Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Mammalian skin healing is a complex orchestrated process that results in scar formation. Scars are problems that arise in all areas of aesthetic and reconstructive surgery. As scars can cause a huge psychological and physical burden for patients, they pose a significant challenge for even the most experienced surgeon. Thus, the prevention and treatment of scar formation is vital to achieving acceptable patient satisfaction and outcomes. Careful planning and execution in wound creation and closure will maximize the ability to create an acceptable scar.
Successful skin wound healing relies on restoring tissue integrity to as close to a homeostasis state as possible. The surgeon must utilize their fundamental tools of plastic surgery, including planning, careful marking, placement of incisions and meticulous postoperative care, to achieve optimal wound healing and reduce scar formation. From appropriate and accurate approximation of wound edges to ensuring an infection and tensile-free environment, the surgeon must appreciate the variable and multiple factors that contribute to scar formation in skin.
In recent years, extensive study has aimed to unlock the mechanisms that initiate and regulate scar formation. Mouse models have demonstrated that specific cell types may be primarily responsible for scar formation following skin injury. Pre-clinical animal models have aided the understanding of skin biology in the context of abnormal and pathological scarring, including keloid and hypertrophic scar. Elucidating the pathways and cell types responsible for scar formation may present new therapeutic targets to potentially overcome and prevent scarring.
Scar treatment is rarely achieved without ensuring that management is patient-focused with a deep understanding of the patient’s experience and expectations. Here we first describe a systematic and strategic approach to assessing scars, emphasizing the need to assess both the physical and psychological need of the patient. We then present non-surgical and surgical approaches to prevent, treat, and revise scars. We further provide an overview of standard and emerging treatments used to treat skin scarring.
![]() Access video lecture content for this chapter online at Elsevier eBooks+
Access video lecture content for this chapter online at Elsevier eBooks+
For physicians scars represent an endpoint in wound healing. For patients, however, scars exemplify something far more deeply personal and emotional. Scars caused by disease, violent trauma, or aberrations of development can result in lifelong physical and psychological burdens. Treating scars thus requires an understanding of the psychological and social distress a patient may experience.
Scars may arise from both culturally sanctioned and prohibited practices. Ritual scarring, or cicatrization, was an important part of identifying tribal belonging in parts of Africa and Australia. In tribal communities in Sudan and Papua New Guinea, the prevalence of keloid formation in certain racial groups was exploited for spiritual and cultural markings. Likewise, the Japanese art of tattoo, or irezumi , carried sufficient cultural weight as to be banned by the Meiji government until 1945, when occupational forces again legalized its practice. Today tattooing and, to a lesser degree, branding and scarification, continues to be a popular form of self-expression.
Gender plays a clear role in the effect of scars in society. A recent study suggested that facial scars in men indicate risk-taking and bravery, and these men are subsequently perceived as more attractive. The same effect was not found when observers were shown similarly scarred women. Nevertheless, many scars carry negative social implications for both genders. Studies of quality of life measures in burn patients reveal that scars cause significant decrease in the quality of patients” social and professional life. For instance, both depression and posttraumatic stress disorder (PTSD) have been identified as potential long-term sequelae, with rates for PTSD in burn patients ranging from 23% to 45% at 1 year following injury. Risk factors include avoidant coping strategies and pre-existing psychiatric history as well as hand and face involvement and burn severity. Therefore understanding the specific aspects of the patient’s life most affected by the presence of scarring can help direct both medical and non-medical therapy.
Psychologist Thomas F. Cash described the importance in reconciling a patient’s “view from the outside” and “view from the inside” when coping with deformity. Understanding the social context and patient’s emotional relationship to scars is vital to treatment. A potentially useful tool in understanding and assessing these variables is Psychological Aspects of Reconstructive and Cosmetic Plastic Surgery: Clinical, Empirical, and Ethical Perspectives . This title reflects a multidisciplinary effort of leading psychologists, psychiatrists, and surgeons in determining and delivering care to patients with real or perceived deformities.
As with the evaluation of most maladies, assessment of a patient with an unsatisfactory scar begins with a focused history and targeted physical examination. While obtaining the history, the etiology of the scar and relevant associated factors (e.g., prior dissatisfactory surgery, relation to violent crime or infection) should be elucidated. In communicating with the patient, the treating physician should be empathetic without attempting to attribute the patient’s current complaints to care provided by prior treating physicians. This can usually be accomplished by focusing on the problem at hand and appropriate next steps.
The physical exam includes an assessment of both the scar and surrounding tissue. With facial scars, attention must be paid to normal folds and features as determined by the aesthetic subunits. Examination of other scars on the patient to determine predisposition to poor scarring can also be helpful. Evaluation of the scar should include written and photographic documentation of size, color, and texture. The relationship of the scar to surrounding structures in motion and repose should be carefully assessed to determine tethering and contracture.
Scar formation can cause functional, aesthetic, and emotional problems. Before initiating treatment, the physician must take the time to understand and address each of these elements. The extent of scar should be considered along with the patient’s goals in getting treatment. Arriving at realistic targets and expectations may require multiple visits or a combination of surgery and counseling. Frequently, scheduling a second or third visit to ensure the patient understands the treatment plan and has realistic expectations prior to undergoing the planned procedure is in the surgeon’s best interest.
Clinical evaluation of a scar is necessary in determining the best course of treatment and effectiveness of therapy. The ideal scale for scar assessment should demonstrate validity, interobserver reliability, and clinical applicability. Though multiple objective and subjective assessment tools have been devised to characterize scars, there is as yet no universal consensus on scar grading. However, the most frequently used measure is the Burn Scar Index, also known as the Vancouver Scar Scale (VSS) ( Table 16.1 ). Originally published in 1990, the VSS is an observer-dependent scale designed to assess changes in burn scars with maturity and in response to treatment. Scars are assessed based on four variables: pigmentation, vascularity, pliability, and height. Scores are then assigned across these four variables based on the degree of variance from normal skin. When applied, the scale can be a useful tool for prognosis and treatment evaluation. In 1995, Baryza and Baryza found that adding a low-cost instrument could improve interobserver reliability. They combined a ruler, a transparent piece of plastic, and a scoring “cheat sheet” to aid in measuring, blanching, and determining the score, respectively.
| Pigmentation | |
| 0 | Normal: color that closely resembles the color of the rest of the body |
| 1 | Hypopigmentation |
| 2 | Hyperpigmentation |
| Vascularity | |
| 0 | Normal: color that closely resembles the color of the rest of the body |
| 1 | Pink |
| 2 | Red |
| 3 | Purple |
| Pliability | |
| 0 | Normal |
| 1 | Supple: flexible with minimal resistance |
| 2 | Yielding: giving way to pressure |
| 3 | Firm: inflexible, not easily moved, resistant to manual pressure |
| 4 | Banding: rope-like tissue that blanches with extension of the scar |
| 5 | Contracture: permanent shortening of the scar, producing deformity or distortion |
| Height | |
| 0 | Normal: flat |
| 1 | <2 mm |
| 2 | <5 mm |
| 3 | >5 mm |
While perhaps the most commonly used assessment tool, the VSS is limited by its historical focus on burn scars, lack of consideration of patient’s own perceptions, exclusion of pain and pruritus, as well as a lesser applicability to larger-scale heterogenous scars. Other evaluation measures, such as the Visual Analog Scale (VAS), the Patient and Observer Scar Assessment Scale (POSAS), the Stony Brook Scar Evaluation Scale (SBSES), and the MCFONTZL classification system, have been created with varying levels of validation and adoption. The large variety of different scales reflects the relative imperfection of each individual system.
The VAS assesses parameters such as color, contour, and texture to correlate intraobserver as well as photographic and histologic findings. The scale can be applied to both burn and surgical scars. Similarly, the POSAS was developed in 2004 for burns and has since been validated for linear scars. This scale has the benefit of incorporating patient opinion to a VSS-like scale and can better assess symptoms such as pain, itchiness, and thickness. A third scale, the SBSES, is a photo-based scale similar to VAS and was initially developed as a tool for emergency medicine physicians to evaluate wounds from 5 to 10 days after wounding up to the time of suture removal. The SBSES has since been adapted for long-term evaluation of scars 3–12 months after injury and has been used as an outcome measure in Food and Drug Administration-mandated clinical trials. Lastly, the MCFONTZL classification system was developed specifically for facial trauma ( Table 16.2 ). This system incorporates billing and uses a mnemonic to divide the face into the maxilla, chin, forehead, orbits, nose, temple, zygoma, and lip. These scales have been useful in comparing outcomes from conventional versus minimally invasive surgery, use of wound closure adhesives, and new therapeutic agents for scar treatment.
| A Area | MCFONTZL aesthetic unit designation |
|---|---|
| S Side | |
| T Thickness | Depth of penetration |
| E Extension | Branching |
| R Relaxed skin tension line conformality | Directionality (relaxed skin tension lines) |
| I Index laceration | Laceration with maximum continuous skin interruption |
| S Soft-tissue defect | |
| K Coding | Current procedural terminology code |
A number of instruments have also been used to facilitate objective assessment of scars. Blood flow has been analyzed by laser Doppler, while depth and color have been studied by ultrasound and spectrometry. Skin elasticity meters, commercialized for evaluation of scleroderma, have also been used to examine scars. Lastly, three-dimensional systems have been used in a number of studies to create high definition and easily reproducible topographic representation of scars. While these instruments demonstrate a high degree of accuracy, reliability and clinical utility, the added expense and additional technical training required have limited them largely to research settings.
Skin wound healing is a complex molecular process, which inevitably results in scar formation. Scars are typically characterized by their abundance of disorganized collagen, loss of mechanical strength, and dermal appendages. Wound repair has been extensively studied, representing a controlled sequence of events involving a network of cell types and molecular pathways.
Wound healing occurs in three distinct overlapping stages: inflammation, proliferation, and remodeling ( Fig. 16.1 ). Following tissue injury, circulating platelets are first activated to initiate a hemostatic cascade, to form a fibrin clot at the injury site. Consequently, during the inflammatory stage, the wound is infiltrated with immune cells to remove cell debris and pathogens. Platelet adhesion during hemostasis activates several inflammatory cytokines that attract immune cells to the wound site. Neutrophils are the key inflammatory cells that are initially needed to debride the wound site. The secretion of cytokines by the neutrophils stimulates the migration of additional inflammatory cells, including monocytes and macrophages. The monocytes differentiate into macrophages at the wound site, which further play both inflammatory and regenerative roles. Two distinct subtypes of macrophages have been described: the “classically” activated (M1) or “alternatively” activated (M2). The “M1” subpopulation represents the proinflammatory phenotype, which are responsible for phagocytosis or removal of damaged tissue and production of proinflammatory cytokines (e.g., interleukin 1(IL-1), IL-6, and transforming growth factor alpha (TGF-α). The alternative “M2” phenotype macrophages exhibit secretion of high levels of anti-inflammatory cytokines to resolve inflammation and promote wound healing. Towards the end of the inflammatory phase, but prior to its resolution, the proliferative phase begins. The ongoing secretion of the cytokines by macrophages during the inflammatory phase encourages fibroblasts, endothelial cells to initiate the proliferative phase.
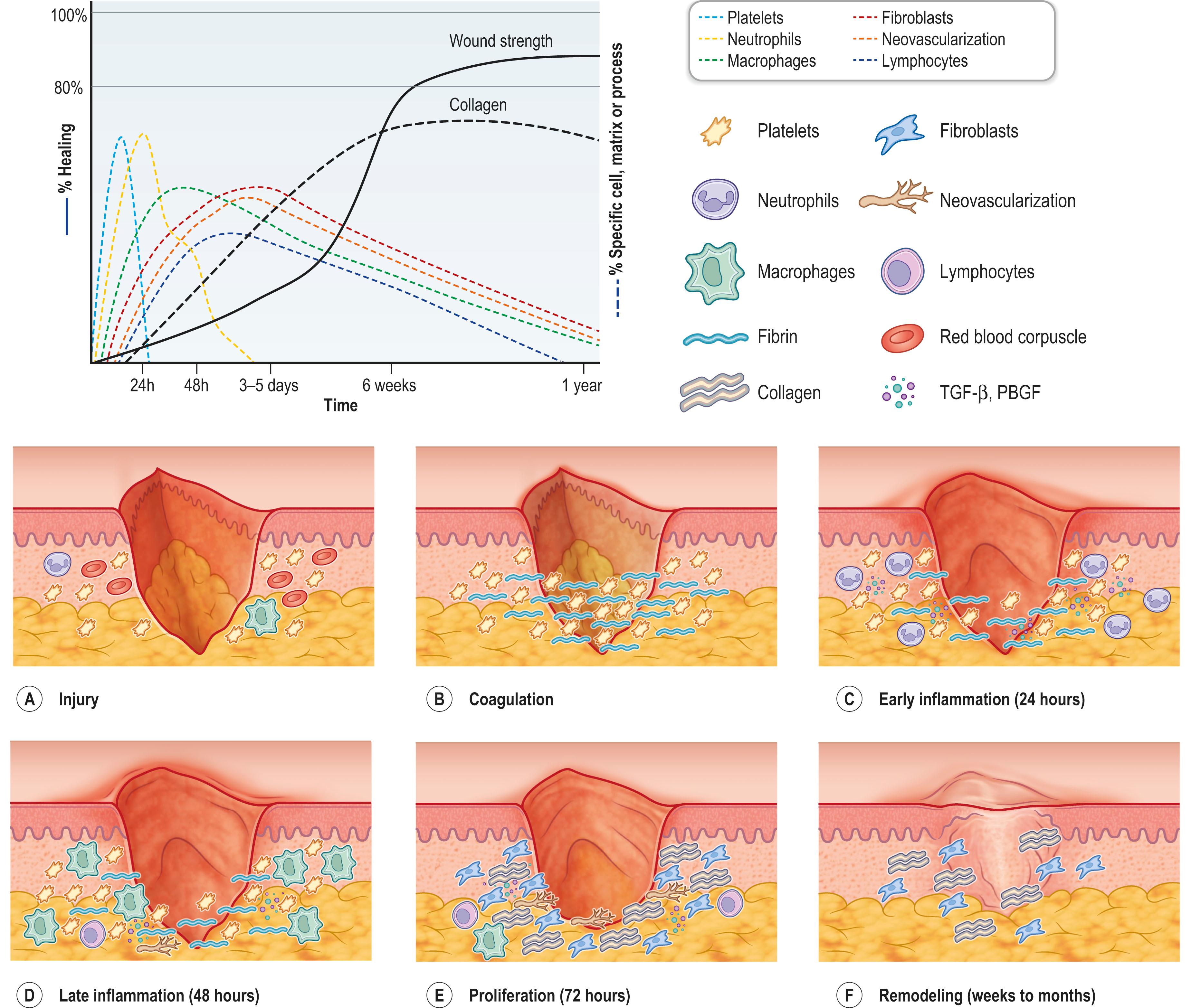
During the proliferative phase, at approximately 4 days post dermal injury, the provisional platelet plug is replaced by a new extracellular matrix (ECM). Fibroblasts are responsible for highly regulated synthesis and deposition of the ECM. The newly formed ECM is termed “granulation tissue” due to its “granular” appearance from its highly vascularized composition. Initially the less dense ECM is comprised of fibronectin, collagen type III, and hyaluronic acid. Scars are initially red due the tissue’s dense capillary network. Over time, the capillaries decrease within the granulation tissue and the red colour fades. In contrast, mature scars are hypopigmented and lighter than the surrounding skin.
During the proliferative phase a subset of fibroblasts transition to an activated myofibroblast phenotype, characterized by their alpha smooth muscle actin expression. These fibroblasts contribute to wound contraction, attaching to the ECM framework to support wound edge approximation. Lastly, in response to hypoxia and the secretion of angiogenic factors such as vascular endothelial growth factor (VEGF) by inflammatory cells, capillaries extend from the wound edges to form a capillary network within the granulation tissue. The oxygenation of the wound bed following vascularization supports tissue regeneration. Furthermore, to achieve successful wound healing, re-epithelialization is required, which occurs within hours of the injury and is mediated by the surrounding epidermal cells.
Lastly, during the remodeling phase, the newly formed granulation tissue is highly organized and aligned, to allow for scar maturation. The remodeling phase is the longest stage of wound healing, lasting from 1 to 2 years. The mature healed scar becomes gradually denser with time, with fibroblasts remodeling the ECM with type 1 collagen. Matrix metalloproteinases (MMPs) secreted by the fibroblasts, inflammatory cells, and epidermal cells are responsible for the ECM remodeling of the granulation tissue into the final scar composition. The reorganization and cross-linking of the ECM by the fibroblast during this phase, gradually strengthens the network of fibers over time. However, the tensile strength of the wounded skin will only reach 80% of the tensile strength of unwounded skin.
Throughout the wound healing process of skin, fibroblasts play the most vital role in tissue repair and invariably the degree of scar formation. Traditionally fibroblasts have been defined as a homogenous population of connective tissue resident cells that secrete the ECM in response to wound injury. However, recent work, has shown that fibroblasts are a heterogeneous cell population within the skin with distinct functional roles. Fibroblasts within murine and human unwounded dermis have been shown to exhibit fibroblast heterogeneity based on three criteria: (1) their embryonic origin, (2) tissue anatomical site, and (3) the microenvironments. Fibroblast subpopulations have been elucidated based on their developmental lineages. In 2015, engrailed-1 lineage-derived fibroblasts were reported to be responsible for scarring in the mouse skin dermis, providing a link between developmental lineage and wound healing. In addition, this study further highlighted that Wnt1 lineage-derived fibroblast population in the oral mucosa are responsible for the decreased scarring response of the fibroblasts within the oral mucosa. More recently, Leavitt et al . demonstrated that paired related homeobox 1 expression defines fibroblasts responsible for ventral scar production in mice.
One of the most intriguing concepts in scar biology is the anatomical diversity of fibroblasts with the skin. In 2013, Driskell et al . demonstrated that skin fibroblasts are derived from a common fibroblast progenitor cell and differentiate into two broad dermal lineages based on their anatomical location within the skin by embryonic day (E12.5) ( Fig. 16.2 ). The upper dermal lineage, the “papillary” fibroblasts, contribute to new hair follicle formation and the lower dermal lineage, the “reticular” fibroblasts, participate in early wound repair. Lastly, fibroblasts have shown to differ by their response to the microenvironmental niche. Recent studies have shown that fibroblast subtypes are able to transition into other fibroblast subtypes and even different cell types due to the exposure of specific external stimuli. For example, in 2017, it was reported that the activated fibroblasts, the myofibroblasts, could give rise to adipocytes under the stimulation of bone morphogenetic protein (BMP) signaling. The reverse of this process has also been reported where adipocytes within the wound site lose their intracellular lipid stores and express fibroblast markers to participate in scar formation.
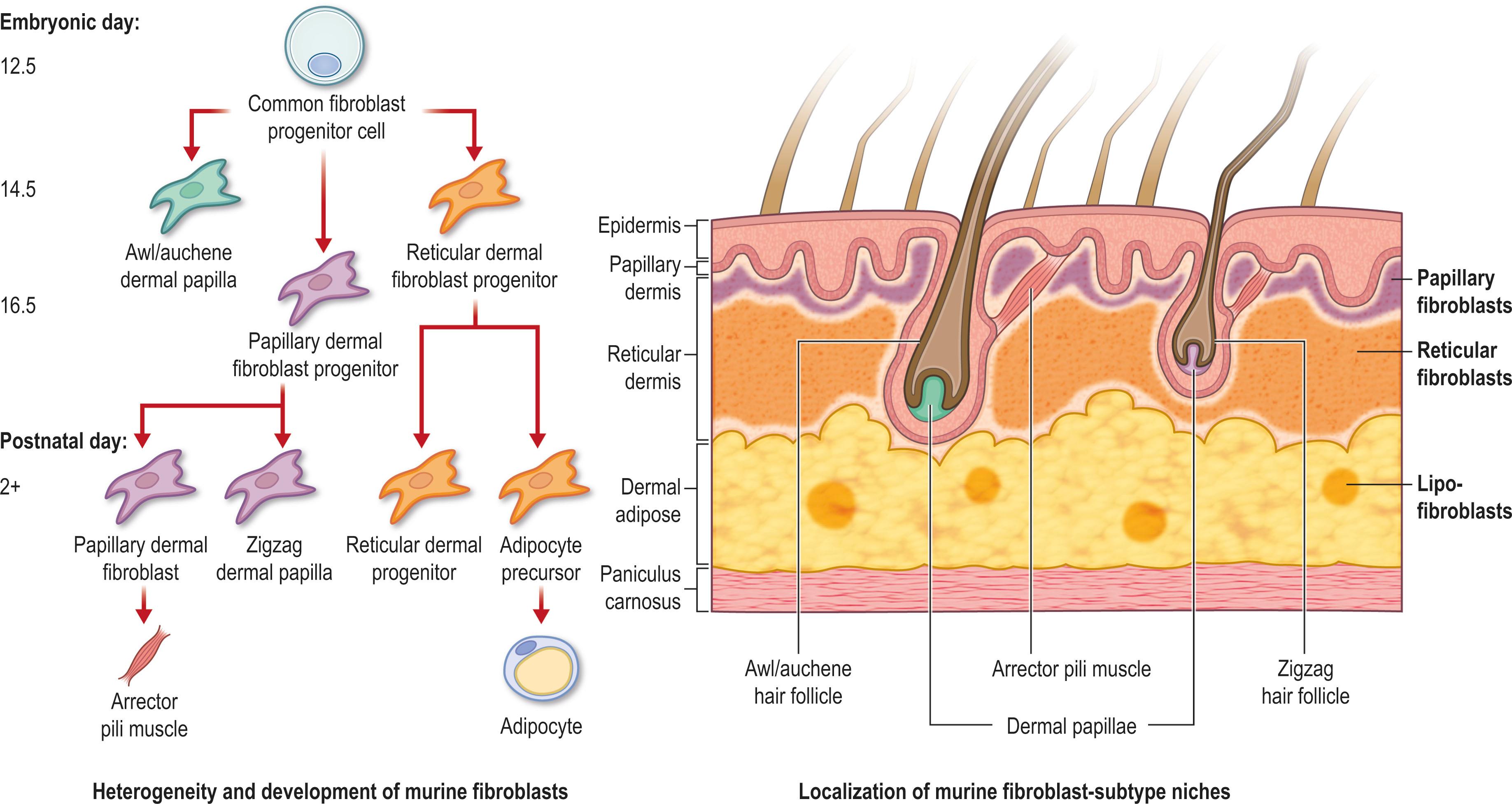
While fibroblast heterogeneity has been highly studied in mouse skin, it remains to be understood how these findings will translate to human fibroblasts. Understanding that fibroblast heterogeneity may dictate wound healing outcomes in skin is crucial for the clinical setting. The identification of unique fibroblast populations that cause skin fibrosis could provide therapeutic targets to overcome skin scar formation.
One exciting approach to decrease scar formation focuses on mechanical tension across the wound. Surgeons have long known that greater tension across skin wounds produces greater scar. Because wound mechanical forces provoke dermal fibroblasts to produce scars, a targeted reduction of wound mechanotransduction signal presents an opportunity to induce tissue regeneration. We have demonstrated that mechanical tension drives engrailed-1 activation in fibroblasts, which when blocked, promotes wound regeneration with recovery of skin appendages, ultrastructure, and mechanical strength. Although these outcomes are promising, further work is needed to translate them to our human patients. These findings also suggest two possible outcomes to postnatal skin wound healing: a fibrotic response and a regenerative response.
Wound healing is a tightly regulated process involving cell–cell signaling and environmental cues. In normal wounds, repair slows when the dermal defect is closed and epithelialization is complete. But when appropriate signals are absent or ineffective, the repair process may continue unabated and result in surplus scarring. Hypertrophic scars and keloids, both fibroproliferative disorders of wound repair, are two examples of this.
In general, both keloid and hypertrophic scar fibroblasts have an upregulation of collagen synthesis, deposition, and accumulation. However, the underlying regulatory mechanisms responsible for this excessive repair are still under investigation. Both profibrotic cytokines such as transforming growth factor-β1 (TGF-β1) as well as a lack of programmed cell death, or apoptosis, of activated fibroblasts secreting ECM components have been implicated in excessive scarring. For unknown reasons, hypertrophic scars and keloids are unique to humans and do not naturally occur in other animals.
Normotrophic, hypertrophic, and keloid scars vary little histologically. Pathologic scar types are therefore distinguished based on clinical characteristics. Hypertrophic scars continue to thicken and rise instead of flattening and shrinking like mature scars, but stay within the original scar boundary. In contrast, keloid scars grow beyond the boundaries of the initial wound ( Table 16.3 ). Because the timeline from immature to mature to hypertrophic and keloid scar formation can vary due to a number of factors, diagnosis is not always clear. Clinical monitoring of scar evolution with frequent serial examinations is the most important strategy for diagnosing and treating the problem scar. Early application of treatment modalities, discussed in depth later in this chapter, can often prevent further progression and even reverse scar pathology.
| Appearance | Growth pattern | Cause | |
|---|---|---|---|
| Normal mature scar | Hypo- or hyperpigmented, flat | Contracts slowly over time | Normal tissue repair |
| Immature scar | Red, slightly elevated, sometimes itchy | Turns into mature scar | Normal tissue repair |
| Hypertrophic | Raised, itchy, painful, confined to its borders | Self-limited, but may take years | Excess mechanical stress |
| Keloid | Raised, itchy, extending into normal tissue | Continued growth; recurs | Genetic predisposition, can result from minor trauma |
Hypertrophic scars are defined as pathologic scars that have not overgrown the original wound boundaries, but are instead abnormally thickened and raised ( Fig. 16.3 ). Hypertrophic scars are often painful and pruritic. This is thought to be due to release of proinflammatory neuropeptide substance P from nerve endings following injury. Hypertrophic scars usually form secondary to excessive tensile forces across the wound and are most common in wounds across flexion surfaces, the extremities, breasts, sternum, and neck.
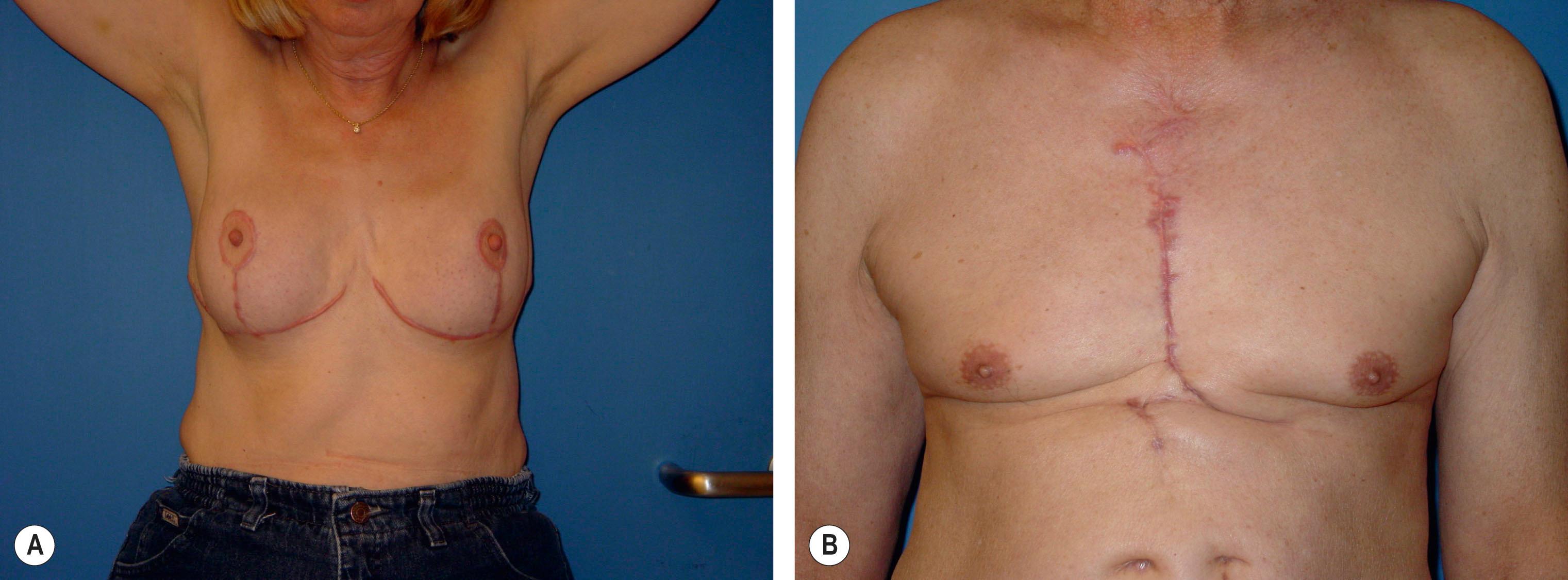
Hypertrophic scarring is a self-limited process and usually regresses with time. These scars eventually fade in color and flatten to the surrounding skin level, though the unaided process may take years. No clear histological differences between hypertrophic scars and keloids exist. Early studies found that keloids contained bundles of collagen around focal nodules of proliferation. Later studies, however, refuted this distinction.
To date, clinical treatment of hypertrophic scars remains unsatisfactory, and is reflective of our incomplete understanding of mechanisms underlying hypertrophic scar formation. The creation of a consistent and reproducible model for testing new treatment modalities, particularly animal models of hypertrophic scars, is key to advancing our knowledge of hypertrophic scar biology.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here