Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Sexual excitement and satisfaction from adequate stimuli are a normal component of a fulfilled life. The somatic and psychosocial factors involved may be compromised by neurologic disease. Sexual dysfunction may occur as the presenting symptom of a developing neurologic disease (e.g., erectile dysfunction in multiple system atrophy) or as an isolated phenomenon after local nerve injury (e.g., painful clitoral dysesthesia after pudendal nerve lesion), or it may be due to more general effects of a neurologic disorder.
Sexual disorders, such as loss of sexual desire, erectile dysfunction in men, decreased lubrication in women, and disturbances of ejaculation and orgasm, are common in patients with neurologic disorders but are usually not communicated by them to the neurologist. Sexual functioning needs to be addressed because it may be relevant for diagnosis, is a major determinant of quality of life, and may respond to treatment. The focus of this chapter is on the more classic neurologic dimension of sexuality as a physiologic function dependent on the integrity of neural control, on sexual dysfunction as a consequence of neurologic disease, and on management of neurologic patients with sexual dysfunction.
To the neurologist, sexual behavior involves a series of neurally controlled phenomena occurring in a hormonally defined milieu. Sexuality depends also on psychosocial factors, however, and the perspective of partnership and social issues should not be forgotten.
The sexual response is traditionally conceptualized as consisting of several phases, including desire, excitation, and orgasm. Although it has been criticized as male oriented, it still serves to structure the relevant observational, neuroanatomic, physiologic, and clinical issues for both genders. Differences in sexual behavior between men and women exist and are determined also by biologic factors.
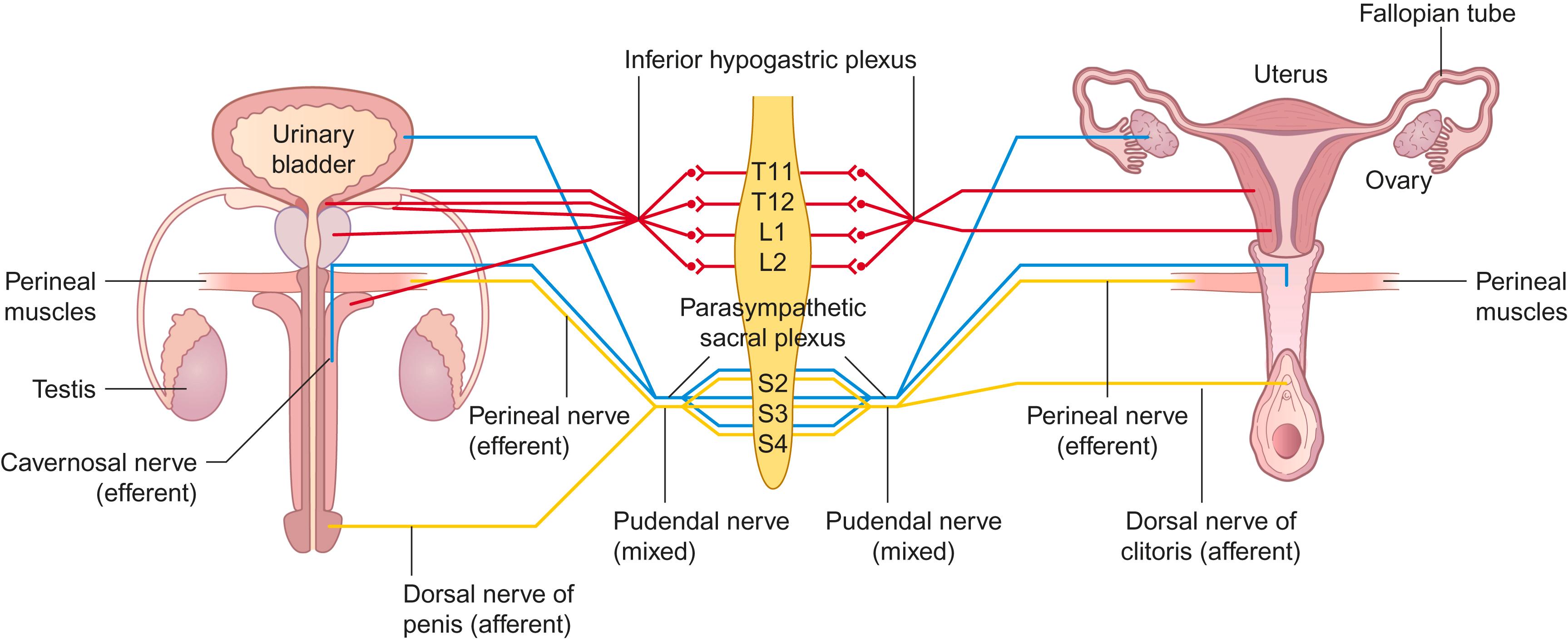
Any lesion involving neural tissue relevant for sexual responses may cause dysfunction, as also may lesions of other neural structures more generally involved in control of sensation, motor function, cognition, and behavior ( Fig. 30-1 ). Thus, all primary sensory areas plus the parietal and inferior temporal lobes process sexual stimuli, the forebrain regulates the initiation and the execution of sexual behavior, the medial preoptic area integrates sensory and hormonal signals, and the amygdala plays a role in the reward aspects of sexual function. Neurons from the paraventricular nucleus project to the thoracic and lumbosacral nuclei concerned with the sexual response, the hypothalamospinal projections being situated in the posterolateral funiculus of the spinal cord. Finally, the sympathetic, parasympathetic, and somatic efferents affect the sexual response, with genital afferents playing a part in the initiation of the cycle and in reinforcing the response. Furthermore, the hypothalamus controls the gonadotropic functions of the pituitary gland and thus prenatal development of genital organs, pubertal development, and the menstrual cycle; hormones finally influence the development of the nervous system.
Fertility and procreation are importantly linked to sexuality, but will not be discussed in depth here.
Desire or sexual interest (libido) refers to the extent that an individual responds to or seeks out erotic stimuli. This varies with time and circumstances, and its measurement depends on self-ratings of such items as the frequency of spontaneous sexual thoughts, the excitement provoked by them, and the resulting behavioral response. Desire is enhanced by sexual activity itself, exciting circumstances, new sexual partners, hypomania, and certain focal brain lesions (particularly of the frontal and temporal lobes). Depressed patients may develop more than their premorbid levels of sexual desire in response to antidepressant medication, as may patients with Parkinson disease in response to dopaminergic therapy. Sexual interest may be lowered by lack of opportunity, age, malnutrition, certain addictive or sedative drugs, debilitating illness, depression, epilepsy, and certain focal brain lesions.
Animal experiments have revealed dopaminergic-stimulating and serotonergic-inhibiting mechanisms controlling sexual interest. Androgens are necessary for normal libido, although there are some uncertainties related to this issue. In women, sexual desire is also associated with levels of free testosterone.
The rhinencephalon, including the limbic cortex, is important for sexual desire and behavior. The basal hypothalamus is particularly relevant; it is affected by tissue levels of the sex steroid hormones, and lesions involving it may lead to loss of desire. The medial preoptic area is involved in regulating sexual motivation and performance, and dopamine may regulate penile erection at this level. A functional magnetic resonance imaging (fMRI) study of sexual interest in men (by comparing erotic to sport clips) showed activation in the right parieto-occipital sulcus, the left superior occipital gyrus, and the precentral gyri. Functional imaging studies have demonstrated that the ovulatory cycle influences sexual interest and that activations in relevant brain areas are relatively greater in premenopausal than menopausal women.
Sexual excitement results from genital stimulation, other sensory stimuli, or sexual ideation. The glans in both genders has a high receptor density: up to 80 to 90 percent of the nerve endings are free in the most superficial layer of mucosa. There are also corpuscular-type endings beneath the mucosal layer. The receptors are of two types: slowly adapting distally and rapidly adapting proximally, with afferent C and A delta fibers. Surrounding the cavernous bodies are large nerve endings that resemble onions, with thick lamellae and a central nerve fiber connected to thick, myelinated nerve fibers. These nerve endings respond to deep pressure and vigorous movement. Receptors close to the cavernous bodies are influenced by the amount of engorgement of cavernous tissues so that touch may be experienced simply as touch or as a sexual stimulus depending on the degree of engorgement.
Sensory information from the glans and skin of the penis and clitoris is conveyed through bilateral branches of the pudendal nerve (the dorsal nerves of penis/clitoris), as shown schematically in Fig. 30-1 . The afferents from the root of the penis (and from the anterior part of the scrotum) join the ilioinguinal nerve. Genital afferents synapse in the spinal cord via interneurons with both somatic and autonomic motor neurons; those afferents destined for supraspinal structures travel in the anterolateral funiculus. “Erotically colored” sensations from the genital region are conveyed by the spinothalamic pathways, and patients with selective damage to these tracts may complain of anorgasmia and ejaculatory failure. Orgasmic sensation is blocked by bilateral anterolateral section of the spinal cord (cordotomy).
Somatic sensory afferents deliver information on tactile sexual stimuli that, after synapsing in the sacral spinal cord, induce local sexual responses (i.e., erectile and glandular responses). Sensory information also passes to suprasacral regions and is operative in other reflex activity, leading to awareness of sexual excitation. Both thalamic and cortical areas receive sensory input from the genitals, and sexual feelings may be elicited when such areas are stimulated. In the primary sensory cortex the genitals are traditionally thought to be represented in the parasagittal area; functional imaging suggests localization on the medial edge of the hemispheric convexity.
Somatosensory input from other body parts (“erogenic zones”) may—subject to somatic and individual psychologic factors and dependent on context—also lead to sexual excitement. Adequate sexual excitation can be achieved by stimuli delivered through cranial nerves. Although such “extrinsic” excitation—similarly to that achieved by stimulation of sexual organs—should also be called reflex (because there may be little “intrinsic” contribution to the excitatory response), it has traditionally been called “psychogenic.” Cortical and subcortical structures related to the limbic system elicit erection when stimulated. (Mental imagery is the “real” psychogenic activator.)
In men during visually evoked sexual arousal, regional cerebral blood-flow measurements by positron emission tomography (PET) have demonstrated activation of the inferior temporal cortex (a visual association region) bilaterally, of the right insula and inferior frontal cortex (regions processing sensory information and motivational state), and the left anterior cingulate cortex (involved in neuroendocrine function). Sexual arousal correlated with the magnitude of hypothalamic activation but was more pronounced in men than women. The occipitotemporal region was more activated in men, and the parietal lobe in women.
Sexual excitement leads to a complex response of the autonomic nervous system, and also to typical posturing in different species; in humans this behavior has been studied little but is understood to be influenced by psychosocial and cultural factors.
Parasympathetic efferents from the S2 to S4 spinal segments, traveling through the sacral plexus and cavernosal nerves, initiate erection ( Fig. 30-1 ). (The male erection has to be firm enough for vaginal penetration, and maintained throughout intercourse to bring about ejaculation, which in turn should deliver sperm to the uterine cervix.)
Blood flow in the penile artery, or the corresponding artery in the clitoris, increases. The smooth muscle of the cavernosal sinuses in the penile corpora relax, and the helicine arterioles branching from cavernosal arteries, selectively shunt blood flow to the lacunar spaces of the cavernosal bodies, filling them with blood. Subtunical venules are compressed so that corporeal venous return is restricted, resulting in increasing intracorporeal pressure. The pressure stabilizes at approximately systolic blood pressure, resulting in penile (and clitoral) tumescence and rigidity. Continued sacral parasympathetic activity maintains this erection.
The nerves of the corpora cavernosa have anatomic characteristics different from other nerves. The intracavernous nerves are located in fibrous tunnels, into which numerous fibrous bundles are attached. Contraction of striated pelvic floor muscles, the ischiocavernous muscles in particular, brought about through pudendal nerve activity, increases the rigidity of erection.
In women, parasympathetic activity causes clitoral erection, engorgement of the labia, and vaginal lubrication. The lubrication during sexual arousal is due to transudation through the vaginal wall. Another source is secretion from paraurethral glands (emptying into the urethra). Increased vaginal blood-flow, lubrication, and erection of cavernous tissue in the clitoris and around the outer part of the vagina are the female homologues of the male erectile response; indeed, lubrication occurs during rapid-eye-movement sleep in women. The response is dependent on innervation and a normal estrogen level.
In the periphery, the main proerectile transmitter is nitric oxide, which is colocalized with vasoactive intestinal peptide and acetylcholine; the main antierectile neurotransmitter is norepinephrine. The same mechanisms are responsible for clitoral erection.
Erections can still occur in men and animals after lesions of the sacral cord and pelvic nerves. This is due to the “alternative” proerectile pathway mediated through the hypogastric nerve. This explains the so-called psychogenic erections of paraplegics with conus or cauda equina lesions but preserved thoracolumbar segments. Similarly, women with injury to the sacral spinal cord and an ability to perceive pinprick in the T12 to L2 segments may retain the capacity for psychogenic genital vasocongestion. In women with complete spinal cord injuries and preserved sacral segments, such a response is as a rule obtained only by manual genital stimulation. Thus, the reflex–“psychogenic” dichotomy of the genital sexual response can be seen in both sexes.
Inhibitory influences on the sexual response also exist. In nucleus paragigantocellularis a majority of the serotonergic neurons project to the spinal cord (traveling in the lateral funiculus) and provide tonic inhibition of sexual reflexes in the rat.
Seminal emission begins during arousal and, with continued sensory stimulation, orgasm is triggered, with ejaculation of the urethral contents resulting from the rhythmic phasic contractions of perineal and pelvic floor muscles. Ejaculation is effected by integrated sympathetic outflow from T11 to L2 segments traveling through the sympathetic chain and hypogastric plexus and along the pelvic and pudendal nerves, and by somatic efferents traveling through the pudendal nerves. Although the predominant neural control of the male accessory sexual organs is sympathetic (adrenergic and purinergic), the secretion of seminal fluid is under parasympathetic control. Sympathetic activity causes smooth muscle contraction in the seminal vesicles, vas deferens, and prostate to deliver seminal fluid to the posterior urethra; in the bladder neck to prevent retrograde ejaculation; and in the corpora cavernosa to cause detumescence. The latter “antierectile” activity is inhibited during erection through spinal coordination of reflex action.
The female orgasmic response consists of rhythmic contractions of pelvic floor muscles, the uterus, fallopian tubes, and paraurethral glands; expulsions from the paraurethral (Skene) glands through the urethra may occur (so-called female ejaculation). Women may achieve orgasms by stimulation outside the genital region and probably do so more easily than men, but the most notable difference from men is that women may achieve multiple consecutive orgasms.
Motor innervation of the pelvic floor muscles as well as the ischiocavernosus and bulbocavernosus muscles is conveyed through pudendal nerve branches from below. However, the most relevant motor innervation of the levator ani (“pelvic floor”) muscle is directly from the sacral plexus via the levator ani nerve. Electromyographic recording of the pelvic floor muscles in women during vibratory clitoral stimulation shows intermittent activity associated with contractions on a background of continuous activity. During ejaculation in males, repeated bursts of electromyographic activity precede and follow expulsion of semen. The sensation of orgasm, however, is not dependent on pelvic muscle contractions. Men report that orgasmic sensation begins before, and lasts longer than, bursts of electromyographic activity in the perineal muscles.
Orgasm can be separated conceptually (but not easily physiologically) from emission and ejaculation. PET imaging during orgasm has shown deactivation of frontal regions and the left temporal lobe; cerebellum and pons were activated ( Fig. 30-2 ). There were only minor differences between the sexes.
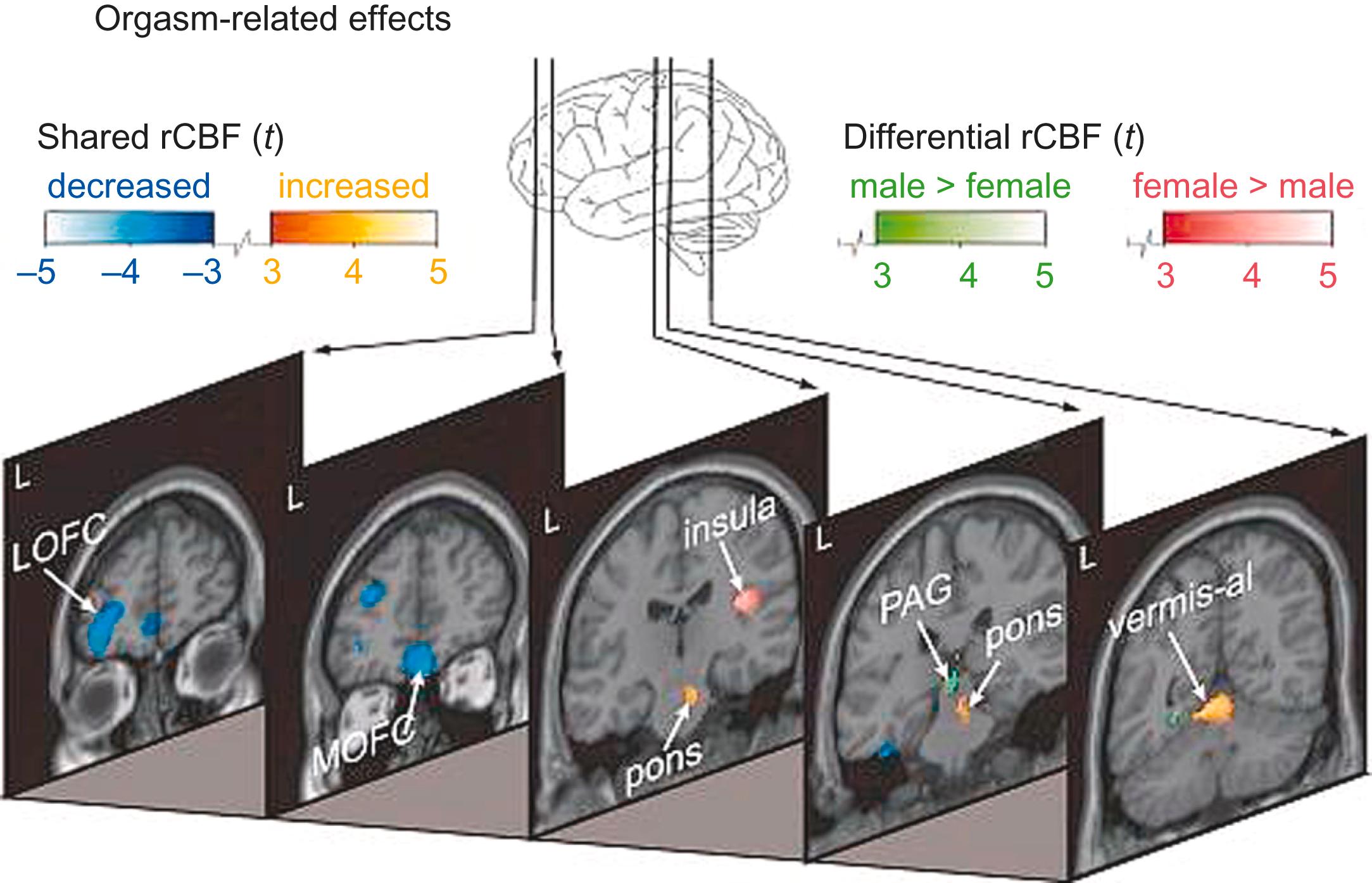
After orgasm (during the “resolution” or “refractory” phase), fMRI reveals activation of amygdala and the temporal lobes, and—for a short period—of the septal area.
Anorgasmia is very rare in neurologically normal men, but 13 percent of women between the ages of 18 and 26 years have never achieved orgasm, with the incidence declining to a minimum of 3 percent in women between the ages of 51 and 64 years.
Ejaculation can be absent with an intact orgasm in lesions of the hypogastric plexus and under the influence of some drugs.
Preservation of the peripheral nerves related to sexual (and bladder and bowel) function during abdominal and pelvic surgery is necessary for good postoperative results. In the pelvis and abdomen, autonomic structures related to genital innervation are situated in the retroperitoneal space. The superior hypogastric plexus is located anterior to the aortic bifurcation at the level of the fifth lumbar vertebral body and sacral promontory between the common iliac arteries. It divides caudally into the right and left hypogastric nerves. Within the pelvis, these nerves become the inferior hypogastric (pelvic) plexus, which is joined on each side by the pelvic nerves. In males, the inferior hypogastric plexus is lateral to the rectum, seminal vesicle, prostate, and the posterior part of the urinary bladder ( Fig. 30-3 ). The lesser and greater cavernosal nerves originate from the anterior part, are joined by fibers from the pudendal nerves, and pass below the pubic arch. In females, the inferior hypogastric (pelvic) plexus gives off uterine nerves, branches for the vagina and cervix, and connections with the paracervical plexus.
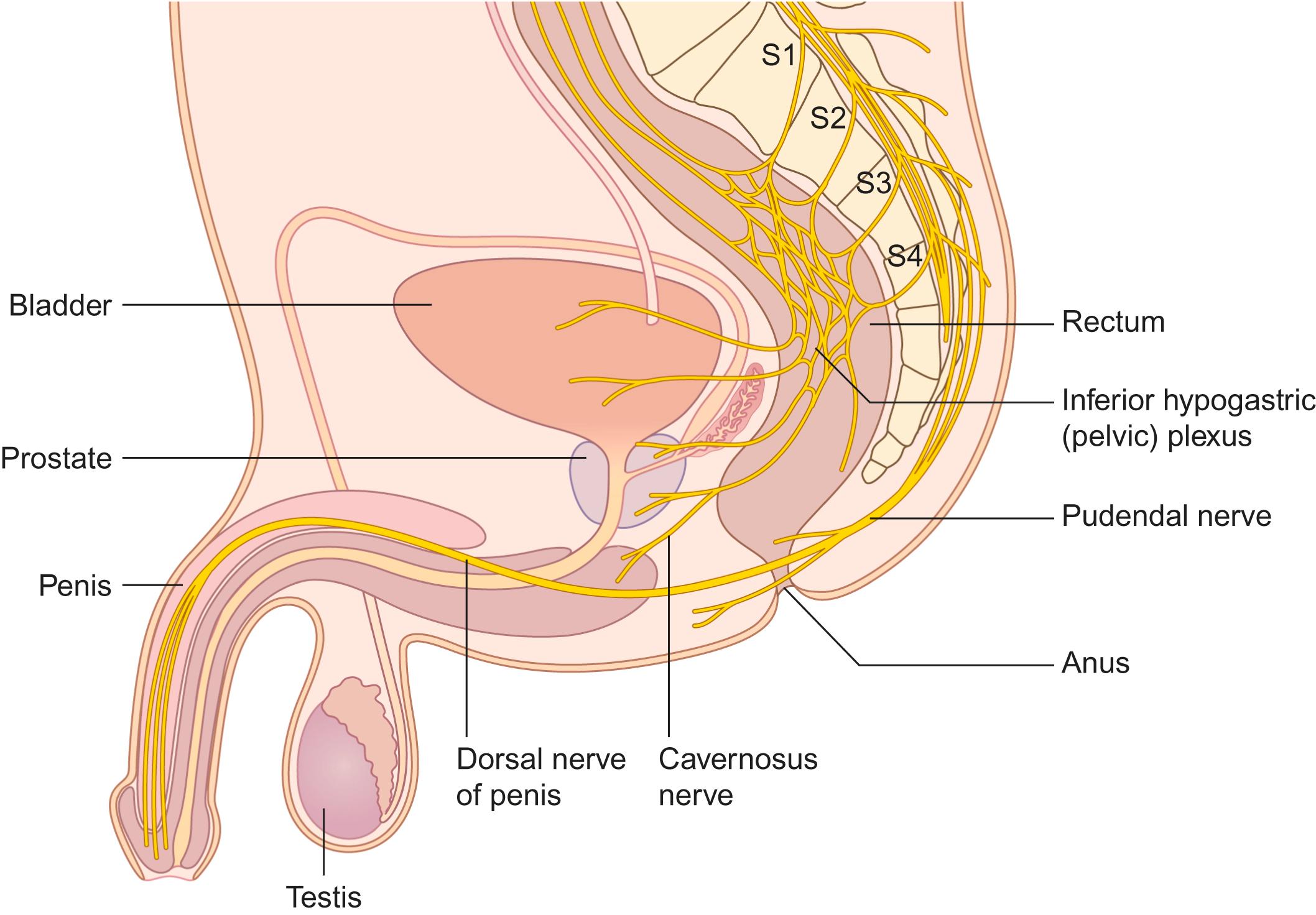
In order to preserve neural structures relevant to sexual function during surgical procedures, the anatomy must be understood, surgical technique must be meticulous, and specific intraoperative “mapping” and “monitoring” procedures may be required.
Further details on the anatomy and physiology of the sexual response in men and women are provided elsewhere.
There is little research on sexual functioning in the elderly. It is not easy to disentangle the changes due to aging and its related psychosocial problems from those due to any of the many different disorders that are common in the elderly (such as hypertension, cardiovascular disorders, lower urinary tract dysfunction, and depression) or to the effects of medication. Regardless, the sexual habits, desires, and preferences of the reasonably healthy elderly do not change, but the frequency of sexual activity generally decreases. Only 26 percent of those aged 75 to 85 years report sexual activity during the previous 12 months. Men need more time and greater stimulation to achieve and maintain erections and to reach orgasm. They have a decreased sensation of impending ejaculation and decreased ejaculatory volume. Their refractory period after detumescence is prolonged. Their testosterone level decreases (but exogenous administration of androgens does not increase libido). Hormonal changes in menopausal women lead to loss of pubic hair and a change in the distribution of body fat tissue; libido diminishes and the vaginal wall thins, with decreased elasticity and lubrication. In some women, however, libido may actually increase after the menopause. In fact, 74% of women above 65 years of age continue to engage in sexual activity at least once a week. Decreased sexual thoughts and frequency of intercourse after menopause are more closely correlated with testosterone than estrogen levels, but there is no clear relationship between low sexual desire and serum testosterone. In the very old (80 to 102 years old), touching and caressing is the most common form of sexual expression.
The breadth of the history and clinical examination will be tailored by the individual physician’s interests and practice habits, but inquiry about sexual function should not be reserved for male patients. With the advent of effective treatment for erectile dysfunction, many physicians take a pragmatic approach to treatment and inquire about little more than whether erectile dysfunction is present. However, there is more to sexual dysfunction than erectile dysfunction, and more to treating any dysfunction than simply prescribing a pill. Dysfunction in an individual patient—even in the presence of neurologic disease—may be due entirely or in part to psychosocial, vascular, endocrine, or other causes.
The history should include details of neurologic disease as well as any past history of urologic/gynecologic, cardiovascular, endocrine, psychologic, and psychiatric disturbances. History of disorders of the sex organs, trauma and surgical procedures, the use of prescription drugs, smoking and alcohol habits, and possible drug abuse should also be elicited. The patient’s sexual expectations, needs, and behavior should be defined as well as any misconceptions. Before diagnosing dysfunction of sexual organs, the level of actual desire should be established. The term hypoactive sexual desire disorder (HSDD) is used to define a persistent or recurrent reduction in desire for sexual activity, alone or with a partner, with inability to respond to sexual cues that would be expected to trigger a sexual response; these symptoms need to cause personal distress. If present in men, the disorder may be associated with impotence; in women it often occurs with the female sexual arousal disorder (FSAD), which is a persistent or recurrent inability to attain or maintain sufficient sexual excitement that causes personal distress.
Men should be asked about erectile function (the occurrence of nocturnal erections, morning erections, and erections evoked by genital, visual, auditory, or psychogenic stimuli) and women about vaginal lubrication. The nature of ejaculation should be determined, and in particular whether it is premature, retarded, absent, or dribbling (i.e., emissions occur through the urethra without the activity of pelvic floor muscles). Retrograde ejaculation, described as “dry ejaculation,” means that ejaculum has entered the bladder. Finally it should be clarified whether the patient can achieve orgasm, and the quality of orgasmic sensations and experiences should be noted. In some circumstances it may be helpful to interview the patient’s partner.
Formal questionnaires can be used to obtain standardized information on male and female sexual function. They are particularly relevant in research.
Sexual development, height and weight, changes in pigmentation and body hair, and the presence of galactorrhea should be noted. The external genitalia should be examined. Palpation of peripheral pulses (arms, legs, penis), auscultation of the heart, and blood pressure measurement are recommended.
A standard neurologic examination, with inspection of the lower back (for nevus, hypertrichosis, or sinus), the feet (for deformity or muscle atrophy), and the anogenital area may reveal signs of underlying neurologic disease. Examination of the anogenital region involves palpation of the bulbocavernosus muscles in the male, testing for voluntary contraction (“move the penis”) and reflex contraction. The anal sphincter (also levator ani) is palpated for tone, voluntary contraction, and reflex contraction in both sexes by rectal examination. The cremasteric reflex (the L1 segment) and the bulbocavernosus and anal reflexes (S2 to S4/5 segments) should be tested.
In men, spontaneous and physiologically induced erection can be studied with a variety of techniques. Spontaneous nocturnal penile tumescence and rigidity can be measured in the sleep laboratory using mercury strain gauges (measuring penile expansion), visual inspection, measurement of buckling force (for assessment of rigidity), and polygraphic confirmation of sleep phases. Continuous monitoring of nocturnal penile tumescence and rigidity can be obtained by a rigidometer during normal sleeping conditions at home and also during daytime napping or in the awake, sexually stimulated state. Various low-cost screening tests for nocturnal penile expansion have been proposed, but their validity is questionable. Testing for nocturnal erections, however, does not reliably distinguish psychologic from central nervous system (CNS) causes of erectile dysfunction. The aforementioned testing remains mainly of research interest.
An intracorporeal penile injection of a vasoactive substance, such as prostaglandin E 1 , will induce an erection (in the absence of major vascular pathology), thus strengthening the suspicion of a neurogenic or psychogenic cause of erectile dysfunction. Intracorporeal injection of vasoactive agents has been proposed as an established diagnostic tool in patients undergoing assessment for possible neurogenic erectile dysfunction and is safe when performed by experienced physicians, with an acceptable complication rate.
Suspected vasogenic erectile dysfunction may require the testing of penile vasculature (blood pressure and vascular competence) by a urologist. The purpose of testing should always be defined; pharmacologic testing may be sufficient for the majority of patients, and invasive tests reserved for those in whom surgery is contemplated.
In women, both direct and indirect methods are used to measure blood-flow changes in the labia and vagina. Noncontrast dynamic magnetic resonance imaging (MRI) can assess female sexual arousal quantitatively. All these tests are only of research interest; it has been stressed that vaginal vasocongestion to erotic stimuli may be unaccompanied by erotic feelings, and subjective indices should therefore be obtained when physiologic measurements are made.
Functional tests are direct extensions of the clinical examination. Special devices and algorithms can be used for quantifying sensory perception on the genital organs and in the perineum. The measurement of vibratory perception (biothesiometry; measuring the vibration perception threshold) on the glans has been advocated for diagnosing sensory neuropathy in male and female patients. The vibration perception threshold on the penis (glans and shaft) in neurologically healthy men is similar to that of the feet, whereas in females this threshold (best measured on the clitoris, labia majora, and perineum) is the same as in the hands. Tests evaluating small-fiber function (e.g., testing for penile thermal sensation) may be more informative about the neural control of erection.
Several neurophysiologic tests have been suggested for assessing sacral or suprasegmental lesions ( Table 30-1 ). Tests measuring conduction through somatic nervous pathways (motor, sensory, and reflex) might be expected to be useful because most lesions should involve both somatic and autonomic neural pathways, and abnormalities obtained on testing the former could be extrapolated to the latter. Even so, these tests are sensitive only to demyelination and not to axonal lesions, which predominate in clinical practice. Electromyography may demonstrate the activation patterns of striated muscles but is mainly used to differentiate normal from denervated (reinnervated) muscle. Controversy exists about the source and nature of the signals recorded in penile and clitoral electromyography, and the findings have no diagnostic relevance. The lumbosacral sympathetic system may be tested by the sympathetic skin response from the perineum (and penis).
| Somatic Sensory |
| Quantitative sensory testing |
| Dorsal penile nerve neurography |
| Pudendal somatosensory evoked potentials |
| Visceral Sensory |
| Somatosensory evoked potentials to proximal urethra/bladder neck stimulation |
| Bladder sensitivity testing |
| Somatic Motor |
| Electromyography |
| Motor evoked potentials |
| Sacral Reflex |
| Bulbocavernosus reflex |
| Anal reflex |
| Sacral reflex to proximal urethra/bladder neck stimulation |
| Autonomic |
| Sympathetic skin response |
| Neurocardiac testing |
| Cystometry |
The role in clinical practice of these and the other tests shown in Table 30-1 is limited. In terms of validity, experience, and available normative values, only electromyography (in muscles of the lower sacral myotomes) and the recording of sacral reflex responses and somatosensory evoked potentials (SEPs) to pudendal nerve stimulation are recommended. The patients with possible or probable neurogenic sexual dysfunction in whom such testing might yield a result relevant for diagnosis and prognosis (though rarely important for decisions on therapy) are those with suspected lesions in the peripheral sacral reflex arc. In these patients, support for the presence or absence of a neurologic lesion can be obtained; this may have medicolegal implications. The relationship between a neurologic lesion (or, for that matter, any neurophysiologic test abnormality) and sexual dysfunction is complex; in women with multiple sclerosis, for instance, latency of pudendal (and tibial) SEPs fails to predict the extent of sexual dysfunction.
When patients with sexual dysfunction have symptoms or signs indicating endocrine dysfunction, hormone assays—or rather, endocrinologic consultation—may be necessary. The hormones to be studied depend on the circumstances (sex, age, and onset and nature of symptoms).
Sexual dysfunction, such as lack of libido (in both sexes), erectile dysfunction and disturbances of ejaculation (in men), and deficient lubrication, dyspareunia, and problems with orgasm (in women), is not uncommon in the general population. In patient populations with disorders of the CNS, the prevalence of sexual dysfunction is reportedly higher, although few comparative studies have been done.
Insofar as many neurologic diseases affect primarily elderly patients and also carry the burdens of any chronic affection, the sexual dysfunction “specific” to the neurologic lesion(s) has to be ascertained against the background of valid control groups.
Some cognitive impairment, personality change, and sensorimotor disability may remain after traumatic brain injury. Sexual dysfunction (either as a consequence of the cerebral lesion or psychosocial factors) occurs at a significantly greater frequency in these individuals. Decreased or increased sexual desire, erectile failure, and retarded ejaculation may occur, at least in part as a consequence of post-traumatic pituitary dysfunction. Frontal and temporal lesions seem to result more often in sexual disturbances than parieto-occipital lesions. Hypersexuality, disinhibited and inappropriate sexual behavior, sexually aggressive behavior, and changes in sexual preference sometimes follow basal frontal and limbic brain injury and may lead to sex offences. Bilateral anterior temporal lesions may result in the Klüver–Bucy syndrome with hypersexuality and pansexuality (i.e., sexual drive that is directed not only toward humans but also toward animals and inanimate objects).
Decline in desire and the frequency of intercourse after stroke is not unexpected; the best predictor of decreased sexuality between partners is the degree of dependence in activities of daily living. From various studies, about 75 percent of patients who were sexually active before the stroke report a subsequent decrease in coital frequency. Poor-sexual functioning may persist even with otherwise good improvement.
Many men become impotent after a stroke; orgasmic and ejaculatory dysfunction is also common. Both erections and ejaculation may return within a year after stroke. Decreased vaginal lubrication and inability to achieve orgasm occur in women.
Sexual problems after a stroke may be complicated by other deficits, poor-personal image, lack of coping and, particularly, depression.
Patients and their partners may avoid sexual intercourse out of concern that another stroke may be precipitated. The heart rate during sexual activity may exceed 180 beats per minute in men and reach similar values in women; the workload during sexual activity is similar to that of climbing stairs or walking briskly. Although the exact risk of stroke during sexual activity is not known, it seems to be low. Patients and their partners may thus be reassured that, in resuming sexual activity, the gains in most instances outweigh any slight risks. These issues should be addressed in counseling.
Hypersexuality after stroke has been described, but seems to be rare.
Epilepsy is associated with sexual problems, more often in men than women. Various types of abnormal behavior, hypersexuality, and, most commonly, hyposexuality have been reported, particularly in temporal lobe epilepsy. Satisfaction with life and sexuality is better in patients who are seizure free. The extent to which social and psychologic factors bear on sexual dysfunction is not clear. It is helpful to determine whether disturbances relate to seizures or occur during the interictal period.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here