Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The senior author (M.B.) has received travel stipends from Codman and Shurtleff, Medtronic, Aesculap, and Sophysa. The author has served on Advisory Boards for Codman and Shurtleff and Medtronic. Clinically, the University of California, Los Angeles, Adult Hydrocephalus Center uses Codman, Medtronic, Sophysa, Aesculap, and Integra products.
Eric Stiner, MD, contributed a significant effort in the writing of the first edition of this chapter while he was a neurosurgery resident at the University of California, Los Angeles.
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
In the initial evaluation of a patient with newly diagnosed hydrocephalus or a previously shunted one, an essential component of the evaluation is to assess for an intraventricular obstruction that may be amenable to an endoscopic procedure.
Every neurosurgeon should have a basic understanding the how a differential-pressure valve (DPV) functions and its effect on intracranial pressure (ICP). For most patients a valve opening-pressure setting should exceed the mean ventricular pressure. In vivo ICP data suggest that the etiology of shunt overdrainage with DPVs is in many, if not most, cases a result of a valve opening-pressure selection that is too low.
Most adult patients with hydrocephalus with adjustable valves require one or more valve adjustments postoperatively, highlighting the potential shortcoming of using a fixed-pressure valve.
In shunted patients, the finding of negative ICP when the patient is in the upright position is expected with DPVs. The diagnosis of overdrainage must be based on appropriate symptoms and/or imaging findings.
There is no evidence that any single shunt valve design is advantageous in the management of hydrocephalus, but individual attributes, including shunt valve prominence, MRI compatibility, and ease of adjustment should be considered for each individual patient and clinical situation.
The use of an antisiphon device (ASD), a flow-restricting device, or a gravitational device may benefit selected patients with symptomatic overdrainage symptoms.
Meticulous attention to detail with regard to surgical site preparation, timing and handling of shunt components, and double gloving by all surgical staff and surgeons should be considered standard of care for all shunt operations.
Hydrocephalus is a commonly encountered disorder that occurs either as a primary condition or as the sequela to an intracranial hemorrhage, a space-occupying lesion, or meningitis. For more than a half-century, a cerebrospinal fluid (CSF) shunt has been the mainstay of treatment for hydrocephalus. Although many consider shunting a relatively simple procedure, problems with CSF shunts are common, costly, and sometimes debilitating. Within the first year, shunts fail at an extraordinary rate of up to 40% and show nearly a 10% infection rate. In a study, pediatric patients underwent an average of 2.66 shunt revisions over 20 years, and although pediatric patients have significantly higher rates of shunt failure than adults, it is clear that the shunt operation has one of the highest associated complication rates in neurosurgery. , Furthermore, cases of hydrocephalus can be some of the most complex and challenging clinical scenarios facing a neurosurgeon. ,
The aim of this chapter is to help neurosurgeons choose the type of shunt, valve setting, and shunt location that will offer the highest probability of a good outcome, while avoiding complications and revisions, in adult patients with hydrocephalus (see Chapter 197, Chapter 198, Chapter 201 for discussions of pediatric hydrocephalus and pediatric shunts). Unfortunately, there are scant level I and II evidentiary data on which to base guidelines pertaining to shunting methods and materials for adult hydrocephalus patients. Our recommendations are therefore derived from the personal experience of thousands of outpatient encounters and over 1000 surgical procedures in adult hydrocephalus patients over a 25-year period, insight drawn from our own clinical studies, and information gleaned from the literature.
Although this chapter is entitled “Shunting,” neurosurgeons should always consider endoscopic third ventriculostomy (ETV) as an alternative when appropriate , (see Chapter 45 for more information on the role of third ventriculostomy in adults and children). Proceeding automatically with a shunt operation, particularly in patients presenting with shunt failure, potentially robs the patient an opportunity to live shunt free. Clinicians should investigate the etiology and ventricular anatomy in every case of hydrocephalus. In some cases, even patients with presumed “communicating” hydrocephalus instead have a ventricular obstruction that clinicians can readily visualize using modern high-resolution MRI technology (such as the constructive interference in steady state [CISS] or fast imaging employing steady-state acquisition [FIESTA] sagittal sequences). , , In our experience, adult patients who underwent shunting procedures in early childhood have a particularly high incidence of noncommunicating (intraventricular) hydrocephalus.
In the initial evaluation of a patient with newly diagnosed hydrocephalus or a previously shunted one, an essential component of the evaluation is to determine whether an ETV (or related procedure) should be considered.
Historically, probably the most challenging unwanted consequence of shunted hydrocephalus has been, and continues to be, CSF overdrainage. In infants, shunting effectively eliminates the forces that normally expand the skull. Later in childhood, this results in an abnormally small cranium, adding to the risk of craniocerebral disproportion. In older children and young adults, chronic overdrainage can result in slit-like ventricles, which in turn negatively influence intracranial compliance and predispose to ventricular shunt obstruction owing to apposition of the ependymal wall or choroid plexus. In older adults, overdrainage can manifest as subdural fluid collections or “spinal headaches.” Each of these conditions increases the likelihood of a shunt revision, which then introduces the specter of shunt infection. The shunt valve currently has the greatest influence on CSF overdrainage.
Probably the most important component of a shunt system is the valve. Neurosurgeons can choose from more than 125 commercially available valves. Since the 1960s the predominant theme in the evolution of valve design has been the goal of preventing CSF overdrainage. This includes the introduction of antisiphon devices (ASDs), flow-restricting elements, multistage valves, and adjustable valves. It is important to understand that manufacturers have little or no direct in vivo intracranial pressure (ICP) or CSF flow data to confirm the efficacy of these devices. Our studies have demonstrated that the in vivo behavior of even the simplest shunt, the ventriculoperitoneal (VP) shunt with a standard differential-pressure valve (DPV), is poorly predicted by the first-order, steady-flow equations that are the basis of the many valve designs.
In our opinion, there is no single valve mechanism, design, or arrangement that is clearly the “best,” nor one that will be adequate for every hydrocephalus patient. There are some valves and valve settings, however, that are poorly suited for adult hydrocephalus and will likely result in a higher complication rate.
Hydrocephalus is a heterogeneous disorder with a wide range of ICPs, ventricular compliance, CSF profiles, and other features across patients. For most pediatric patients, most valve designs work satisfactorily at least in the short term, and surveys have shown significant variations in valve selection for pediatric neurosurgeons. For unclear reasons, adult hydrocephalus is less forgiving, presumably owing to the less elastic brain in addition to the augmented effect of gravity-dependent drainage. Knowledge of valve design and function can greatly help in preventing and managing complications. The following is a primer on shunt valve design and characteristics with which every neurosurgeon placing shunts should be familiar.
The basic building block of most shunt valves is a differential-pressure “check valve” mechanism. The basic design of John Holter continues in some form more than a half-century after its development. In most modern valve designs, it consists of a tiny sphere situated on a ring, with a spring pushing the sphere downward on the ring. CSF passes through the ring orifice, elevating the ball if the pressure exceeds the pressure exerted by the spring. This creates a one-way flow mechanism because reverse flow will not occur as the ball seats down onto the ring.
Fluid flow depends on the differential pressure across the ring: not necessarily the absolute CSF pressure. For example, if the spring is exerting 100 mm H 2 O of pressure downward, CSF will flow if the difference between the inlet and outlet pressure is greater than 100 mm H 2 O, regardless whether the inlet pressure is positive or negative.
A common misconception is that the valve opening pressure must be lower than the measured CSF pressure for CSF to flow across the valve. Our in vivo studies have demonstrated that the interaction between CSF pressure and valve function is not straightforward and that, in general, more CSF drainage occurs than one might predict. We studied a group of patients with idiopathic normal-pressure hydrocephalus (NPH) in whom the mean preoperative recumbent ICP was 164 ± 64 mm H 2 O. Each patient underwent a VP shunt with a standard DPV (as the only valve mechanism) initially set at an opening pressure of 200 mm H 2 O. Whereas one might predict that no CSF flow would occur at this valve setting, the postoperative ICP was statistically lower at 125 ± 69 mm H 2 O ( P = .04).
The concepts of CSF opening pressure (which, by default, is a mean pressure) and bulk CSF flow have been the standards of hydrocephalus pathophysiology teaching for decades. The reality, however, is that the ICP waveform is pulsatile, with superimposed significant elevations of ICP occurring because of coughing and Valsalva maneuvers, as well as intrinsic vasomotor changes. New research is attempting to further the understanding of hydrocephalus by using computational modeling of ICP variations driven by arterial pulsations and inspiration-expiration cycles, as well as changes in CNS compliance that are involved in pathologic states. The phenomenon of how CSF flow can occur with valve pressure settings exceeding the “measured” CSF pressure (ICP, ventricular or lumbar pressure) can be explained by the combination of pulsatile-dynamic ICP physiology interacting with a one-way DPV.
The interaction between pulsatile ICP and the one-way valve mechanism (inherent to DPVs) is paramount to the understanding of shunt overdrainage. Our continuous ICP recordings demonstrate that peak ICPs often exceed 200 mm H 2 O among patients with a mean ICP of 164 mm H 2 O. Even taking into account distal intra-abdominal pressure, one-way CSF egress occurs during these peaks across the DPV. The minute boluses of CSF exiting the cranial vault with every pulsation exceeding the valve pressure results in a reduction of the mean ICP. This “check valve” one-way flow mechanism acts like a tiny pump of sorts, theoretically resulting in more drainage through a DPV set at zero compared with a valveless shunt. This phenomenon is amplified with the use of valve pressure settings lower than the mean preoperative ICP and likely accounts in large part for excessive CSF drainage in clinical practice.
There is a basic misconception that the valve opening pressure must be lower than the mean ventricular pressure for the shunt to flow.
Most commercially available CSF shunt valves contain a DPV mechanism in one form or another. For some it is the sole valve mechanism, whereas in others it is the first-in-series component of the valve assembly. Examples of ball-spring valves are the Strata Valve (Medtronic), Codman Hakim Programmable and Certas Valves (Integra), and the proGAV (Aesculap). A simpler, less accurate mechanism consists of a valve mechanism derived from two apposing semirigid membranes. These valves, which include the Pudenz (Integra), and Codman distal slit valves, are manufactured and then individually tested to determine the approximate opening pressure. They are then segregated into different bins covering a range of pressures. For example, the “medium-pressure valve” bin would contain valves ranging from 50 to 90 mm H 2 O opening pressure.
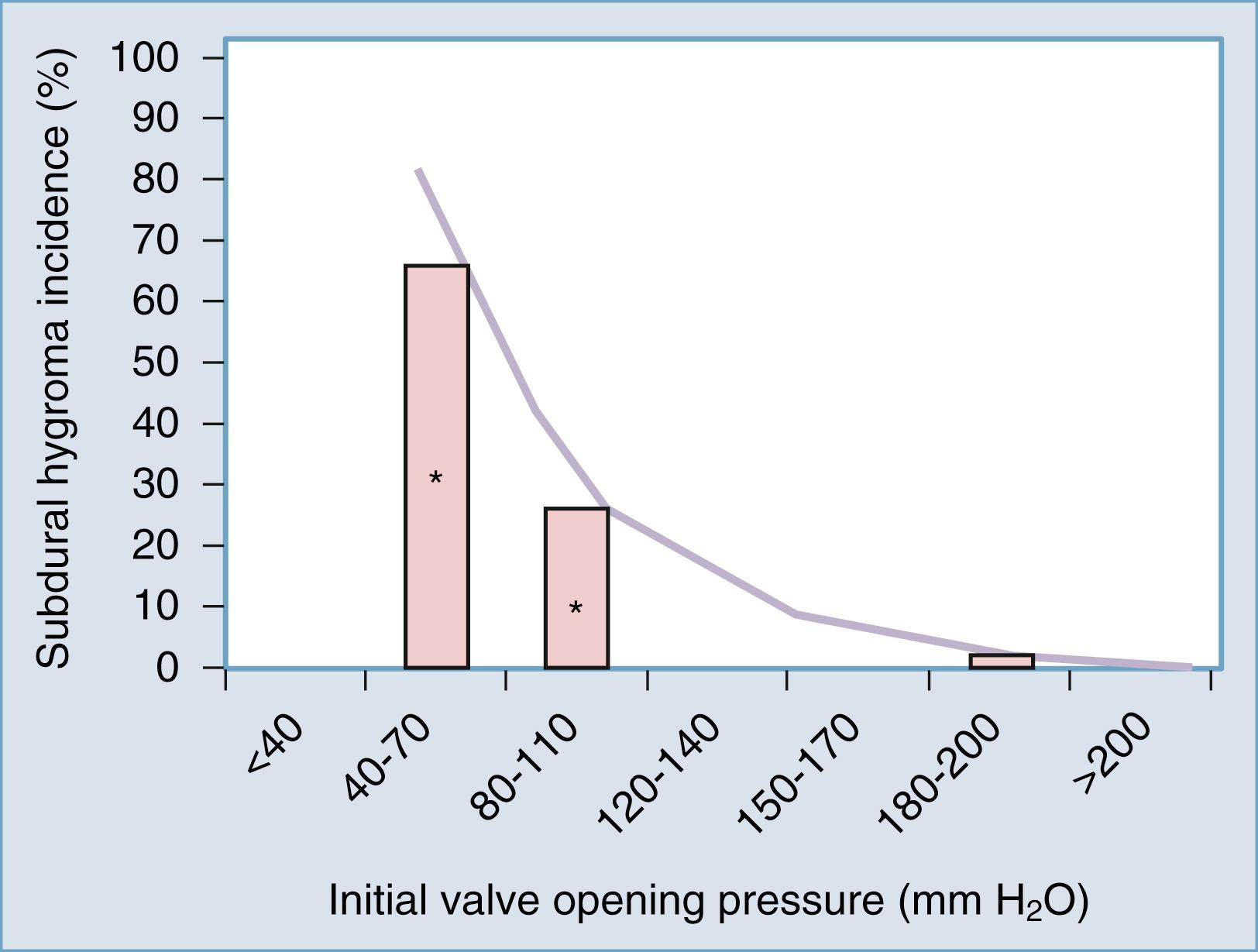
A “programmable,” or adjustable, valve is created by adding a mechanism that enables precise changes of the spring tension of a DPV. There are several competing designs enabling this, all incorporating a magnetic actuation of a rotor. Strictly speaking, these valves are not truly programmable and are better considered as merely adjustable valves. Adjustable valves arose from the realization that fixed-pressure DPVs result in either overdrainage or underdrainage in a significant number of adult patients. The Dutch Normal-Pressure Hydrocephalus Study, a prospective, randomized study, demonstrated that subdural hygromas occurred in 71% of patients with fixed low-pressure valve shunts compared with 34% in patients randomized to medium-pressure shunts. Given the likelihood that expanding or large subdural hygromas pose a risk for subdural hematoma, this is one example showing that there is clearly a risk for selecting an opening pressure that is too low. The analysis of our series of 114 consecutive patients with idiopathic NPH, each treated with an initial valve opening pressure of 200 mm H 2 O, revealed a subdural hygroma incidence of 4%. As shown in Fig. 44.1 , combining the results of the Dutch Normal-Pressure Hydrocephalus Study with our experience suggests a seemingly exponential relationship between subdural hygromas and valve opening pressure.
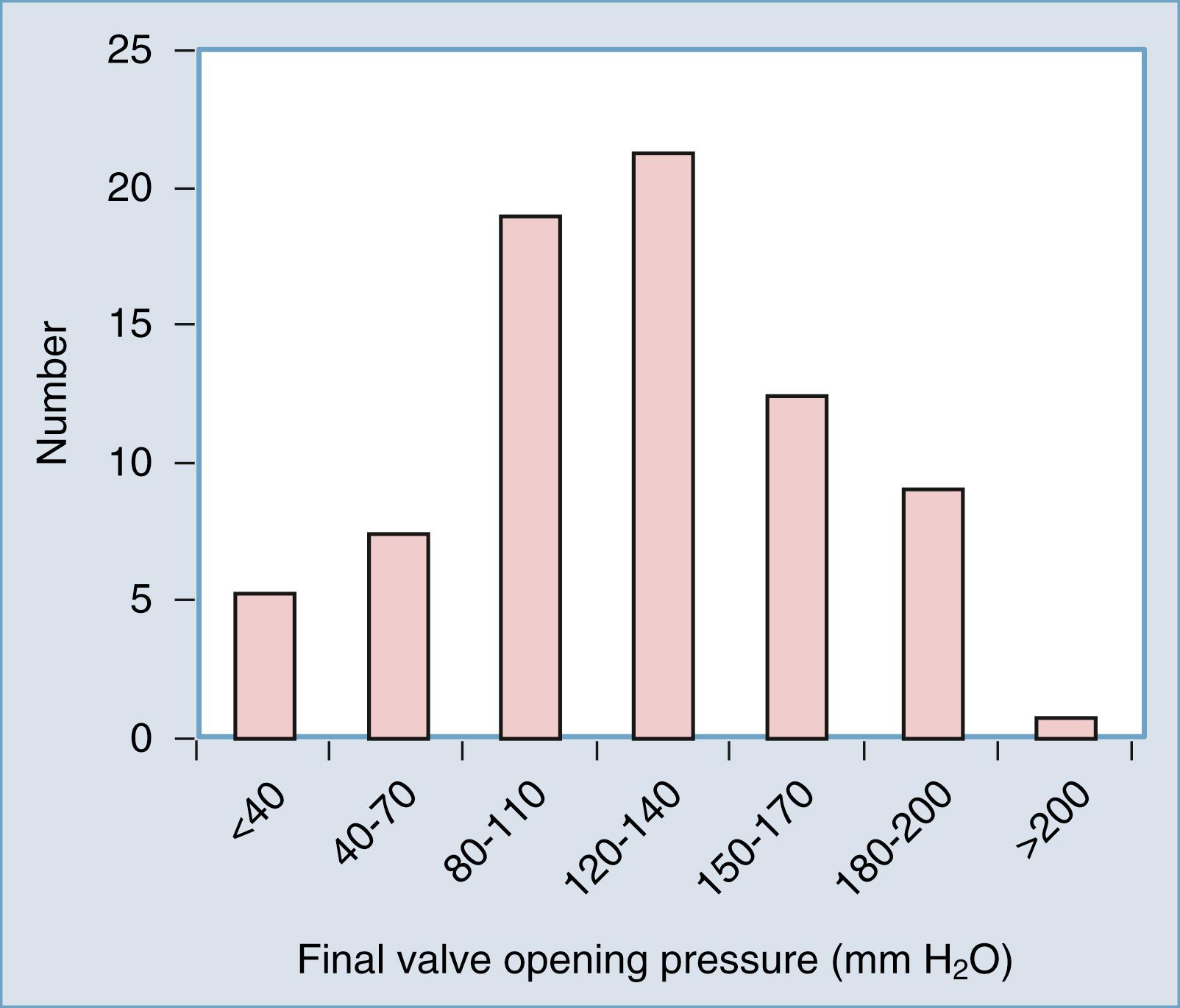
Another justification for the routine use of adjustable valves is based on the range of “final” valve opening pressures when these valves are used. In our retrospective evaluation of 114 consecutive patients with NPH surgically treated with a CSF shunt, the histogram distribution of the final valve opening pressure revealed a roughly gaussian distribution, with most patients in the 120 to 140 mm H 2 O range ( Fig. 44.2 ). This finding closely agrees with those of other large NPH studies. With the wide distribution of final valve pressures shown in Fig. 44.2 (from <40 to >200 mm H 2 O), it is difficult to fathom how a fixed-pressure valve could adequately serve this population unless there were a way of selecting the appropriate valve pressure preoperatively. Although some have suggested algorithms to do so, , none has been independently evaluated or validated.
Most adult patients with hydrocephalus with adjustable valves will require at least one valve adjustment, highlighting the risk for and downside of using a fixed-pressure valve design.
Some neurosurgeons remain reluctant to use adjustable valves on a routine basis (or at all). A systematic review and meta-analysis of patients with normal-pressure hydrocephalus displayed a significant reduction in shunt revision rates as well as reduced incidence of subdural collections with the use of adjustable valves. Arguments that these valves are unreliable or malfunction more frequently than fixed valves are not supported by any clinical study (nor our clinical experience with the implantation of more than 1000 of these devices). Perhaps the biggest drawback is cost. Currently, adjustable valves are 2 to 3 times more expensive than fixed-pressure valves, and there is no clinical study comparing cost-effectiveness. A direct comparison of cost utilization would have to factor in the morbidity associated with repeat operations and associated operative risks with use of fixed-pressure valves.
Another drawback of adjustable valves has been MRI compatibility. Because the rotors harbor permanent magnets, some valve designs (first generation) have a higher susceptibility to large magnetic fields, especially MRI scanners. In our practice, we specifically use valves with locking mechanisms in patients in whom it is anticipated that future MRI studies are required (e.g., any patient with a brain tumor). Many modern valves, including Certas (Integra) and Polaris (Sophysa) are considered to be MRI resistant up to 3 T per manufacturer specifications. Table 44.1 summarizes characteristics of the most commonly used adjustable valves.
| Valve Manufacturer | Valve Model | Variations | Primary Valve Mechanism Type | Ease of Adjustment | MRI Susceptibility | Valve Opening Pressure (range, mm H 2 O) | No. of Gradations | Integral Antisiphon, Flow-Restricting, or Gravitational Device | Antisiphon or Gravitational Valve Name | Clinical Considerations |
|---|---|---|---|---|---|---|---|---|---|---|
| Codman | Hakim Programmable | Various form factors | Ball-spring DPV | Typically easy Not possible to read valve setting Large cumbersome suitcase |
High | 30–200 | 17 | No | ||
| Codman | Hakim Programmable with SiphonGuard | Various form factors | Ball-spring DPV | Same as above | High | 30–200 | 17 | Yes | SiphonGuard | No studies demonstrating efficacy of SiphonGuard |
| Codman | Certas Plus | Various form factors | Ball-spring DPV | Very easy to adjust | Very low | 25–400 (virtual off) | 8 | Yes | ||
| Medtronic | Strata | Various form factors | Ball-spring DPV | StrataVarius adjustment tool very easy to use | High | 5 | Yes | Siphon Control Device | Siphon control device mechanism may fail if thick scar forms over it Use in low-pressure hydrocephalus states may be contraindicated |
|
| Medtronic | Strata NSC | Various form factors | Ball-spring DPV | Same as above Must insert separate SIM card | High | No (NSC means no siphon control) | ||||
| Sophysa | Polaris | Range of adjustable opening pressures | Ball-spring DPV | Can be very challenging with thick scalp | Very low | 10–140, 30–200, 40–300, 50–400 | 5 | No | ||
| Aesculap | proGAV | Ball-spring DPV | Can be very challenging with thick scalp Moderately painful for most patients |
Very low | No | |||||
| Aesculap | proGAV with ShuntAssistant (also called proSA) | Ball-spring DPV | Same as above | Very low | Yes | ShuntAssistant | ||||
| Integra | Orbis Sigma II | Self-adjusting pin in orifice design: flow restricting | Not externally adjustable | None | N/A | Basic design is flow restricting | No clinical evidence demonstrating clinical efficacy |
Soon after the first DPVs were implanted in the 1950s, CSF overdrainage complications were recognized. , Early studies documented significantly negative ICPs in shunted patients in the upright position. At the time, it was assumed that overdrainage complications (such as subdural hematomas) were due to this gravity-dependent drainage (which was originally termed “siphoning”). Over the ensuing years, multiple device types have been manufactured to counteract excessive drainage, most of which are add-on devices placed immediately distal, in series with the primary DPV mechanism.
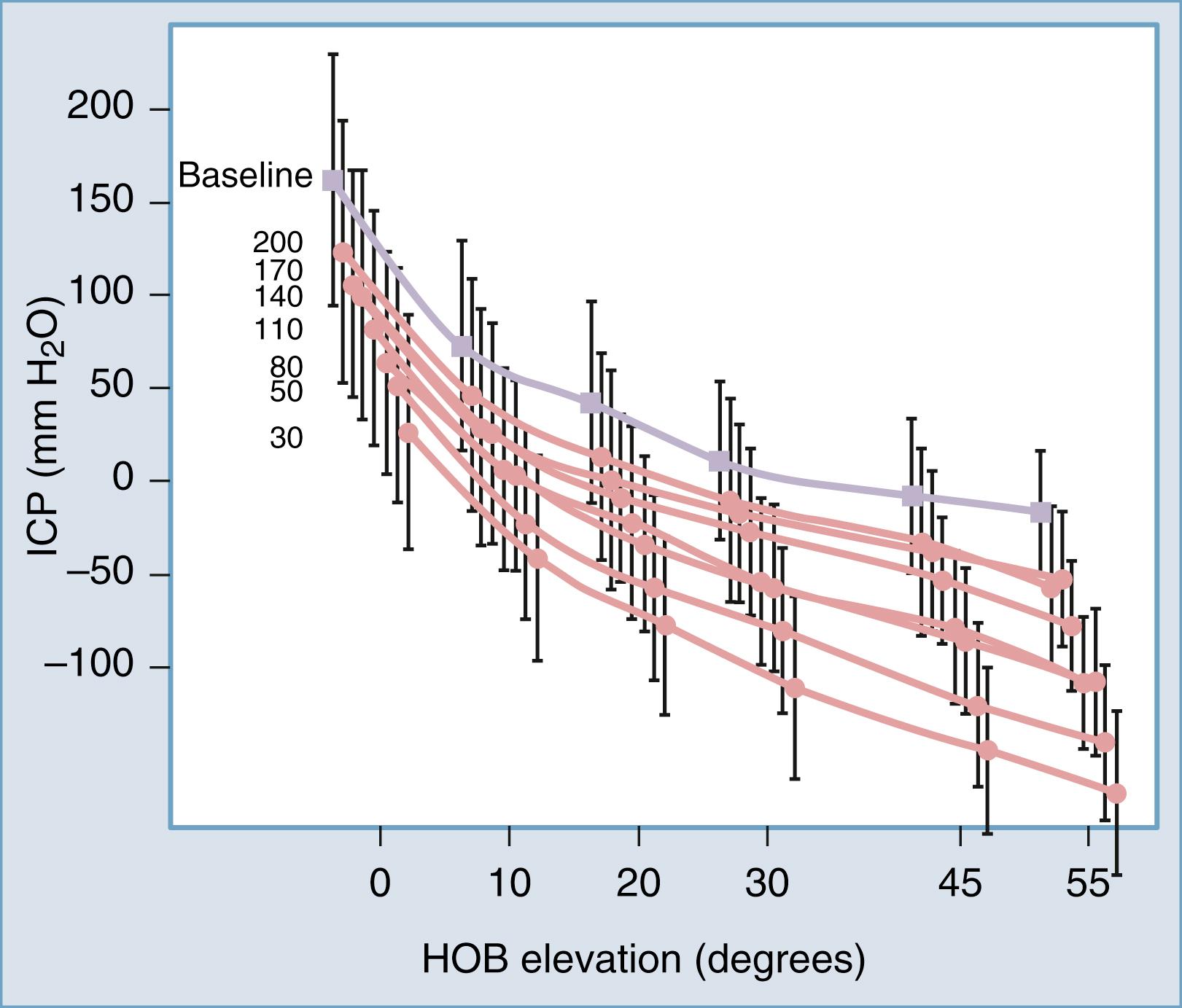
In our opinion, the etiology of shunt-related CSF overdrainage is multifactorial and heterogeneous, with an excessive emphasis being placed on the role of siphoning. Our ICP studies in patients with idiopathic NPH, as well as others, suggest that historically the primary problem has been the use of DPV settings that were too low, with gravity-dependent drainage being a compounding phenomenon. It is important to note that as any person assumes an upright position, ICP decreases whether they have a shunt or not. As a matter of fact, in the standing position, healthy adults have a slightly subatmospheric ICP (measured level with the foramen of Monro). When you place a shunt with solely a DPV, the curve of ICP compared with head-of-bed elevation in shunted patients nearly parallels that of the preshunt state ( Fig. 44.3 ). In other words, a shunt with a DPV essentially lowers the ICP nearly equally across the head-of-bed angulation range. The degree of ICP reduction is largely a function of the valve opening pressure, again supporting the notion that shunt overdrainage occurs primarily as a result of valve pressure selection.
In vivo ICP data suggest that the etiology of shunt overdrainage with DPVs is in many, if not most, cases a result of selection of a valve opening pressure that is too low, rather than so-called siphoning.
Even with the use of properly selected DPV opening-pressure settings, gravity-dependent drainage can still be problematic, albeit in a minority of patients. Currently, it is not possible to predict which patients will require a second valve mechanism in addition to the DPV.
The ASD design is the oldest, consisting of a membrane that is mechanically coupled to the subcutaneous tissue overlying it. The pressure differential between the internal valve lumen and the atmosphere, transmitted across the skin and ASD membrane, determines the flow-pressure characteristics of the ASD device. When the intraluminal pressure becomes significantly negative relative to atmospheric pressure, the membrane is drawn inward, interacting with other fixed components of the ASD and thereby creating an increased ASD-valve pressure gradient. A newer generation ASD design (termed a siphon control device ) is found in the Delta Chamber Valve (Medtronic), which is incorporated in the Delta Valve (fixed pressure) and the Strata Valve (adjustable DPV). For patients with overdrainage headaches, the addition of an ASD can be very effective.
For some patients with hydrocephalus, the presence of an ASD is detrimental. This so-called low-pressure hydrocephalus syndrome, of which the incidence has not been quantified but has been presumed to be less than 5%, occurs in both children and adults. Given the low incidence of low-pressure hydrocephalus, we do not feel that this risk constitutes a contraindication to the general use of products with ASDs. For clinicians who routinely use ASD devices, however, it is important that they become familiar with the signs and symptoms of low-pressure hydrocephalus. ,
A patient who does not improve clinically (or deteriorates), continues to have significant ventriculomegaly that does not change, has low measured ICP, or has a patent shunt with an ASD should not be diagnosed as a nonresponder until the diagnosis of low-pressure hydrocephalus has been ruled out.
Another approach taken to counteract shunt overdrainage is the incorporation of a CSF flow-restriction mechanism. The premise is that shunt overdrainage can be averted if the rate of CSF drainage is limited. There are several different design approaches to achieve flow restriction. The Orbis-Sigma II valve (Integra) was designed to directly address flow restriction by using a multistage needle-valve design. Depending on the differential pressure, a needle is raised or lowered through a small orifice. The diameter of the needle at any given point will determine the cross-sectional area through which the CSF can flow. The manufacturer claims that in stage I it functions as a low-pressure DPV to minimize underdrainage complications. When conditions favor postural or vasogenic overdrainage, the needle moves to stage II, and the valve functions as a flow regulator to maintain flow within physiologic limits. Lastly, the manufacture claims that if ICP elevates abruptly, the valve opens widely to function as a safety valve, allowing rapid CSF flow.
There is limited in vivo clinical evidence, however, to support the efficacy of an ASD or the Orbis-Sigma valve design. A large, prospective, randomized study comparing a standard DPV, the Delta Valve, and the Orbis-Sigma valve (the original design, predating the Orbis-Sigma II) found no statistical difference in the rate of ventricular reduction, the final ventricle size, or the incidence of clinical shunt failure. , In a retrospective study comparing the Orbis-Sigma valve with a standard DPV in NPH, Weiner and colleagues found no significant difference in the time to initial malfunction (shunt survival) between the Orbis-Sigma valve and the DPV shunts. There were three subdural hematomas and one infection in the Orbis-Sigma valve group compared with no complications in the DPV group ( P = .11). Nearly 90% of all patients experienced improvement in gait after shunting, regardless of the valve system that was used. The Orbis-Sigma II valve was studied in a single-arm, prospective, multicenter clinical study that included 270 adult hydrocephalic patients. Shunt obstruction occurred in 14% of patients. The probability of having experienced a shunt failure–free interval at 1 year was 71%, and at 2 years, it was 67%, with no difference in shunt survival in pediatric versus adult groups. According to the authors, overdrainage occurred in only 2% of patients, although their definition of overdrainage was very narrow. Clinical underdrainage was not assessed. One retrospective observational cohort study of pediatric and adult patients who received an ASD in series with a standard adjustable valve found a significant decrease in proximal catheter obstruction rates at 1 year and 5 years compared with a system without an ASD.
Another approach to flow restriction is the incorporation of an add-on high–flow resistance element. The Codman SiphonGuard (Integra) is a coiled helical device that is placed immediately distal to a DPV (adjustable or fixed pressure), and is also available integrated with existing Codman valves. The SiphonGuard device is unaffected by scar tissue encapsulation or external pressure. To our knowledge, to date there have been no published clinical studies evaluating the SiphonGuard device in vivo. In vitro benchtop testing from an independent laboratory demonstrated that switching between the primary and secondary pathways was initiated at a fluid flow rate between 700 and 1800 μL/min.
Miyake and associates created an externalized loop connected to an indwelling VP shunt and measured CSF flow rates in patients with NPH. They assessed the Codman adjustable (DPV) and Orbis-Sigma valves. They demonstrated that shunt flow differed across patients, but in general, flow increased as the adjustable valve setting was lowered regardless of whether the patient was recumbent or sitting. At higher opening pressures of the adjustable valve (140–200 mm H 2 O) in the recumbent position, the flow was intermittent, whereas at the lowest setting of 30 mm H 2 O, the flow rate was 100 to 200 μL/min. In the sitting position, the shunt flow rates were higher, ranging from 200 to 600 μL/min. Based on these data in which measured CSF flow rates did not exceed 600 μL/min, it is unclear whether the flow-restricting circuit in the SiphonGuard would be activated at all in NPH. Similar flow data do not exist for younger hydrocephalus patients to our knowledge, although presumably the flow rates may be higher than in NPH patients. For the Orbis-Sigma valve, the flow rates were very similar to the adjustable valve set at 200 mm H 2 O in both the recumbent and sitting positions. This actual in vivo flow data would appear to contradict the Orbis-Sigma manufacturer’s concept that in stage I, it functions as a low-pressure DPV. There are no in vivo data available to either confirm or refute the manufacturer’s claims regarding stage II and III activity.
Medtronic manufactures a peritoneal catheter with a smaller internal diameter, which also achieves a fixed added flow resistance. This catheter is intended to be used in conjunction with a DPV. To date, there have been no published clinical studies addressing the clinical efficacy or pitfalls of this approach. Interesting to note, Sotelo and colleagues reported the use of a valveless shunt that incorporated solely a peritoneal catheter with a highly precise cross-sectional internal diameter of 0.51 mm to limit CSF flow (a much higher resistance compared with the Medtronic catheter). At the end of the observation period of 44 ± 17 months, the failure rate of the shunting device was 14% for the high-resistance valveless shunt compared with 46% for controls ( P < .0002). Shunt endurance was 88% for patients with the valveless shunt and 60% for patients with conventional valve shunts. Signs of overdrainage developed in 40% of patients treated with valved shunts, but apparently were not observed in patients with the high-resistance valveless shunt.
Another approach to combat overdrainage has been with the so-called gravitational devices. This is accomplished through a mechanical mechanism using dense spheres that divert the CSF into one of two parallel DPVs. When the patient is in the recumbent position, the opening pressure is zero, whereas CSF is diverted to a DPV with a higher opening pressure in the upright position. This approach is not new (the Integra Horizontal-Vertical Valve has been marketed for more than two decades), but recent improved designs have offered a graded transition.
Aesculap manufactures both fixed (ShuntAssistant) and adjustable (proSA) gravitational valves. When used alone without a series adjustable DPV, the fixed gravitational devices have shown mixed results with regard to preventing overdrainage or underdrainage clinical conditions. , It was subsequently recommended that these gravitational devices be used in series distal to an adjustable DPV, although this, too, has been beset with technical problems. Our anecdotal experience with the add-on Aesculap ShuntAssistant (fixed) valve is that although it could be effective in alleviating overdrainage headaches, underdrainage symptoms led to a large proportion of these devices being explanted.
The combination of an adjustable DPV together with the proSA allows the maximal degree of shunt management. Analogous to the concept that a fixed-pressure DPV does not allow tailoring to individual patient needs, it is likely that addressing the heterogeneous overdrainage problems (in patients in whom an adjustable DPV failed) is best done with an adjustable gravitational device. We have encountered patients with NPH who cannot tolerate further lowering of an adjustable DPV because of overdrainage symptoms (headache, hearing loss) and yet who do not improve clinically (gait, bladder control). The use of the adjustable DPV and the proSA has been highly effective in correcting both the simultaneous overdrainage and underdrainage states in many of these cases in our anecdotal experience. In other patients with chronic headaches without postural dependence, this approach has not been effective. A major drawback to the combined adjustable valve approach is high cost. See Table 44.2 for a summary of antisiphon, flow-restricting, and gravitational device characteristics.
| Valve Manufacturer | Valve Model | Form Factors | Mechanism | Adjustable? | Ease of Adjustment | MRI Susceptibility | Valve Opening Pressure (range, mm H 2 O) | No. of Adjustment Gradations | Integration with DPV | Available as Stand-Alone? | Clinical Considerations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Codman | SiphonGuard | Low profile, cylindrical | At flow rates exceeding 0.7–1.5 μL/min, primary pathway closes and flow is diverted to the high-resistance secondary pathway | No | N/A | None | N/A | N/A | Available integrated with various Codman valves | Yes | Lack of clinical study documenting efficacy or corroborating manufacturer claims |
| Medtronic | Delta Chamber | Low profile, button sized | Siphon control device is a membrane housed within chamber that changes flow resistance relative to atmospheric pressure | No | N/A | None | N/A | N/A | Integrated into Strata II and Delta valves | Yes | Siphon control device mechanism may be ineffectual owing to scar formation or in low-pressure hydrocephalus states |
| Aesculap | ShuntAssistant | Low profile cylindrical | Tantalum spheres direct CSF to differential pressure valve in upright position Supine valve pressure always zero |
No | N/A | None | Various upright pressure level settings available: 100, 150, 200, 250, 300, and 350 | N/A | No | Yes | Unclear how to determine which model to use for any given patient |
| Aesculap | proSA | Moderate low profile | Same as ShuntAssistant | Yes | Programming device causes moderate discomfort and can be very challenging with thick scalp | None at 3 T | Upright pressure level setting adjustable between 0 and 400 | 24 | No | Yes | Combined with adjustable DPV provides highest degree of management options but at greatly added cost |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here