cells organize into a perichondrium. Cartilage cells enlarge and degenerate, and the matrix surrounding them calcifies. This calcification begins in the center of the diaphysis and becomes the primary ossification center. Vascular buds enter the ossification center and transport new mesenchymal cells capable of differentiating into osteoblasts, chondroclasts, and osteoclasts. These cells align themselves on the calcified cartilage and deposit bone. Primary cancellous bone is thus formed, and ossification expands toward the metaphyseal regions.
Long bone growth continues as the terminal ends of the cartilage model keep growing in length by cartilage cell proliferation, hypertrophy, and production of extracellular matrix. This growth continues in this manner until after birth, when secondary ossification centers (epiphyses) develop.
The mass of cartilage found between the epiphyseal and diaphyseal bones in later postnatal development thins to become the epiphyseal plate, which continues as the principal contributor to the growth (in length) of long bones until maturation is reached. The girth of the long bone is provided by the cambium layer of the periosteum. Successive surfaces of compact bone are added to the exterior while remodeling by resorption of the interior (endosteal) surface takes place.
Once the physis is established between the epiphysis and metaphysis, the periosteal ring becomes relatively firmly attached at the level of the zone of hypertrophied cells. This periphyseal periosteal collar is referred to as the fibrous ring of LaCroix. The zone of Ranvier, the cellular segment responsible for growth in diameter of the physis, is located in the same area. The periosteum is firmly attached at this level. Even when the periosteum is torn over the metaphysis or diaphysis, it usually remains attached at the physis.
Factors affecting skeletal growth vary and are incompletely understood. Although commonly used growth curve charts suggest that growth is smoothly continuous throughout childhood, a saltation and stasis model of human growth is now recognized, with bursts of growth in length (growth spurts) occurring after prolonged periods of stasis. Growth in length occurs only at the physis and can occur through three mechanisms: an increase in the number of cells, an increase in the size of cells, or an increase in the amount of extracellular matrix. The physis responds to various growth-regulating hormones (e.g., growth hormone, thyroxine, estrogen, and testosterone), parathyroid hormone, and corticosteroids, as well as the peptide-signaling proteins—transforming growth factor β (TGF-β), platelet-derived growth factor (PDGF), and bone morphogenetic proteins (BMPs)—and immunoregulatory cytokines (interleukin-1 [IL-1] and IL-6). Further research is still needed to better understand these growth pathways. However, recently it was suggested that differential expression of gene pathways specifically for BMP-2 and BMP-6 may contribute to further physeal growth.
Local paracrine regulators have recently been identified as critical in controlling bone development and remodeling. An important feedback loop controlling chondrocyte development involves Indian hedgehog protein (Ihh) and parathyroid hormone-related peptide. These paracrine factors control the decision for chondrocytes to leave the proliferative pool and undergo hypertrophic differentiation. Fibroblast growth factor (FGF) signaling also appears crucial in regulating chondrocyte proliferation and differentiation in the physis and appears to have an opposite effect from the BMPs, decreasing chondrocyte proliferation, increasing production of Ihh, and accelerating differentiation of hypertrophic chondrocytes. An example of abnormal growth related to FGF signaling is in achondroplasia. A gene mutation affecting FGF receptor 3 suppresses proliferation and maturation of growth plate chondrocytes, causing decreased growth plate size and decreased bone elongation. Thyroxine is also involved in the cellular and molecular events of terminal chondrocyte differentiation and morphogenesis of columnar cartilage.
Diurnal variation in the growth of bone has been shown to reflect the levels of the different hormones, and animal studies suggest mechanical factors may also be critical because 90% of growth occurred during periods of recumbency in a study of growth in sheep. Physeal growth is slowed by excessive compression and accelerated by distraction, recognized in the American literature as the Hueter-Volkmann law, but noted earlier by Delpech in 1829. This theory affects growth but also fracture healing, which is one of many differences between pediatric and adult fracture healing.
Growth in length ceases at skeletal maturity with fusion of the physes and occurs at different times in individual bones; it also varies based on gender, hereditary factors, and hormone levels. Physiologic physiodesis is the normal, gradual replacement of the growth plate by bone during adolescence, and physeal closure is induced at skeletal maturity by estrogen levels in both males and females.
Fracture healing is usually divided into three stages: (1) inflammatory, (2) reparative, and (3) remodeling. Fracture healing involves both membranous and endochondral ossification. Injuries to the pediatric skeleton always involve a variable amount of surrounding soft tissue injury. Unlike the soft tissues, which heal by replacement of the injured tissue with collagen scar tissue, bone heals by replacing the area that is injured with normal bony tissue.
The blood supply to the bone is an important part of fracture healing, and significant soft tissue injury delays healing because the blood supply to bone enters at sites of soft tissue attachment. The normal process of fracture healing in any part of the bone follows a set chronologic order. Any of these phases may be disrupted or delayed by excessive adjacent soft tissue injury.
The inflammatory phase of fracture healing “sets the stage” for cartilage and bone formation by supplying the building blocks necessary for repair and remodeling. When bone is injured, the bone, periosteum, and soft tissue (mostly muscle) around the fracture begin to bleed. Hematomas form at the fracture site, both inside and outside the bone. The hematoma may dissect along the periosteum, which is easily elevated or was elevated at the time that the fracture was maximally displaced. The more severe the soft tissue injury, the more displaced the fracture; in addition, the more the periosteum is torn, the larger the area that fills with the hematoma. The role of a hematoma is to serve as a source of signaling agents capable of initiating cellular events critical to fracture healing. This also explains why some minimally displaced or greenstick fractures (minimal to no hematoma) may be slow to heal.
Research has focused on factors controlling fracture healing in two groups: peptide-signaling proteins (TGF-β, FGF, PDGF, and BMPs) and immunoregulatory cytokines (IL-1 and IL-6). The peptide-signaling proteins are derived from platelets and extracellular bone matrix and are critical for regulation of cell proliferation and mesenchymal stem cell differentiation. TGF-β is a multifunctional growth factor that controls tissue differentiation in fracture repair. FGFs increase the proliferation of osteoblasts and chondrocytes and may stimulate the formation of new blood vessels. PDGF acts on mesenchymal cell precursors to stimulate osteoblast differentiation. BMPs are a class of proteins produced in the early stages of fracture repair and strongly stimulate endochondral ossification. The sole criterion for BMP classification is the induction of bone formation in a standard in vivo rodent assay, and at least 14 BMPs, grouped in the TGF superfamily of growth and differentiation factors, have been identified. BMPs are present in bone matrix in a form that allows for presentation to marrow stromal cells to induce differentiation into osteoblasts. Furthermore, osteoblasts have been shown to synthesize and secrete BMPs. Cells that synthesize new bone during fractures also have been shown to be targets of BMPs and to possess BMP receptors. BMPs (BMP 2, 3, 4, 5, and 8) and BMP receptors are upregulated in the periosteum as early as 3 days after fracture.
Studies utilizing microarray analysis of the genetic response to a fracture demonstrate that the genomic response to a fracture is complex and involves thousands of genes, including the BMPs and other growth factors noted earlier, as well as immunoregulatory cytokines. The immunoregulatory cytokines are released from inflammatory cells present in the hematoma and serve to regulate the early events in fracture healing.
Pediatric bone is more vascular than that of an adult and is able to generate a greater hyperemic and inflammatory response. The more mature (less porous) the cortex, the slower the vascular response to injury. Vasodilatation and the cellular inflammatory response begin shortly after a fracture, and the injured area is filled with inflammatory cells such as polymorphonuclear leukocytes and macrophages. The hematoma and inflammatory response also incite the release of molecules such as growth factors and cytokines from the platelets. In the initial phase of fracture healing, after the hematoma has formed, a scaffolding of fibrovascular tissue replaces the clot with collagen fibers. These fibers eventually become the collagen of the woven bone of the primary callus that forms around the fracture.
The primary callus is later ossified as the microvascular supply returns to the area. However, the bone, for at least a millimeter or two directly adjacent to the fracture site, loses its blood supply early in the inflammatory stage. After initial reabsorption of the dead bone along the fracture line, the fracture line in children usually becomes more visible radiographically 2 or 3 weeks after injury. The dead bone at the fracture surface is revascularized in a process that occurs faster in more vascular areas such as the metaphysis (as compared with the diaphysis).
The vascular response aids in initiating the cellular response to the fracture. A number of TGF-β subtypes help mediate cellular and tissue responses to inflammation and tissue repair. During the inflammatory phase of fracture healing, TGF-β from the extracellular matrix of bone and also from platelets controls the mesenchymal precursor cells that may form osteoblasts and osteoclasts. The maximal cellular response is ongoing within 24 hours of injury and occurs first in the subperiosteal region of the fracture.
Osteogenic induction is stimulation by growth factors to convert the multipotential cells into osteoprogenitor cells. The osteoprogenitor cells on the undersurface of the periosteum help form periosteal bone. The osteogenic cells that originate from the periosteum help manufacture the external callus. Endochondral bone formation from the endosteal areas combines with subperiosteal bone formation to bridge the fracture.
The subperiosteal callus in children initially stabilizes the area so that the external callus may clinically heal the fracture by the end of the reparative phase. During remodeling, this callus decreases and is replaced with the endochondral ossified bone that has formed at the fracture surface.
The reparative phase of fracture healing is highlighted by the development of new blood vessels and the onset of cartilage formation. The surrounding soft tissue provides vascular ingrowth initially to the periosteal area and subsequently to the endosteal area. Before the fracture, the cortical blood supply was primarily from endosteal bone and branched out radially from inside the medullary canal. During the reparative phase, most of the blood supply to the cortex arises from outside the bone rather than inside.
Rat models of fracture healing reveal that intramembranous and endochondral bone formation is initiated during the first 10 days. Inflammatory mediators in the fracture hematoma recruit chondrocytes capable of producing fracture callus. The hematoma is eventually replaced by the ingrowth of fibrovascular tissue. This developing construct provides structural support to stabilize the bone ends. This primitive tissue is eventually replaced through endochondral and intramembranous bone formation.
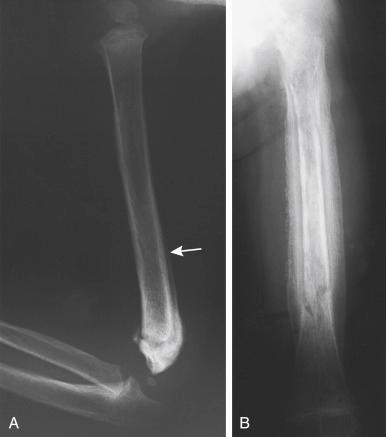
Tissue differentiation during the reparative phase is strongly influenced by local mechanical factors. Fracture stability has a critical effect on bone healing. Fracture healing is classically divided into primary and secondary healing. Primary healing results from rigid stabilization (i.e., plate immobilization) and involves a direct attempt by the cortex to bridge the fracture gap. Bridging occurs through direct haversian remodeling by intramembranous bone formation ( Fig. 1.2A, B ).
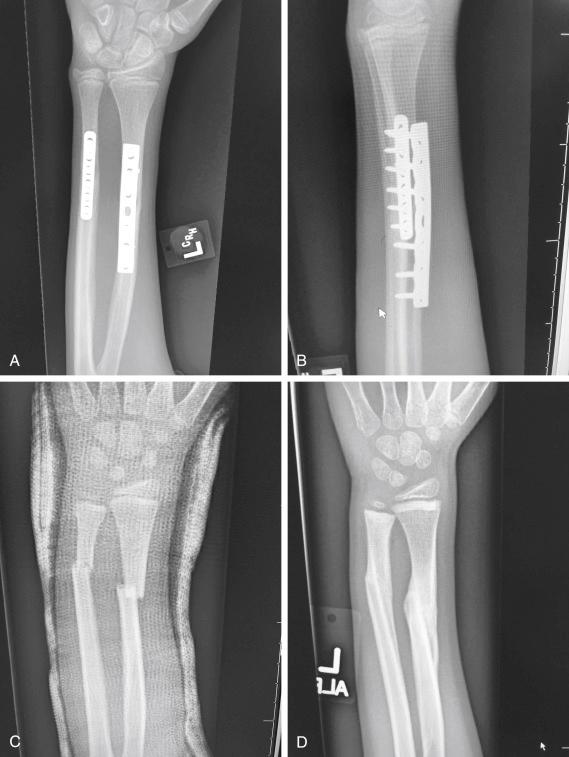
Secondary healing results from treatment of fractures with less rigid methods (i.e., fracture bracing, casts). In secondary healing, more motion at the fracture site leads to lower oxygen tension, and more cartilage is formed. Motion at the fracture site, the presence of a fracture gap, and an intact soft tissue envelope all encourage the formation of abundant callus ( Fig. 1.2C, D ). The increased diameter of the callus enhances biomechanical stability because the rigidity of the bone is proportional to its radius. The callus formed subsequently undergoes endochondral ossification. Ideal fracture treatment involves enough rigidity to ensure adequate vessel ingrowth, followed by progressive loading and motion to stimulate ample callus formation.
As the periosteum produces bone beneath it, the periosteum is pushed away from the bone and makes a collar of bone around the area of injury. Initially, this tissue is more cartilaginous and fibrous and is not very well ossified. It may not show up well on a radiograph until the blood supply is adequate enough to allow mineralization and conversion to bone.
An important process that occurs between the reparative and remodeling phases is clinical union of the fracture, which takes place when the bony callus surrounds the fracture fragments and joins the callus coming from the other side. At this point, the bone may be stable clinically, and although some plastic deformation is still possible with force, the bone is usually strong enough that the patient can begin to use the extremity in a more normal way.
Although there are many ways suggested in the literature to determine union, clinical examination with radiographic evidence of healing is the most important in assessing union. Clinical union has occurred when the fracture site is no longer tender and does not move during examination and when physiologic loading does not cause pain. Radiographic union occurs later when radiographs demonstrate bone bridging across the fracture. This point demarcates the end of the reparative phase and the beginning of the remodeling phase.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here