Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Shock is an acute process characterized by the body's inability to deliver adequate oxygen to meet the metabolic demands of vital organs and tissues. Insufficient oxygen at the tissue level is unable to support normal aerobic cellular metabolism, resulting in a shift to less efficient anaerobic metabolism. As shock progresses, increases in tissue oxygen extraction are unable to compensate for this deficiency in oxygen delivery, leading to progressive clinical deterioration and lactic acidosis. If inadequate tissue perfusion persists, adverse vascular, inflammatory, metabolic, cellular, endocrine, and systemic responses worsen physiologic instability.
Compensation for inadequate oxygen delivery involves a complex set of responses that attempt to preserve oxygenation of the vital organs (i.e., brain, heart, kidneys, liver) at the expense of other organs (i.e., skin, gastrointestinal tract, muscles). Of importance, the brain is especially sensitive to periods of poor oxygen supply given its lack of capacity for anaerobic metabolism. Initially, shock is often well compensated, but it may rapidly progress to an uncompensated state requiring more aggressive therapies to achieve clinical recovery. The combination of a continued presence of an inciting trigger and the body's exaggerated and potentially harmful neurohumoral, inflammatory, and cellular responses lead to the progression of shock. Irrespective of the underlying cause of shock, the specific pattern of response, pathophysiology, clinical manifestations, and treatment may vary significantly depending on the specific etiology (which may be unknown), the clinical circumstances, and an individual patient's biologic response to the shock state. Untreated shock causes irreversible tissue and organ injury (i.e., irreversible shock ) and, ultimately, death.
Shock occurs in approximately 2% of all hospitalized infants, children, and adults in developed countries, and the mortality rate varies substantially depending on the etiology and clinical circumstances. Of patients who do not survive, most do not die in the acute hypotensive phase of shock, but rather as a result of associated complications and multiple-organ dysfunction syndrome (MODS). MODS is defined as any alteration of organ function that requires medical support for maintenance, and the presence of MODS in patients with shock substantially increases the probability of death. In pediatrics, educational efforts and the utilization of standardized management guidelines that emphasize early recognition and intervention along with the rapid transfer of critically ill patients to a pediatric intensive care unit (PICU) have led to decreases in the mortality rate for shock ( Figs. 88.1 and 88.2 ).
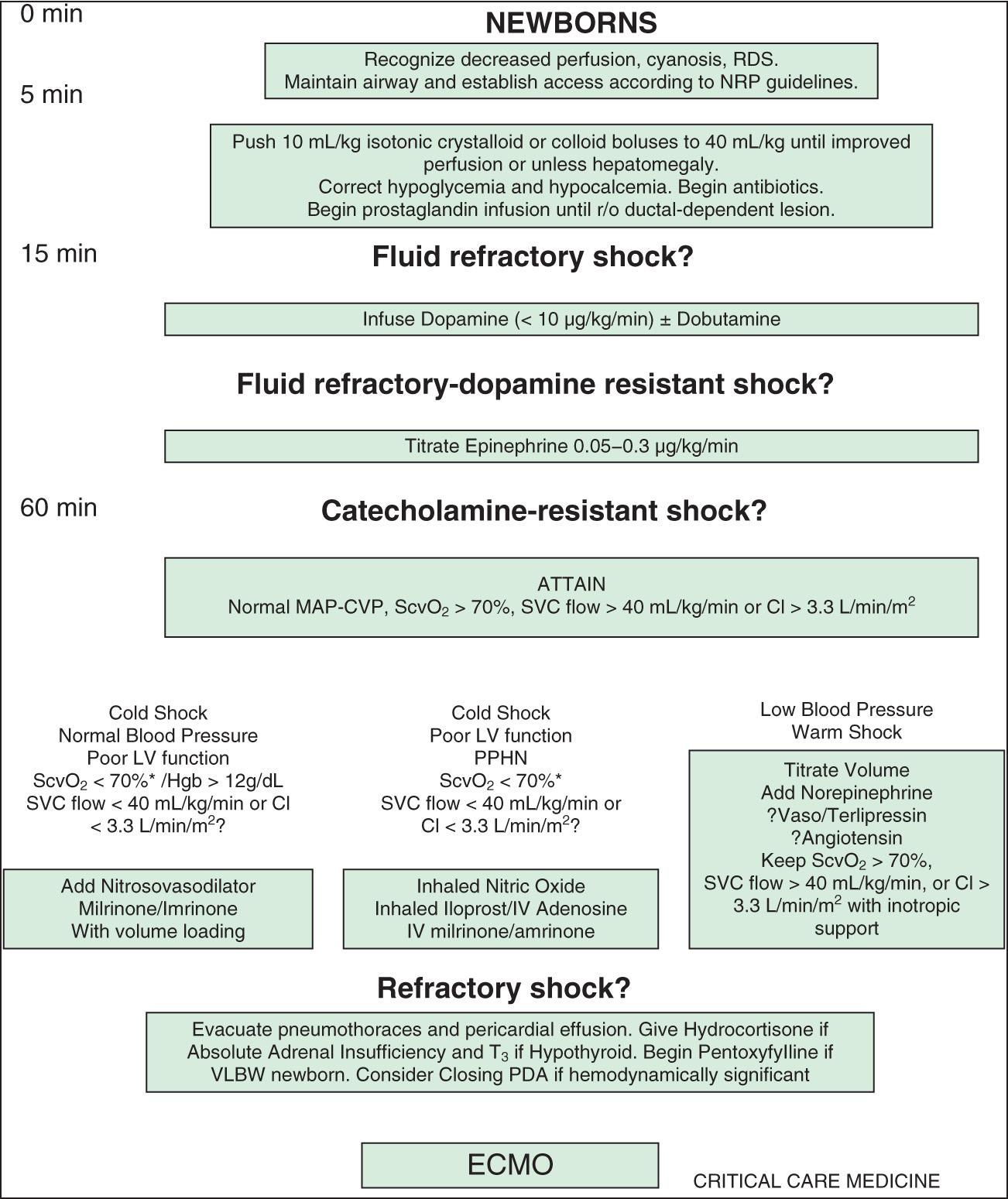
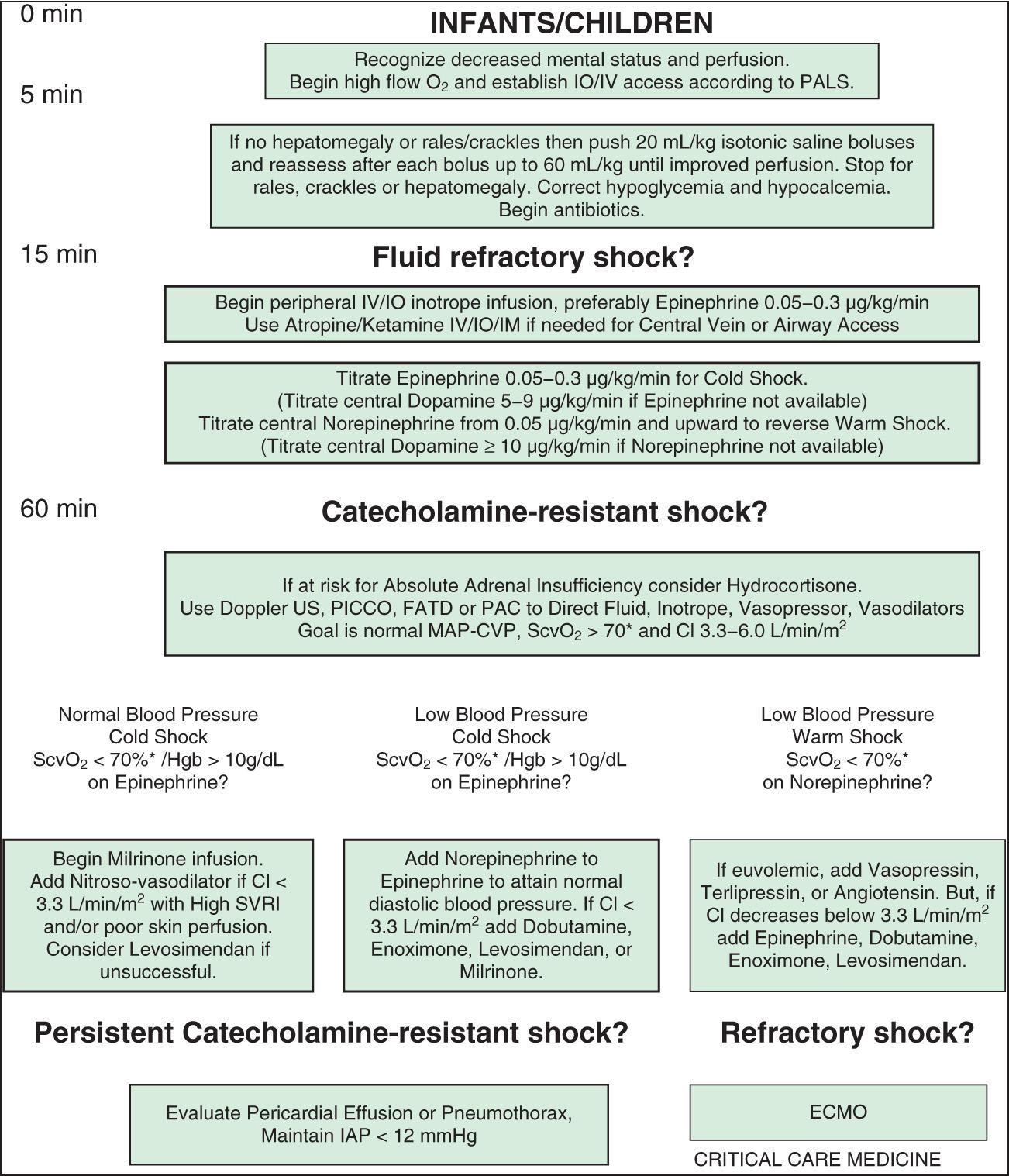
Shock classification systems generally define 5 major types of shock: hypovolemic, cardiogenic, distributive, obstructive, and septic ( Table 88.1 ). Hypovolemic shock , the most common cause of shock in children worldwide, is most frequently caused by diarrhea, vomiting, or hemorrhage. Cardiogenic shock is seen in patients with congenital heart disease (before or after surgery, including heart transplantation) or those with congenital or acquired cardiomyopathies, including acute myocarditis. Obstructive shock stems from any lesion that creates a mechanical barrier that impedes adequate cardiac output, which includes pericardial tamponade, tension pneumothorax, pulmonary embolism, and ductus-dependent congenital heart lesions. Distributive shock is caused by inadequate vasomotor tone, which leads to capillary leak and maldistribution of fluid into the interstitium. Septic shock is often discussed synonymously with distributive shock, but the septic process usually involves a more complex interaction of distributive, hypovolemic, and cardiogenic shock.
| HYPOVOLEMIC | CARDIOGENIC | DISTRIBUTIVE | SEPTIC | OBSTRUCTIVE |
|---|---|---|---|---|
| Decreased preload secondary to internal or external losses | Cardiac pump failure secondary to poor myocardial function | Abnormalities of vasomotor tone from loss of venous and arterial capacitance |
|
Decreased cardiac output secondary to direct impediment to right- or left-sided heart outflow or restriction of all cardiac chambers |
| POTENTIAL ETIOLOGIES | ||||
|
|
|
|
|
An initial insult triggers shock, leading to inadequate oxygen delivery to organs and tissues. Compensatory mechanisms attempt to maintain blood pressure (BP) by increasing cardiac output and systemic vascular resistance (SVR). The body also attempts to optimize oxygen delivery to the tissues by increasing oxygen extraction and redistributing blood flow to the brain, heart, and kidneys at the expense of the skin and gastrointestinal (GI) tract. These responses lead to an initial state of compensated shock in which BP is maintained. If treatment is not initiated or is inadequate during this period, decompensated shock develops, with hypotension and tissue damage that may lead to multisystem organ dysfunction and, ultimately, death ( Tables 88.2 and 88.3 ).
| ORGAN SYSTEM | CRITERIA FOR DYSFUNCTION |
|---|---|
| Cardiovascular |
|
| Respiratory |
|
| Neurologic | GCS score ≤11 or Acute change in mental status with decrease in GCS score ≥3 points from abnormal baseline |
| Hematologic |
|
| Renal |
|
| Hepatic |
|
| ORGAN SYSTEM | ↓ PERFUSION | ↓↓ PERFUSION | ↓↓↓ PERFUSION |
|---|---|---|---|
| Central nervous system | — | Restless, apathetic, anxious | Agitated/confused, stuporous, coma |
| Respiration | — | ↑ Ventilation | ↑↑ Ventilation |
| Metabolism | — | Compensated metabolic acidemia | Uncompensated metabolic acidemia |
| Gut | — | ↓ Motility | Ileus |
| Kidney | ↓ Urine volume | Oliguria (<0.5 mL/kg/hr) | Oliguria/anuria |
| ↑ Urinary specific gravity | |||
| Skin | Delayed capillary refill | Cool extremities | Mottled, cyanotic, cold extremities |
| Cardiovascular system | ↑ Heart rate | ↑↑ Heart rate | ↑↑ Heart rate |
| ↓ Peripheral pulses | ↓ Blood pressure, central pulses only |
In the early phases of shock, multiple compensatory physiologic mechanisms act to maintain BP and preserve tissue perfusion and oxygen delivery. Cardiovascular effects include increases in heart rate (HR), stroke volume, and vascular smooth muscle tone, which are regulated through sympathetic nervous system activation and neurohormonal responses. Respiratory compensation involves greater carbon dioxide (CO 2 ) elimination in response to the metabolic acidosis and increased CO 2 production from poor tissue perfusion. Renal excretion of hydrogen ions (H + ) and retention of bicarbonate (HCO 3 − ) also increase in an effort to maintain normal body pH (see Chapter 68.7 ). Maintenance of intravascular volume is facilitated via sodium regulation through the renin-angiotensin-aldosterone and atrial natriuretic factor axes, cortisol and catecholamine synthesis and release, and antidiuretic hormone secretion. Despite these compensatory mechanisms, the underlying shock and host response lead to vascular endothelial cell injury and significant leakage of intravascular fluids into the interstitial extracellular space.
Another important aspect of the initial pathophysiology of shock is the impact on cardiac output. All forms of shock affect cardiac output through several mechanisms, with changes in HR, preload, afterload, and myocardial contractility occurring separately or in combination ( Table 88.4 ). Hypovolemic shock is characterized primarily by fluid loss and decreased preload. Tachycardia and an increase in SVR are the initial compensatory responses to maintain cardiac output and systemic BP. Without adequate volume replacement, hypotension develops, followed by tissue ischemia and further clinical deterioration. When there is preexisting low plasma oncotic pressure (caused by nephrotic syndrome, malnutrition, hepatic dysfunction, acute severe burns, etc.), even further volume loss and exacerbation of shock may result from endothelial breakdown and worsening capillary leak.
Hypovolemic shock may be a result of direct blood loss through hemorrhage or abnormal loss of body fluids (diarrhea, vomiting, burns, diabetes mellitus or insipidus, nephrosis).
Hypovolemic shock may also result from hypoproteinemia (liver injury, or as a progressive complication of increased capillary permeability).
Distributive shock (neurogenic, anaphylaxis, or septic shock) occurs when there is loss of vascular tone—venous, arterial, or both (sympathetic blockade, local substances affecting permeability, acidosis, drug effects, spinal cord transection).
Sepsis may change the capillary permeability in the absence of any change in capillary hydrostatic pressure (endotoxins from sepsis, excess histamine release in anaphylaxis).
Peripheral hypoperfusion may result from any condition that affects the heart's ability to pump blood efficiently (ischemia, acidosis, drugs, constrictive pericarditis, pancreatitis, sepsis).
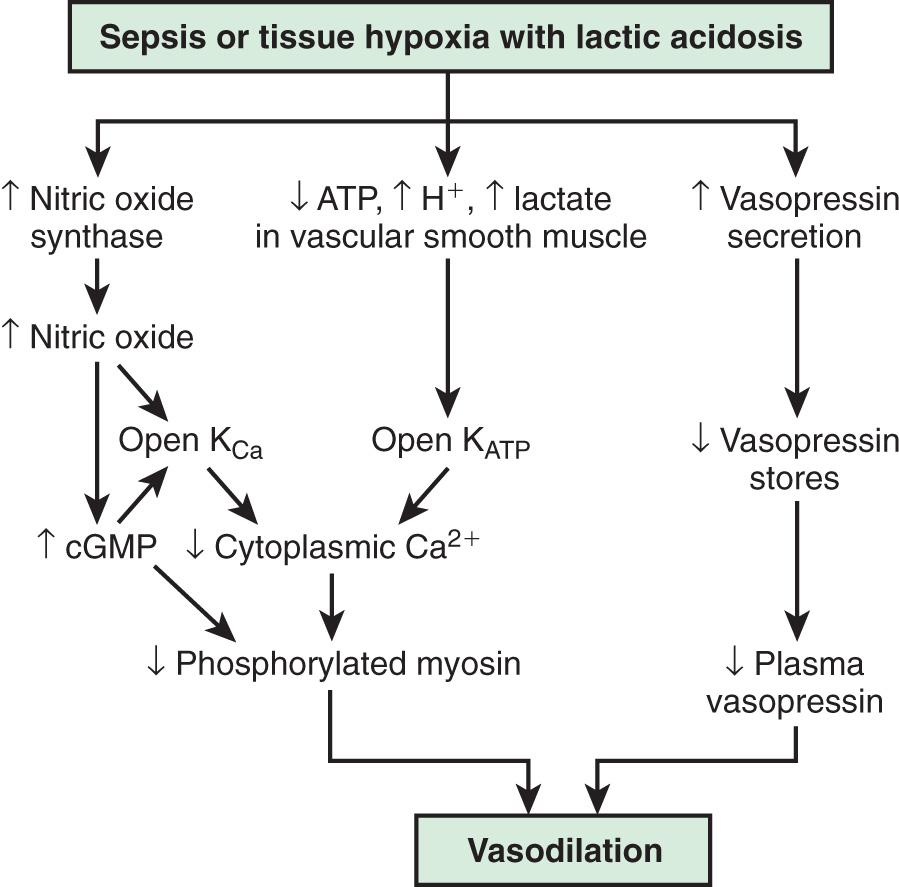
In contrast, the underlying pathophysiologic mechanism leading to distributive shock is a state of abnormal vasodilation and decreased SVR. Sepsis, hypoxia, poisoning, anaphylaxis, spinal cord injury, or mitochondrial dysfunction can cause vasodilatory shock ( Fig. 88.3 ). The lowering of SVR is accompanied initially by a maldistribution of blood flow away from vital organs and a compensatory increase in cardiac output. This process leads to significant decreases in both preload and afterload. Therapies for distributive shock must address both these problems simultaneously.
Cardiogenic shock may be seen in patients with myocarditis, cardiomyopathy, arrhythmias and congenital heart disease (generally following cardiac surgery) (see Chapter 461 ). In these patients, myocardial contractility is affected, leading to systolic and/or diastolic dysfunction. The later phases of all forms of shock frequently have a negative impact on the myocardium, leading to development of a cardiogenic component to the initial shock state.
Septic shock is generally a unique combination of distributive, hypovolemic, and cardiogenic shock. Hypovolemia from intravascular fluid losses occurs through capillary leak. Cardiogenic shock results from the myocardial-depressant effects of sepsis, and distributive shock is the result of decreased SVR. The degree to which a patient exhibits each of these responses varies, but there are frequently alterations in preload, afterload, and myocardial contractility.
In septic shock, it is important to distinguish between the inciting infection and the host inflammatory response. Normally, host immunity prevents the development of sepsis through activation of the reticular endothelial system along with the cellular and humoral immune systems. This host immune response produces an inflammatory cascade of toxic mediators, including hormones, cytokines, and enzymes. If this inflammatory cascade is uncontrolled, derangement of the microcirculatory system leads to subsequent organ and cellular dysfunction.
The systemic inflammatory response syndrome ( SIRS ) is an inflammatory cascade that is initiated by the host response to an infectious or noninfectious trigger ( Table 88.5 ). This inflammatory cascade is triggered when the host defense system does not adequately recognize and/or eliminate the triggering event. The inflammatory cascade initiated by shock can lead to hypovolemia, cardiac and vascular failure, acute respiratory distress syndrome (ARDS), insulin resistance, decreased cytochrome P450 activity (decreased steroid synthesis), coagulopathy, and unresolved or secondary infection. Tumor necrosis factor (TNF) and other inflammatory mediators increase vascular permeability, causing diffuse capillary leak, decreased vascular tone, and an imbalance between perfusion and metabolic demands of the tissues. TNF and interleukin (IL)-1 stimulate the release of proinflammatory and antiinflammatory mediators, causing fever and vasodilation. Proinflammatory mediators include IL-6, IL-12, interferon-γ, and macrophage migration inhibitory factor; antiinflammatory cytokines include IL-10, transforming growth factor-β, and IL-4. Arachidonic acid metabolites lead to the development of fever, tachypnea, ventilation-perfusion abnormalities, and lactic acidosis. Nitric oxide (NO), released from the endothelium or inflammatory cells, is a major contributor to hypotension. Myocardial depression is caused directly by myocardial-depressant factors, TNF, and some interleukins and is further depressed by depleted catecholamines, increased β-endorphin, and production of myocardial NO.
Bacteremia or meningitis (S treptococcus pneumoniae, Haemophilus influenzae type b, Neisseria meningitidis, group A streptococcus, Staphylococcus aureus )
Viral illness (influenza, enteroviruses, hemorrhagic fever group, herpes simplex virus, respiratory syncytial virus, cytomegalovirus, Epstein-Barr virus)
Encephalitis (arboviruses, enteroviruses, herpes simplex virus)
Rickettsiae (Rocky Mountain spotted fever, Ehrlichia, Q fever)
Syphilis
Vaccine reaction (pertussis, influenza, measles)
Toxin-mediated reaction (toxic shock, staphylococcal scalded skin syndrome)
Pneumonia (bacteria, virus, mycobacteria, fungi, allergic reaction)
Pulmonary emboli
Heart failure
Arrhythmia
Pericarditis
Myocarditis
Adrenal insufficiency (adrenogenital syndrome, Addison disease, corticosteroid withdrawal)
Electrolyte disturbances (hypo- or hypernatremia; hypo- or hypercalcemia)
Diabetes insipidus
Diabetes mellitus
Inborn errors of metabolism (organic acidosis, urea cycle, carnitine deficiency, mitochondrial disorders)
Hypoglycemia
Reye syndrome
Gastroenteritis with dehydration
Volvulus
Intussusception
Appendicitis
Peritonitis (spontaneous, associated with perforation or peritoneal dialysis)
Necrotizing enterocolitis
Hepatitis
Hemorrhage
Pancreatitis
Anemia (sickle cell disease, blood loss, nutritional)
Methemoglobinemia
Splenic sequestration crisis
Leukemia or lymphoma
Hemophagocytic syndromes
Intoxication (drugs, carbon monoxide, intentional or accidental overdose)
Intracranial hemorrhage
Infant botulism
Trauma (child abuse, accidental)
Guillain-Barré syndrome
Myasthenia gravis
Anaphylaxis (food, drug, insect sting)
Hemolytic-uremic syndrome
Kawasaki disease
Erythema multiforme
Hemorrhagic shock–encephalopathy syndrome
Poisoning
Toxic envenomation
Macrophage activation syndrome
Idiopathic systemic capillary leak (Clarkson) syndrome
The inflammatory cascade is initiated by toxins or superantigens through macrophage binding or lymphocyte activation ( Fig. 88.4 ). The vascular endothelium is both a target of tissue injury and a source of mediators that may cause further injury. Biochemical responses include the production of arachidonic acid metabolites, release of myocardial-depressant factors and endogenous opiates, activation of the complement system, and production and release of other mediators, which may be proinflammatory or antiinflammatory. The balance among these mediator groups for an individual patient contributes to the progression (and resolution) of disease and affects the prognosis.
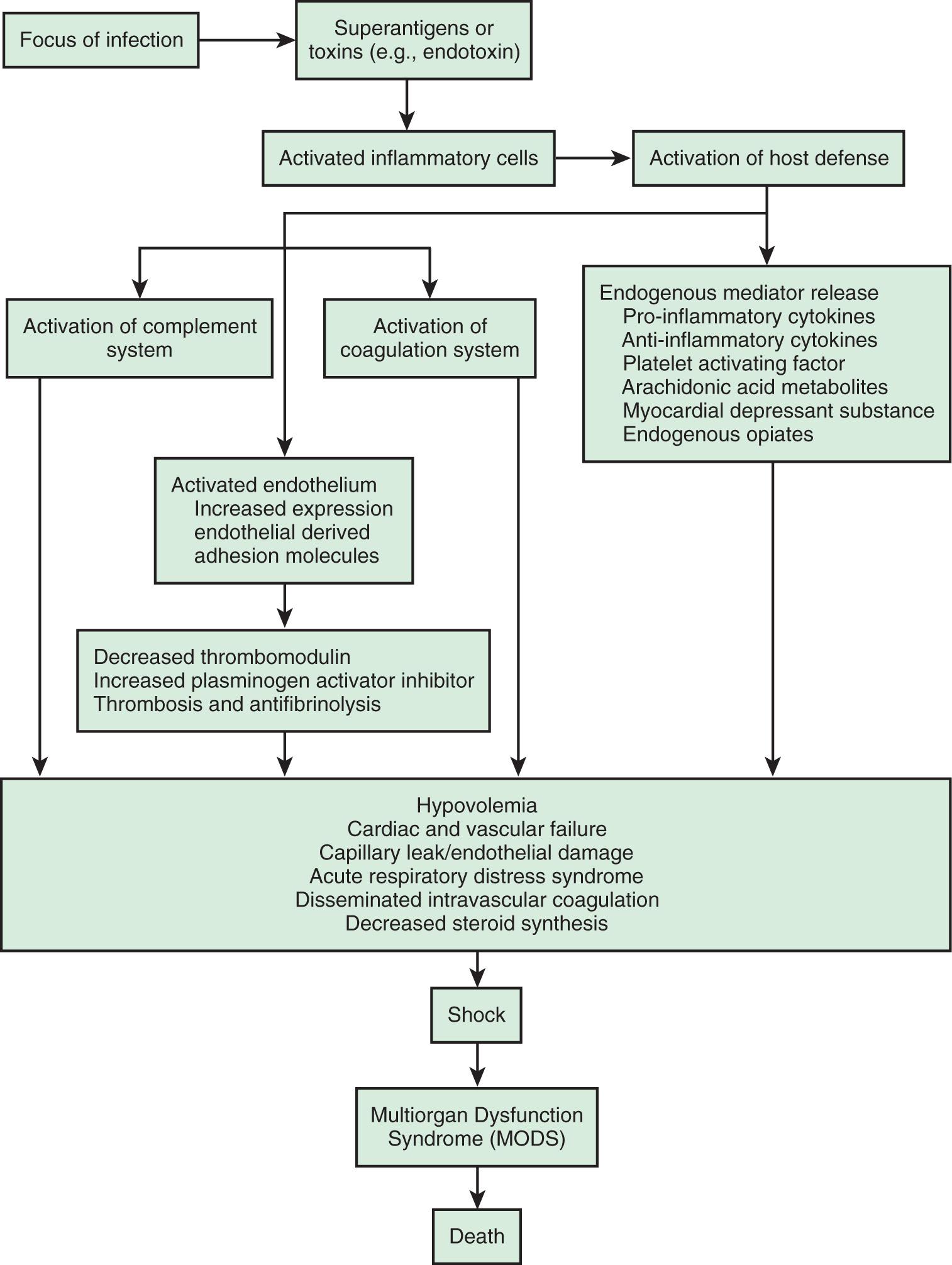
Table 88.1 shows a classification system for shock. Categorization is important, but there may be significant overlap among these groups, especially in septic shock. The clinical presentation of shock depends in part on the underlying etiology, but if unrecognized and untreated, all forms of shock follow a common and untoward progression of clinical signs and pathophysiologic changes that may ultimately lead to irreversible organ injury and death.
Shock may initially manifest as only tachycardia, with or without tachypnea. Progression leads to decreased urine output, poor peripheral perfusion, respiratory distress or failure, alteration of mental status, and low BP (see Table 88.3 ). A significant misconception is that shock occurs only with low BP; hypotension is often a late finding and is not a criterion for the diagnosis of shock because of a complex set of compensatory mechanisms that attempt to preserve BP and peripheral perfusion. Hypotension reflects an advanced state of decompensated shock and is associated with increased morbidity and mortality.
Hypovolemic shock often manifests initially as orthostatic hypotension and is associated with dry mucous membranes, dry axillae, poor skin turgor, and decreased urine output. Depending on the degree of dehydration, the patient with hypovolemic shock may present with either normal or slightly cool distal extremities, and pulses may be normal, decreased, or absent depending on disease severity. The presenting signs of cardiogenic shock are tachypnea, cool extremities, delayed capillary filling time, poor peripheral and/or central pulses, declining mental status, and decreased urine output caused by the combination of decreased cardiac output and compensatory peripheral vasoconstriction (see Chapter 469.1 ). Obstructive shock often also manifests as inadequate cardiac output because of a physical restriction of forward blood flow, and the acute presentation may quickly progress to cardiac arrest. Distributive shock manifests initially as peripheral vasodilation and increased but inadequate cardiac output.
Regardless of etiology, uncompensated shock, with hypotension, high SVR, decreased cardiac output, respiratory failure, obtundation, and oliguria, occurs late in the progression of disease. Table 88.6 lists the hemodynamic findings in various shock states. Additional clinical findings in shock include cutaneous lesions such as petechiae, diffuse erythema, ecchymoses, ecthyma gangrenosum, and peripheral gangrene. Jaundice can be present either as a sign of infection or as a result of MODS.
| TYPE OF SHOCK | CARDIAC OUTPUT | SYSTEMIC VASCULAR RESISTANCE | MEAN ARTERIAL PRESSURE | CAPILLARY WEDGE PRESSURE | CENTRAL VENOUS PRESSURE |
|---|---|---|---|---|---|
| Hypovolemic | ↓ | ↑ | ↔ or ↓ | ↓↓↓ | ↓↓↓ |
| Cardiogenic * | |||||
| Systolic | ↓↓ | ↑↑↑ | ↔ or ↓ | ↑↑ | ↑↑ |
| Diastolic | ↔ | ↑↑ | ↔ | ↑↑ | ↑ |
| Obstructive | ↓ | ↑ | ↔ or ↓ | ↑↑ † | ↑↑ † |
| Distributive | ↑↑ | ↓↓↓ | ↔ or ↓ | ↔ or ↓ | ↔ or ↓ |
| Septic | |||||
| Early | ↑↑↑ | ↓↓↓ | ↔ or ↓ ‡ | ↓ | ↓ |
| Late | ↓↓ | ↓↓ | ↓↓ | ↑ | ↑ or ↔ |
* Systolic or diastolic dysfunction.
† Wedge pressure, central venous pressure, and pulmonary artery diastolic pressures are equal.
Sepsis is defined as SIRS resulting from a suspected or proven infectious etiology. The clinical spectrum of sepsis begins when a systemic (e.g., bacteremia, rickettsial disease, fungemia, viremia) or localized (e.g., meningitis, pneumonia, pyelonephritis, peritonitis, necrotizing fasciitis) infection progresses from sepsis to severe sepsis (i.e., presence of sepsis combined with organ dysfunction). Further clinical deterioration leads to septic shock (severe sepsis plus the persistence of hypoperfusion or hypotension despite adequate fluid resuscitation or a requirement for vasoactive agents), MODS, and possibly death ( Table 88.7 ). This is a complex spectrum of clinical problems that is a leading cause of mortality in children worldwide. Mortality can be mitigated and outcomes improved with early recognition and treatment.
Suspected or proven infection or a clinical syndrome associated with high probability of infection.
Two of 4 criteria, 1 of which must be abnormal temperature or abnormal leukocyte count:
Core temperature >38.5°C (101.3°F) or <36°C (96.8°F) (rectal, bladder, oral, or central catheter)
Tachycardia:
Mean heart rate >2 SD above normal for age in absence of external stimuli, chronic drugs or painful stimuli
or
Unexplained persistent elevation over 0.5-4 hr
or
In children <1 yr old, persistent bradycardia over 0.5 hr (mean heart rate <10th percentile for age in absence of vagal stimuli, β-blocker drugs, or congenital heart disease)
Respiratory rate >2 SD above normal for age or acute need for mechanical ventilation not related to neuromuscular disease or general anesthesia
Leukocyte count elevated or depressed for age (not secondary to chemotherapy) or >10% immature neutrophils
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here