Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Radiology testing in the trauma setting including radiography, fluoroscopy, ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) has increased substantially in the emergency room and trauma setting over the last decade. The use of CT, which is most concerning because of expense and radiation dose, increased 330% from 1996 to 2007 such that almost one quarter of all CT scans are performed in the emergency department. The increase in total number of patients seeking emergency room care during that period only increased by 30%. Substantial costs to the health care system and patient safety issues derive from overutilization of imaging resources. Safety concerns include the theoretical risk of cancer in patients exposed to radiation, the risk for renal failure or allergic reactions from contrast media, and nephrogenic systemic fibrosis from MRI contrast agents. While these risks are often outweighed by the benefits of diagnosis, radiologists from all across the country report an excessive number of negative examinations performed for questionable indications. While medical-legal issues are often cited as a reason for imaging, the not-too-distant future may herald litigation for physicians who overorder radiation-based tests. Despite these concerns, imaging will remain an important tool in the diagnostic armamentarium of trauma physicians. Determining the best study to order for a particular indication requires an understanding of each modality’s uses and risks as well as an understanding of how pretest probability and evidence influence diagnostic decision making.
Plain radiography (plain films or “x-rays”) uses ionizing radiation (potentially a cause of cancer) to produce an image based on the relative densities of tissues. Metal appears most white and air appears black with all other tissues filling in the range of appearances. The spatial resolution of plain radiographs is excellent and with portable units, images are acquired with minimal patient transfer. Before CT came into common use, radiographs were the most reliable assessment for musculoskeletal injuries and evaluation of the chest. It is still commonly used in the trauma setting for a rapid evaluation of the chest, pelvis, and extremities.
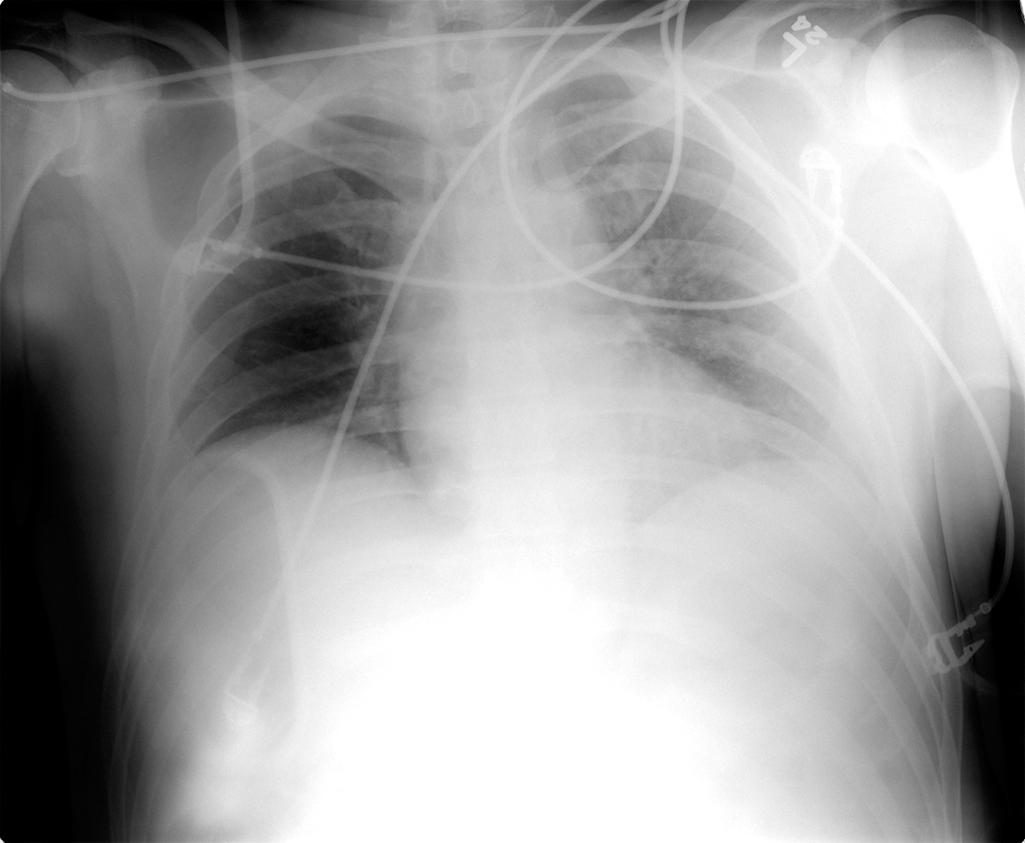
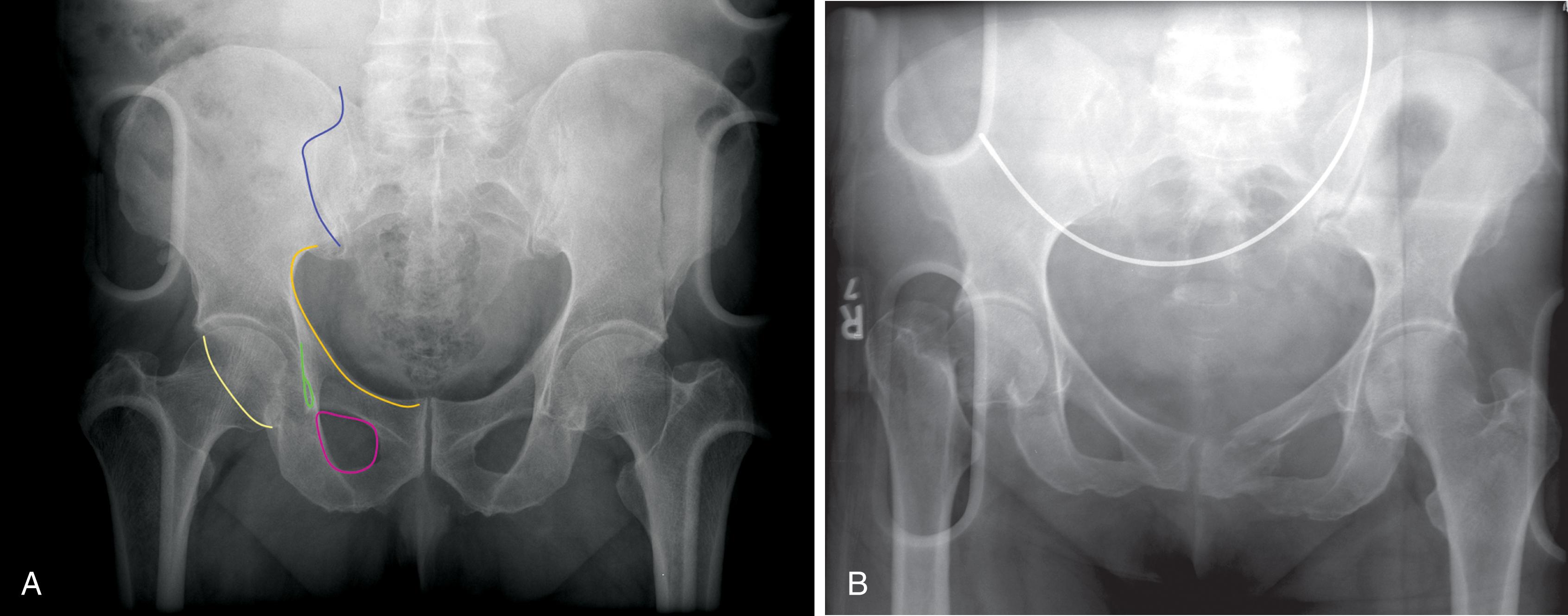
The initial radiograph of the chest and pelvis is useful as a quick screening tool to assess extent of traumatic injuries, though sensitivity to detect soft tissue and bony injuries should be considered low or moderate at best if patient position is not optimal. Sensitivity also depends partly on radiograph quality ( Fig. 1 ). Overlying structures such as backboards and electrocardiogram leads limit assessment of soft tissues and obscure the lung apices where small pneumothoraces may reside. Because lung volumes are typically low, the heart and mediastinum normally appear wide or enlarged. Subtle findings that suggest aortic injury such as thickening or obscuration of the paraspinal stripe is difficult to appreciate. Retrocardiac density may obscure the descending aorta and left diaphragm due to atelectasis common to the supine patient in expiratory phase of respiration. Anterolateral rib fractures in particular may be missed. The pelvis radiograph may also miss subtle injuries, and evidence of pelvic trauma through physical examination is key to interpreting these films with respect to pretest probabilities ( Fig. 2 ).
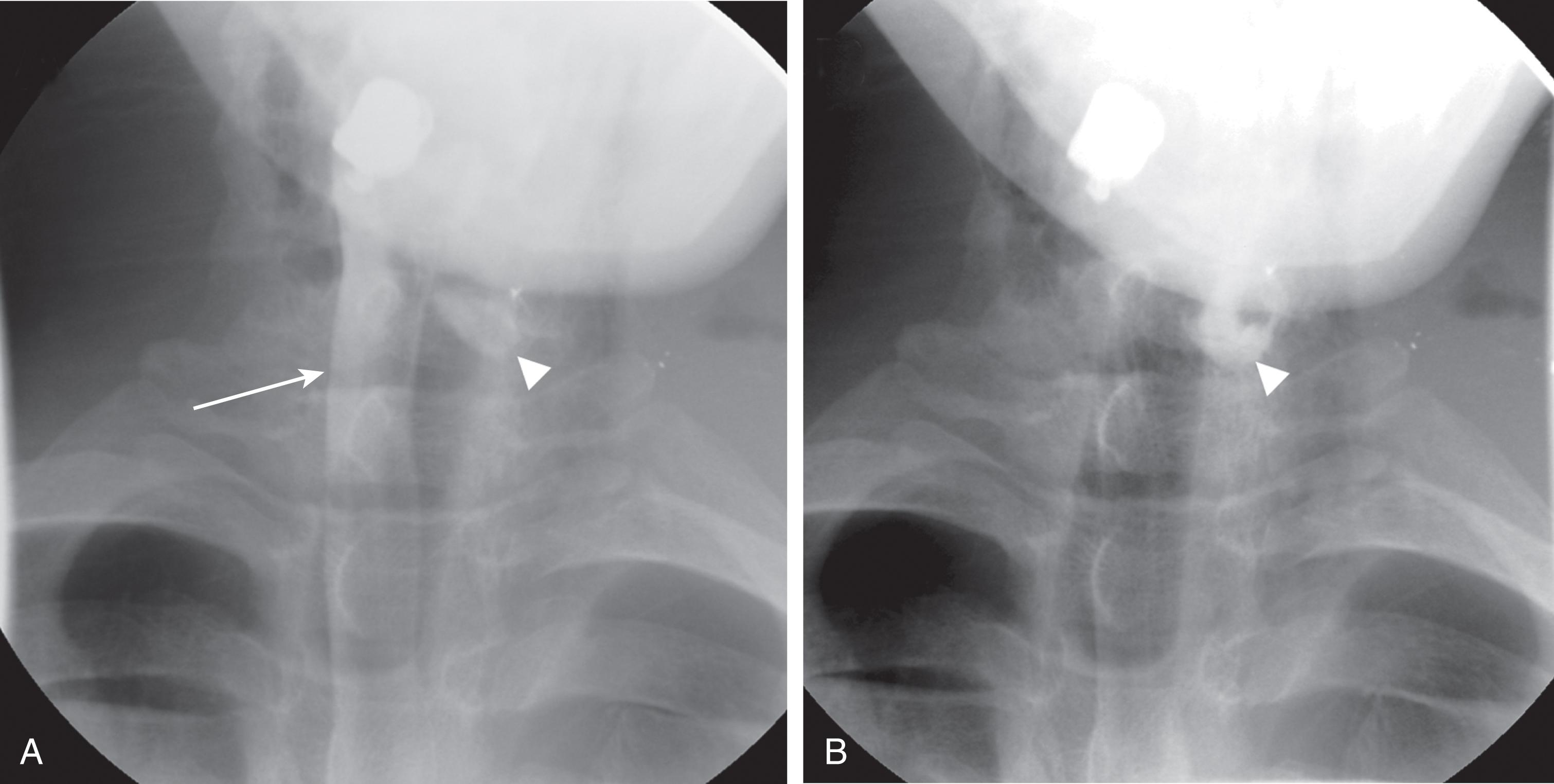
Fluoroscopy uses x-rays in a similar fashion as plain radiographs, but the images are viewed in real time, typically with the introduction of contrast agents. The radiation dose administered during a burst of fluoro is typically less than for a conventional radiograph; however, since multiple bursts of fluoro are given during a typical procedure, the radiation dose can increase rapidly. Diagnostic fluoroscopic procedures are useful for evaluating hollow viscus or any structure into which contrast can be placed. The image is then a lumenogram, useful for identifying leaks in the bowel ( Fig. 3 ), bladder, or extravasation during angiograms. Fluoroscopy is often used by orthopedic surgeons to set bones. Operators who use fluoroscopy should wear leaded gowns, thyroid shields, and radiation badges when appropriate.
US uses a transducer to generate sound waves, which pass into the body and bounce off tissues that have features that reflect sound. The reflected sound returns to and is detected by the transducer. By knowing how much sound returned to the transducer and how long it took for the sound wave to make the round trip, the computer calculates the depth and relative brightness of the tissue. Therefore, the image is not a density map; rather, the image is based on the ability of various tissues to reflect sound. For this reason, something that is bright is called echogenic or hyperechoic (many echoes) and something that is darker than surrounding tissues is hypoechoic (less echoes) or completely black is anechoic (no echoes). Because water conducts sound, it appears black since no sound waves are reflected. In contrast, air is a strong reflector of sound so most of the sound is reflected to the transducer. Therefore, air appears as a white line with “dirty shadowing” or an indistinct fuzzy appearance beneath it that obscures features of tissues deeper than the air. Radiology technologists sometimes say that a structure like the pancreas is “gassed out,” meaning that gas from bowel is obscuring that structure. If gas is a problem, you can give ask the patient to drink water, which fills the stomach and duodenum and allows better images of structures in the upper abdomen. When structures are obscured by gas or bone, it is said that the patient has “poor windows” and giving water can improve those “windows” ( Fig. 4 ).
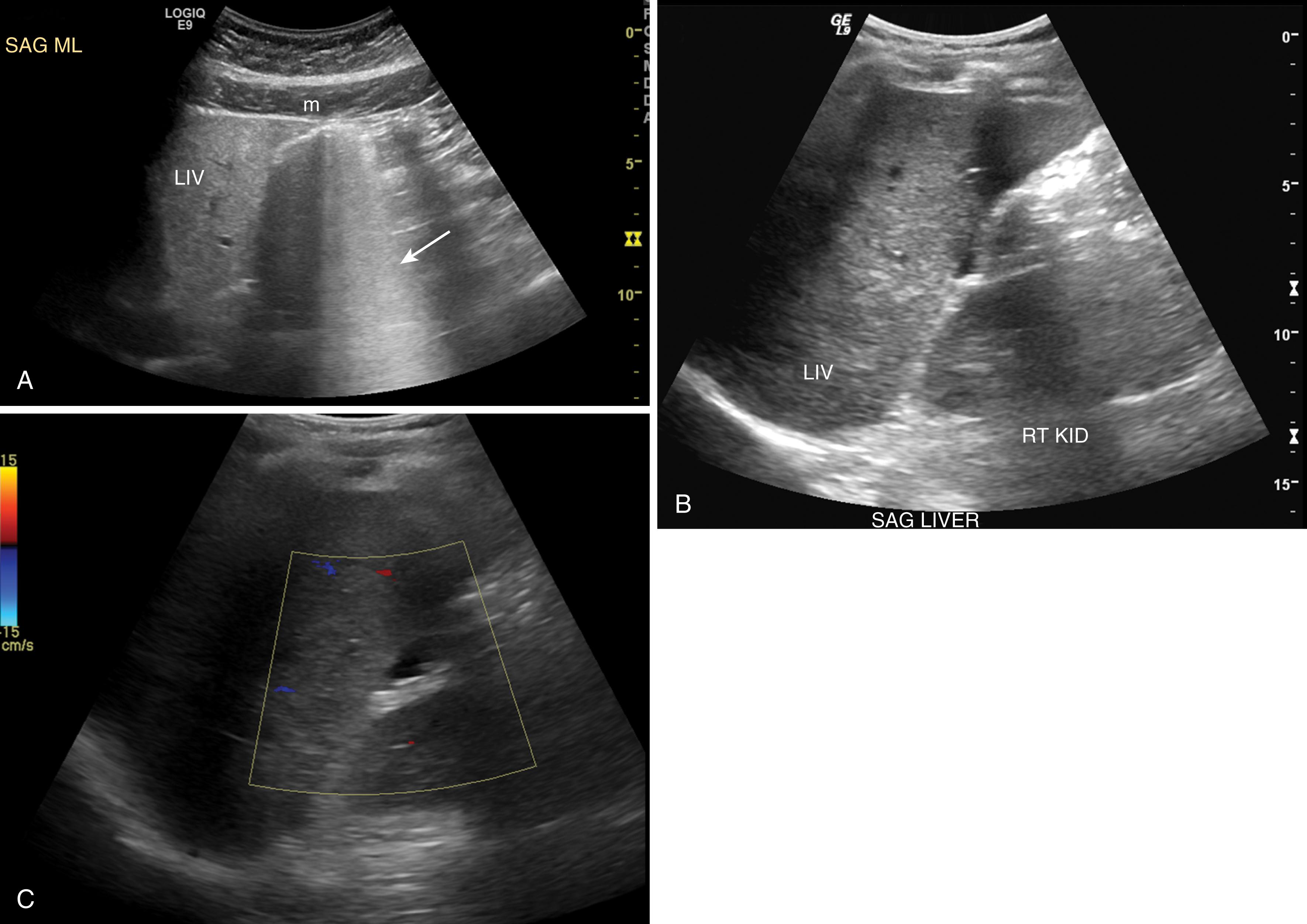
US has excellent temporal resolution and allows real-time imaging of the body. It is particularly useful for detecting fluid and the characteristics of fluid such as hemoperitoneum in the trauma setting. It is also useful for detecting injuries to solid organs and confirming the patency of vasculature using Doppler techniques. Importantly, since US uses sound waves, there is no ionizing radiation. Because US contrast agents are not typically used in the trauma setting, there is no risk of contrast or allergic reactions, so the safety profile of US is excellent. However, the sensitivity to detect disease such as solid organ injuries is lower than other modalities such as CT.
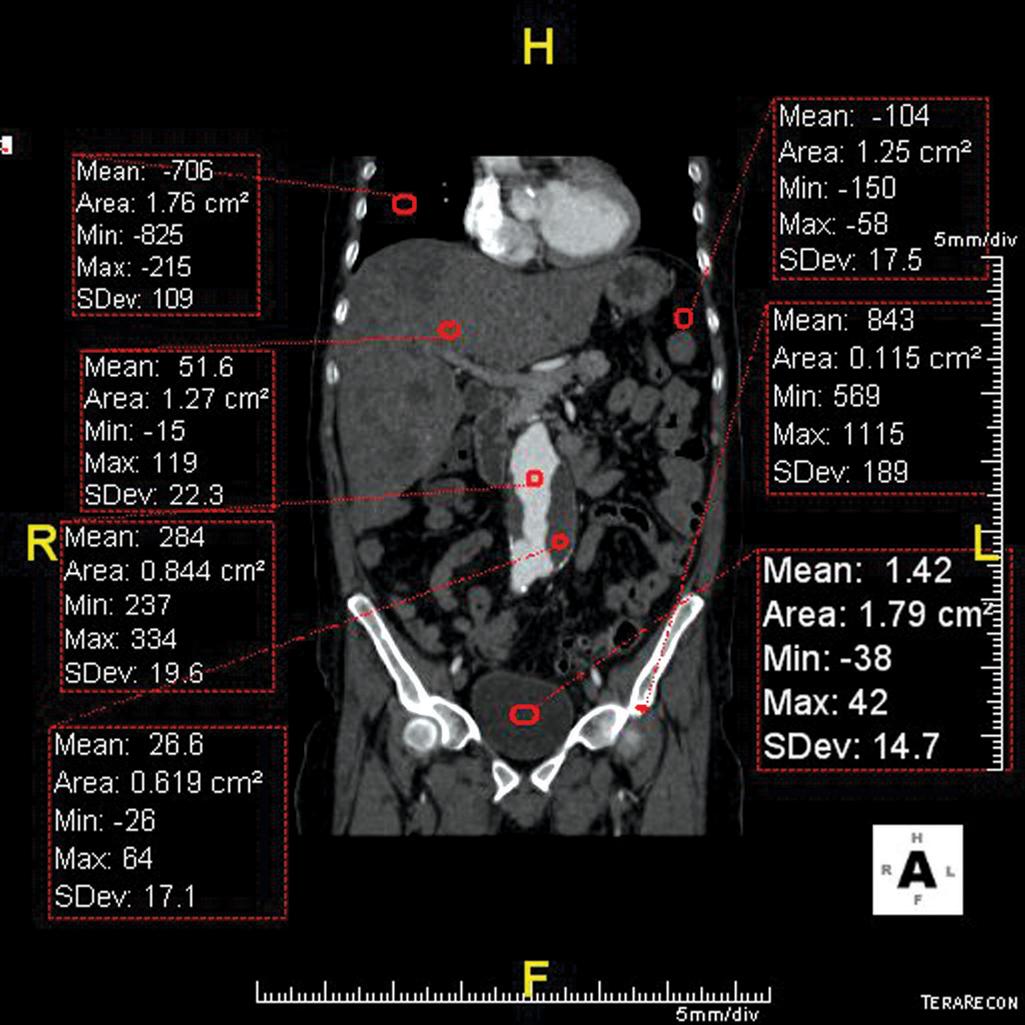
CT has become the workhorse in the trauma setting due to improved availability and the brief time it takes to acquire images. CT does use x-rays, a potentially cancer-causing form of ionizing radiation. By measuring the attenuation of x-rays of a particular spot in the body using a variety of projections, a computer assigns a value to that area based off the Hounsfield scale where water is assigned a value of 0 and air is assigned −1000. All other densities are then assigned Hounsfield units reflecting their relative density ( Fig. 5 ). CT has excellent spatial resolution and good contrast resolution. Spatial resolution for CT is the ability to differentiate two lines separated by a very small distance. Spatial resolution allows a viewer to see small structures. Contrast resolution for CT is the ability to differentiate two adjacent tissues of slightly varying density. The ability to see a liver contusion is very dependent on contrast resolution and image noise. A noisy image appears grainy to the viewer and can obscure findings by decreasing contrast resolution. Generally, the more radiation dose used, the less noise and better overall image quality will result. Another way to improve contrast resolution is to use intravenous (IV) CT contrast. IV contrast agents for CT are made up of iodinated molecules that attenuate x-rays and appear bright on the image.
IV contrast reflects the vascularity of a tissue or pathologies within the tissue, and the timing of the contrast bolus is important as the different phase of contrast impacts what can be seen in the image. This is important to understand because it explains why radiologists have to scan the patient multiple times and why it’s important to let the radiologist know exactly what is being looked for before the study is performed. The arterial phase is obtained between 15 and 30 seconds after injection of contrast depending on what part of the body is being scanned and how fast the CT scanner is. A CT obtained in the arterial phase is called a CT angiogram or CTA. The arterial phase only shows arterial supply and is excellent for seeing extraluminal contrast (active extravasation) against the background of unenhanced tissue ( Fig. 6 ). Unfortunately, it is sometimes difficult to decide if a particular line of contrast seen on the image is part of a small vessel or actually a spurt of extravasated contrast. For this, a more delayed phase of contrast is required. Splenic parenchyma is also particularly difficult in the arterial phase as the heterogeneous blood supply causes a mixed pattern that can be mistaken for a splenic injury.
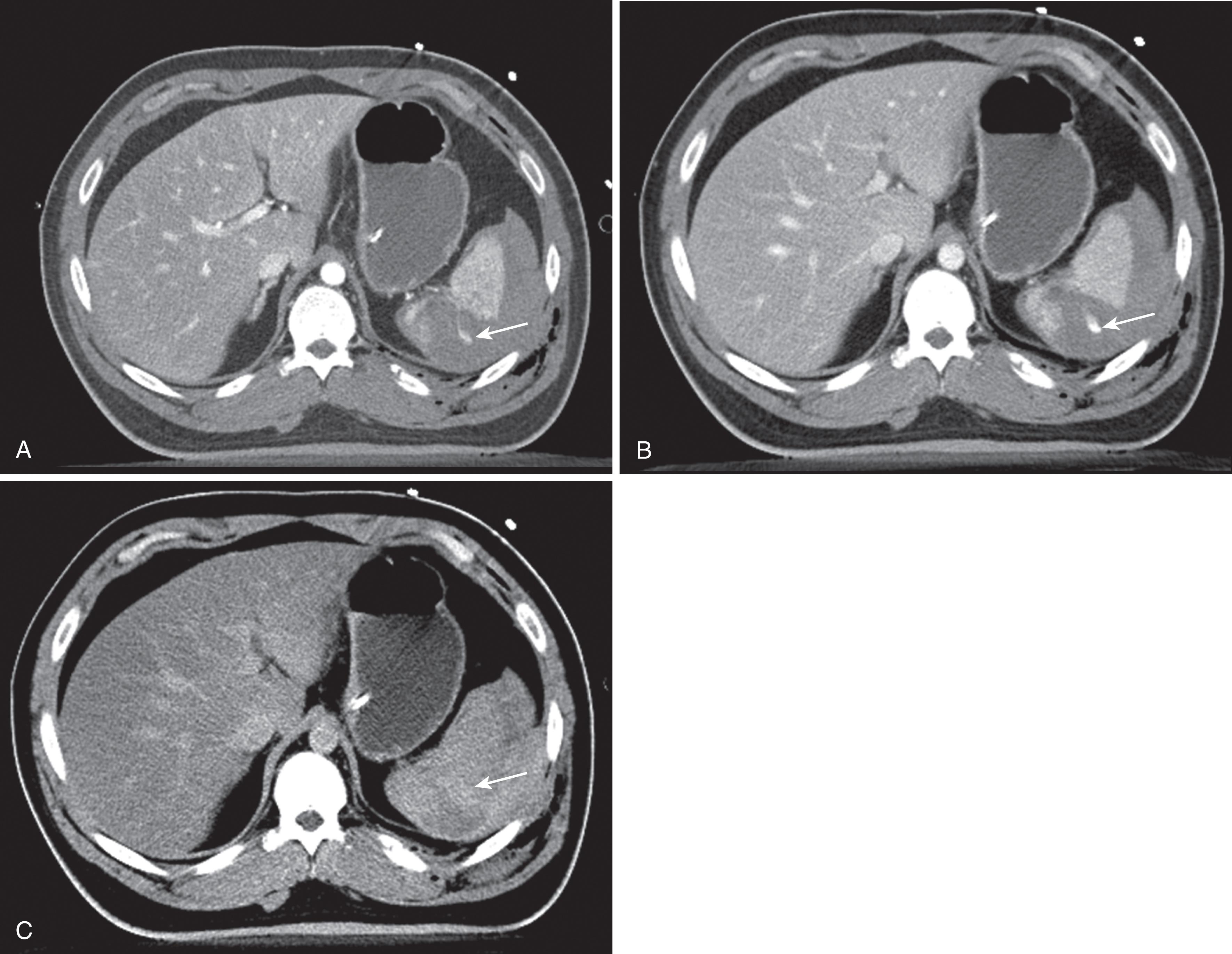
The “portal venous” phase is usually acquired between 60 and 75 seconds after injection of contrast. During this time, the portal veins and solid organs including the liver, kidneys, pancreas, and spleen should be optimally enhanced for evaluation of the parenchyma. It is ideal for detecting subtle contusions or lacerations. The arteries remain enhanced but because the parenchyma of solid organs is enhanced, extravasation may be difficult to evaluate. Large veins are also enhanced at this phase, and venous injuries may be detected. The delayed phase is acquired 5 minutes after contrast injection and is best for evaluating the renal collecting system and urinary bladder as they should be filled with contrast. Solid organs have lost most of their parenchymal enhancement by 5 minutes. Because arteries are certainly not enhanced during the delayed examination, extravasated contrast will appear as a larger, more diffuse area of contrast enhancement (a blush) than the focus of extravasated contrast seen on the arterial phase. By comparing the delayed phase or sometimes even the portal venous phase to the arterial phase, active extravasation can be diagnosed. The downside of obtaining multiple phases of contrast is the doubling or tripling of radiation dose. To reduce the chances of obtaining unnecessary scans, the radiologist may view the images before the delay phase and decide whether or not additional phases are necessary.
CT technology has dramatically changed over the past 15 years. Conventional CT scanners obtained one image (a slice) of a given thickness. After acquiring the image, the table moved the patient to the next position and another image was obtained. This is called step and shoot. For various reasons, reconstructed images were limited, and the scans were relatively slow. With helical or spiral CT, the table moves at a constant rate throughout image acquisition as the gantry rotates around the patient. Software reconstructs the images into a volumetric data set that can be reconstructed in any plane. Multidetector computed tomography (MDCT) uses multiple rows of detectors to scan a wider area of the body with each rotation. Since the scan covers a greater area, the table speed can be increased and the total time decreases. This is particularly helpful in the trauma setting as patients with altered mental status may not hold still and motion degrades image quality, making diagnosis difficult. Some of the newest and fastest units have acquired diagnostic images while a child tried to sit up on the CT table during the scan. All new CT units are MDCT and detector rows of 64, 128, and 320 have revolutionized CT imaging.
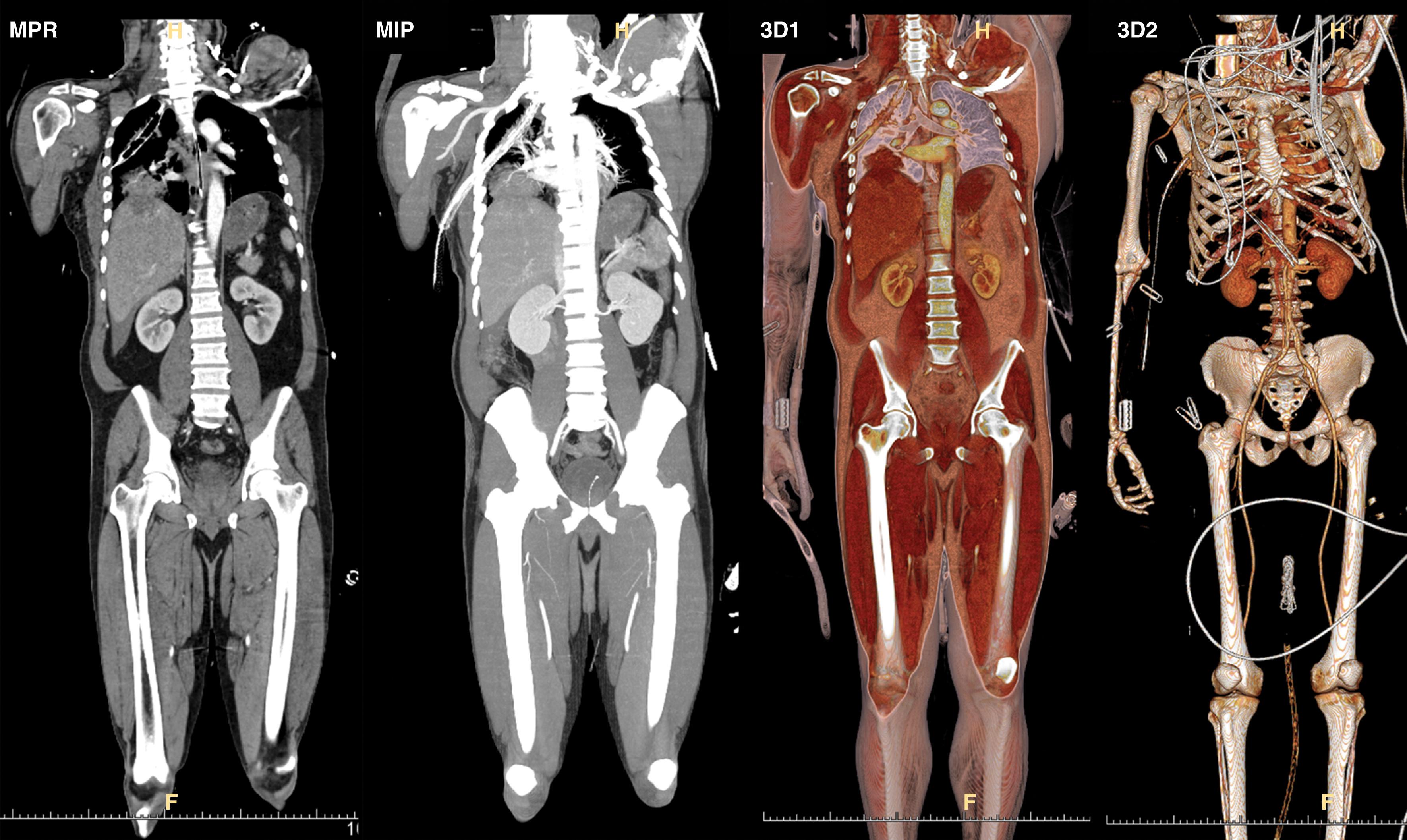
With the advent of spiral CT and isotropic data sets (each voxel or part of the image makes up a cube of equal sides), the image can be reconstructed into any plane. By changing the color or brightness that a particular density is assigned, a variety of images can be generated. Three-dimensional images are produced by assigning colors to various densities in the volume data set ( Fig. 7 ). Maximum intensity projected images emphasize high-density structures such as contrast-enhanced vessels and bone. Lower-density structures are suppressed. Maximum intensity projected images are very useful for assessing arteries in the trauma patient. These image processing techniques help show spatial relationships between injuries, anatomy, and foreign bodies ( Fig. 8 ).
MRI has limited use in the early trauma setting because it is generally not available in most trauma centers, it takes a relatively long time to acquire the images, and the patient must be free of metallic foreign bodies. MRI has excellent contrast resolution, but spatial resolution of CT is superior. CT is able to make most of the diagnoses important to stabilize the patient. Detailed evaluation of the spinal cord or evaluation of subtle diffuse axonal injury lesions may be improved with MRI.
If the patient has a known severe iodinated contrast allergy, then CT may be performed without the administration of IV contrast, although it will be significantly limited in evaluation for vascular, solid organ, and hollow viscus injury. Another option would be to premedicate the patient with corticosteroids and diphenhydramine; however, the fastest widely accepted protocol requires a 6-hour preparation time. IV contrast administration after premedication should only be performed in patients who report prior minor reactions to IV contrast, history of moderate to severe asthma, severe reaction to one substance, or multiple known food, medication, or other substance allergies. Contrary to prior beliefs, studies have shown that a history of minor shellfish allergy does not pose a significant risk of reaction to contrast administration. Premedication should not be administered if there is a history of anaphylaxis or bronchospasm after IV contrast exposure. Caution should also be taken in administering contrast despite premedication in patients on chronic corticosteroid therapy, those having severe allergic reaction to any one substance, and those with severe allergies. Keep in mind that even with premedication a patient may still adversely react to IV contrast. No set premedication protocol exists. Tables 1 and 2 list widely accepted premedication protocols.
| Prednisone | 50 mg PO | Administer 13, 7, and 1 hour prior to IV contrast administration |
| or | Administer 12 and 2 hours prior to IV contrast administration | |
| Methylprednisolone | 32 mg PO | Administer 13, 7, and 1 hour before IV contrast administration |
| or | ||
| Hydrocortisone | 200 mg IV | |
| Diphenhydramine | 50 mg PO, IV, or IM | Administer 1 hour prior to IV contrast administration |
| Hydrocortisone | 200 mg IV | Administer 6 hours prior to IV contrast administration |
| Diphenhydramine | 50 mg IV | Administer 1 hour prior to contrast administration |
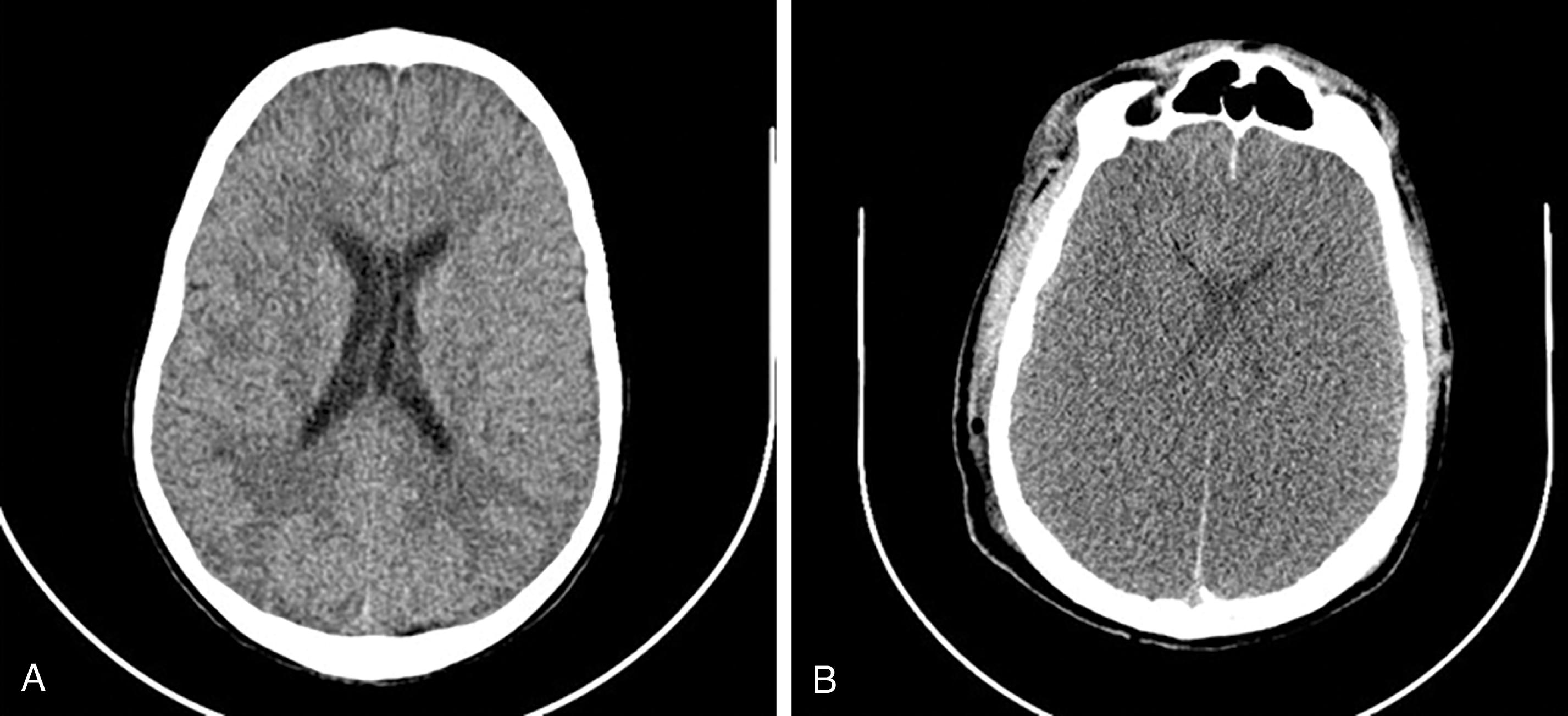
Traumatic brain injuries lead to significant morbidity and mortality in trauma patients. Evaluation with a noncontrast CT of the brain is routine and should be the first step to exclude traumatic brain injury. Candidates for imaging include those who suffer loss of consciousness or altered mental status; those with neurological deficits; intoxicated patients; patients with battle signs, fluid or bloody otorrhea, suspected CSF leaks, and facial trauma ( Figs. 9 and 10 ).
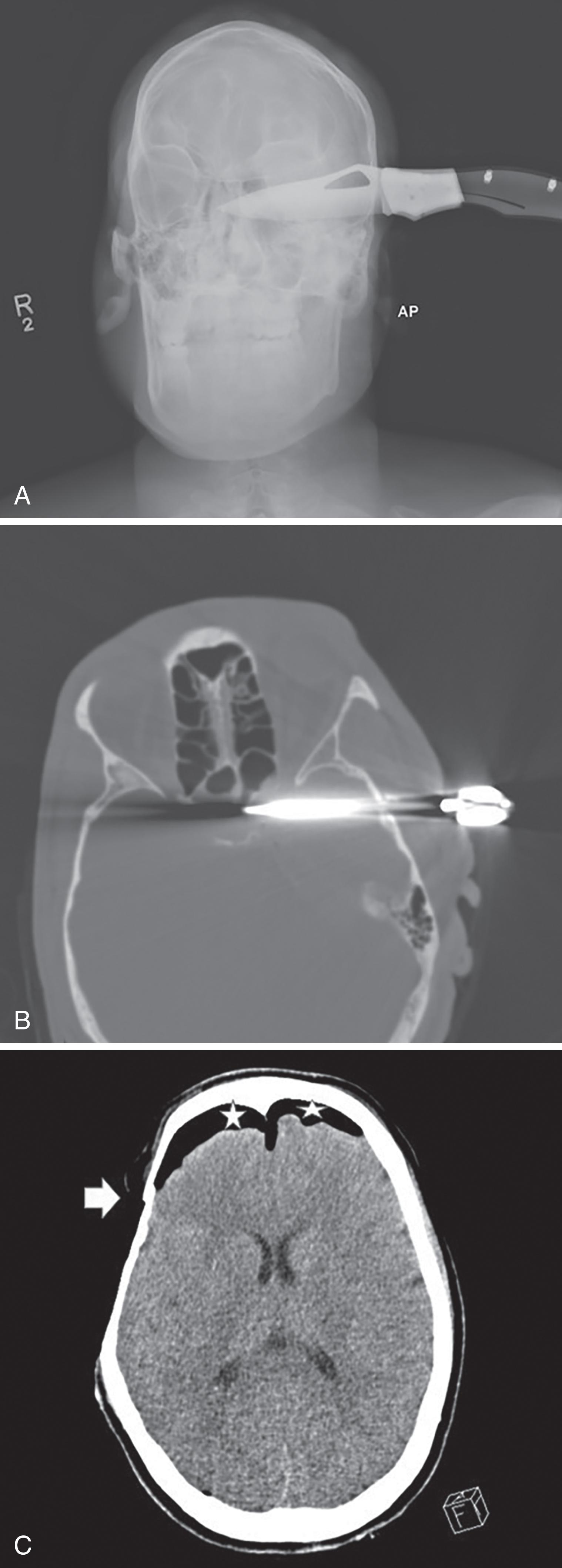
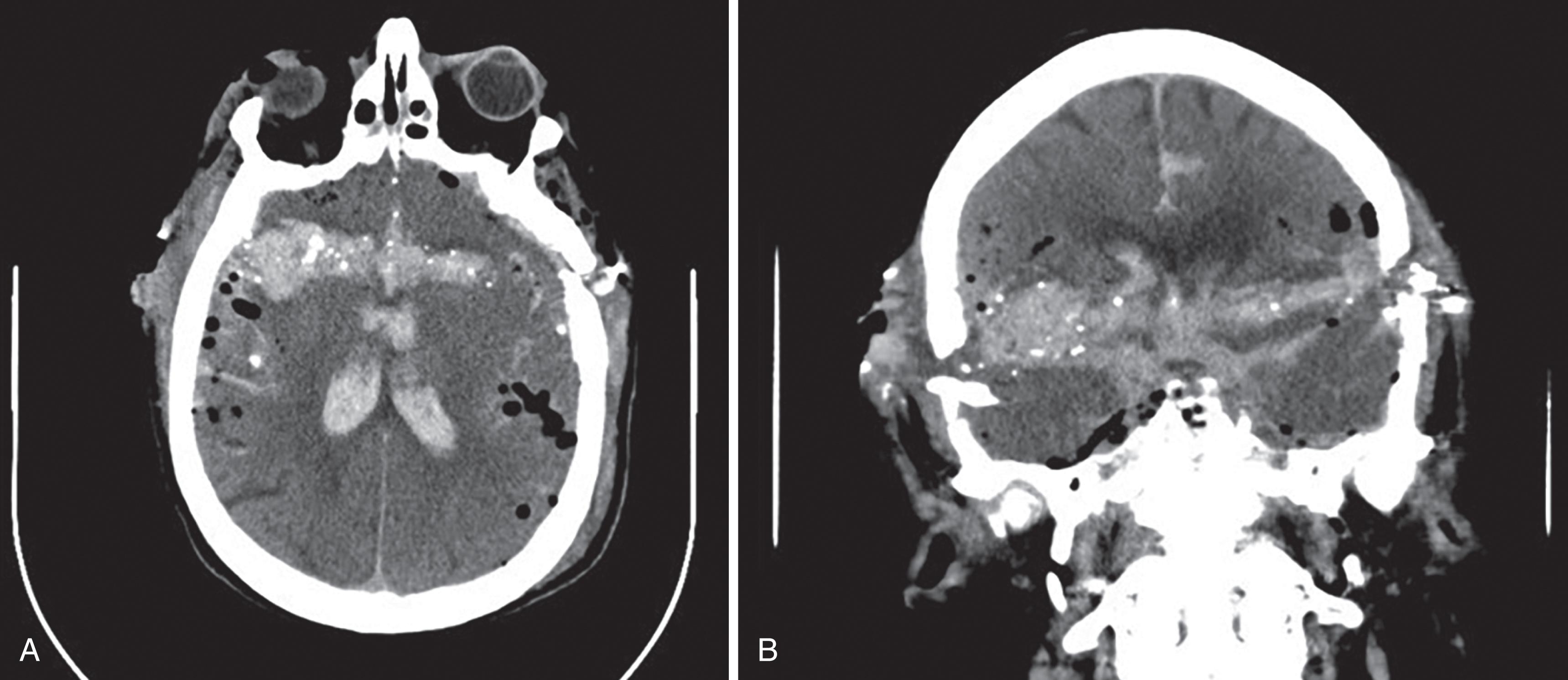
Subarachnoid hemorrhage will appear as extra-axial serpiginous areas of high density, high density within the interpeduncular cistern, focal high-density collections in sulci and cerebellar folia, high density along the interhemispheric fissure or along the tentorium ( Fig. 11 ). Acute subdural hemorrhage usually appears as dense extra-axial crescent-shaped collections; however, they may also present as dense thickening of the interhemispheric fissure or blood layering along the tentorium. Acute epidural bleeds present as lentiform or convex-shaped extra-axial dense collections and commonly have an adjacent skull fracture. Hemorrhagic cortical contusions appear as high-density foci within the cerebral cortex. Diffuse axonal injuries, injury to the white matter tracts, are difficult to diagnose. MRI is more sensitive than CT in identifying these lesions and therefore the preferred imaging choice to identify these lesions. MRI gradient echo sequences and diffusion tensor imaging are the best sequences to identify these lesions. On MRI 1- to 15-mm foci of edema or hemorrhage may be seen in the white matter tracts. Diffuse axonal injuries may present on CT as foci of hemorrhage (high density) at the gray-white matter junction and within the corpus callosum, thalami, cerebral peduncles, and basal ganglia. Diffuse axonal injuries are a leading cause of persistent unconsciousness and vegetative states as well as persistent neurological impairment in the trauma patient. In a patient with persistent coma and negative CT, MRI should be obtained to evaluate for diffuse axonal injuries.
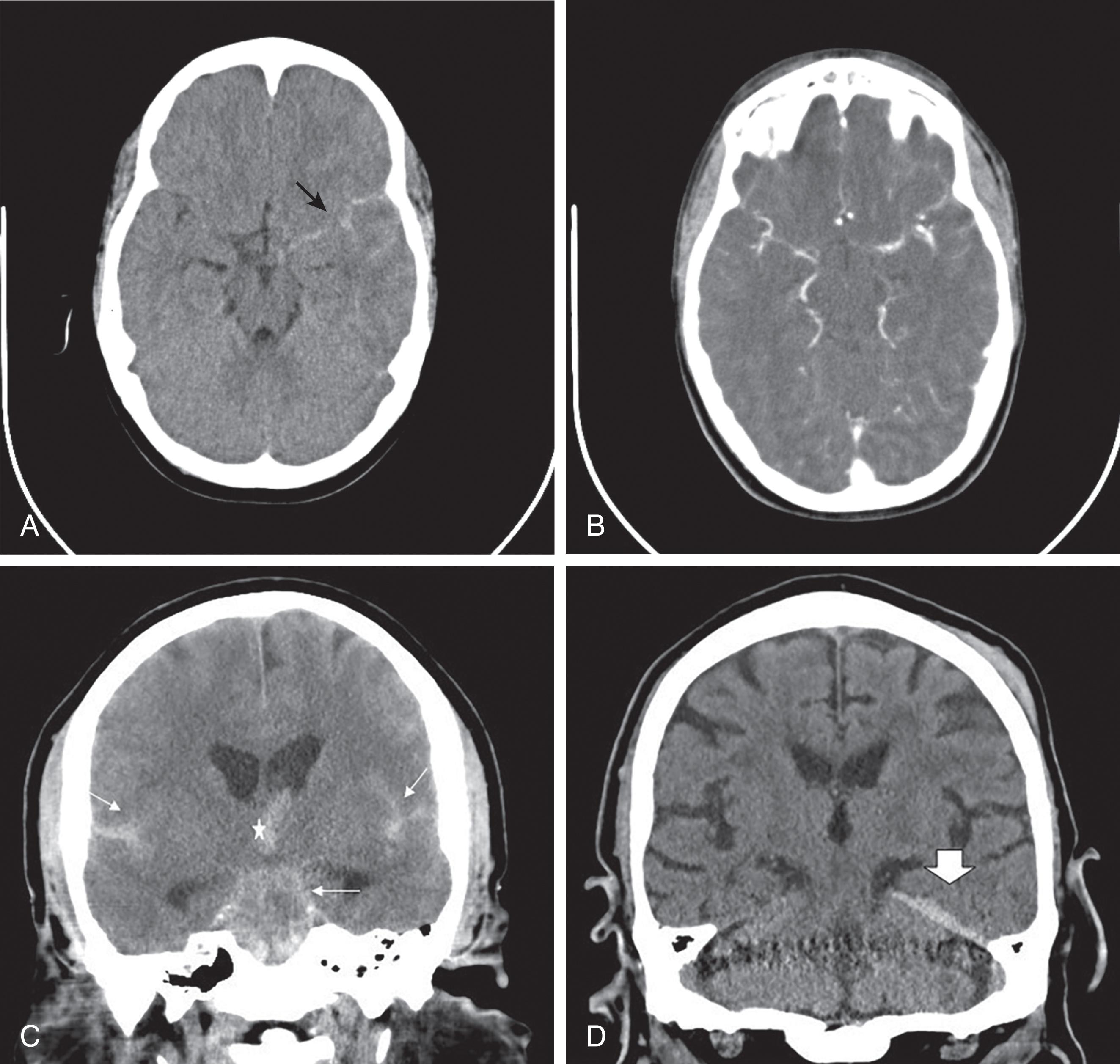
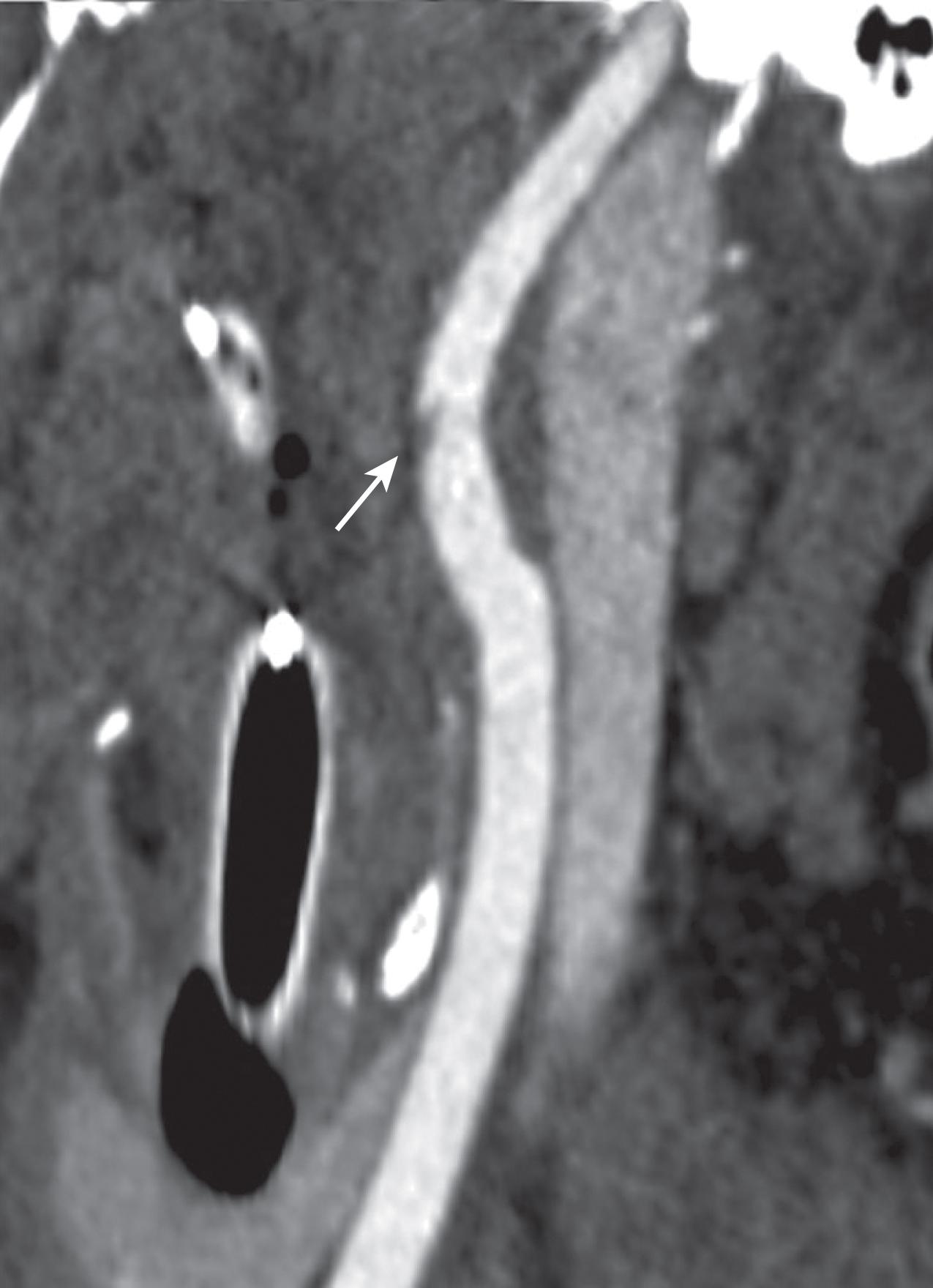
Both penetrating injuries and blunt trauma to the head and neck may result in vascular injury. CTA (computed tomography arteriography) of the head and neck should be performed if there is concern for vascular occlusion, dissection, transection, vascular extravasation, or carotid cavernous sinus fistula ( Fig. 12 ). CTA should be performed following a noncontrast brain CT as contrast typically obscures hemorrhage (see Fig. 11 B). Incidentally CTA images of the head and neck may demonstrate brain death. In brain death there is nonopacification of the arteries above the level of the terminal internal carotid arteries. The most sensitive and specific marker of brain death on CTA is nonopacification of the cortical middle cerebral arteries and internal cerebral veins ( Fig. 13 ). Confirmation may be obtained with a dedicated nuclear medicine brain perfusion study.
If the patient exhibits signs of skull base injury, then a dedicated temporal bone CT should be performed. Axial 1- to 2-mm thin section CT images are obtained through the temporal bones. Sagittal and coronal images should be reformatted to aid in the detection of subtle nondisplaced fractures and to evaluate the extent and path of injury.
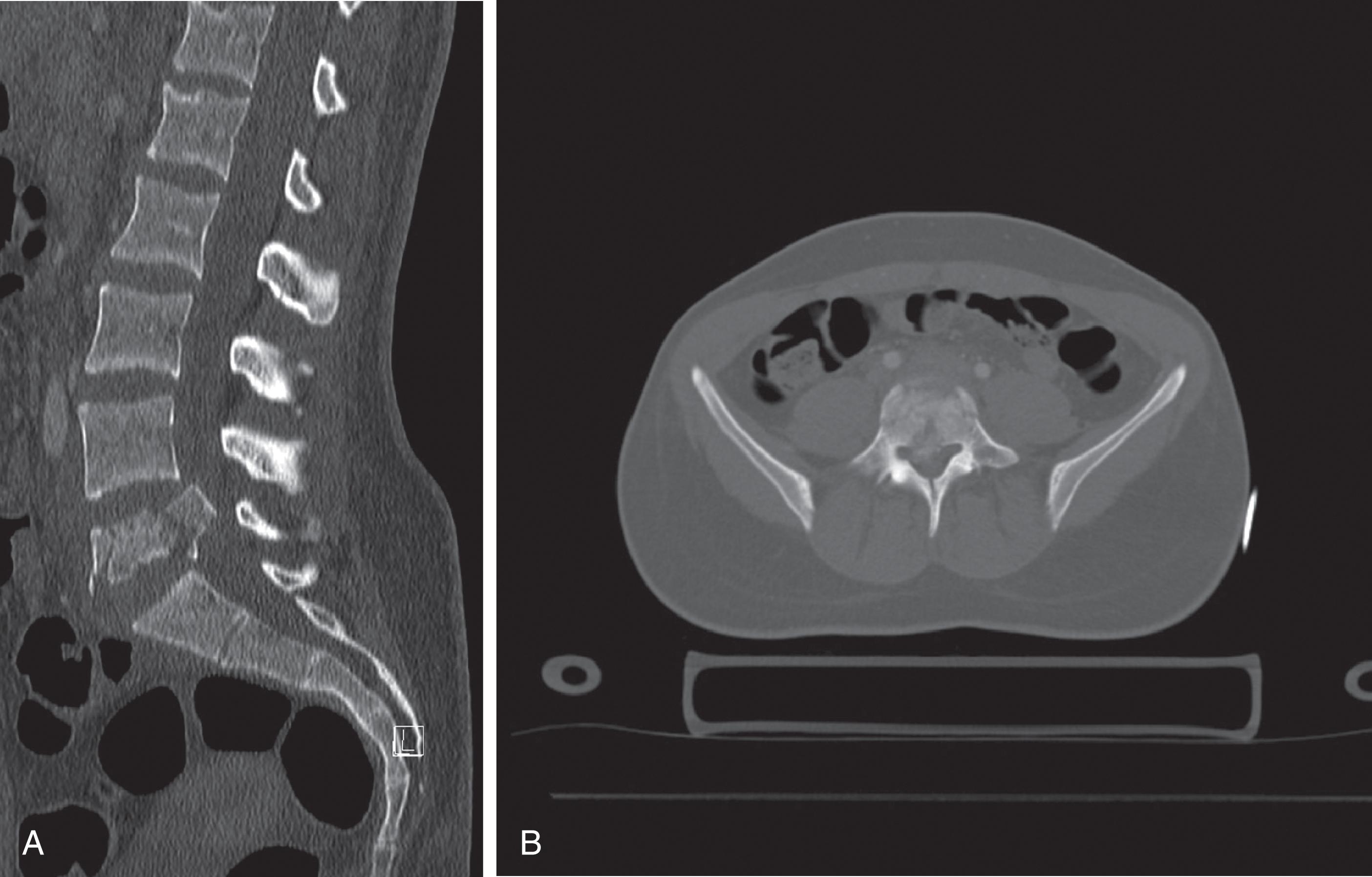
Traditionally x-rays of the spine were used to evaluate for fractures and ligamentous injuries. With the advent of CT their use has diminished as the limitations of conventional radiography are better recognized. CT is not only faster but has been proven to be more accurate in the evaluation of spine injuries. The spine is now routinely incorporated in whole body CT protocols. Sagittal and coronal reformatted images aid in the evaluation of spine injuries ( Fig. 14 ). MRI remains the best modality to evaluate for ligamentous injury, spinal cord injury, and epidural hematomas.
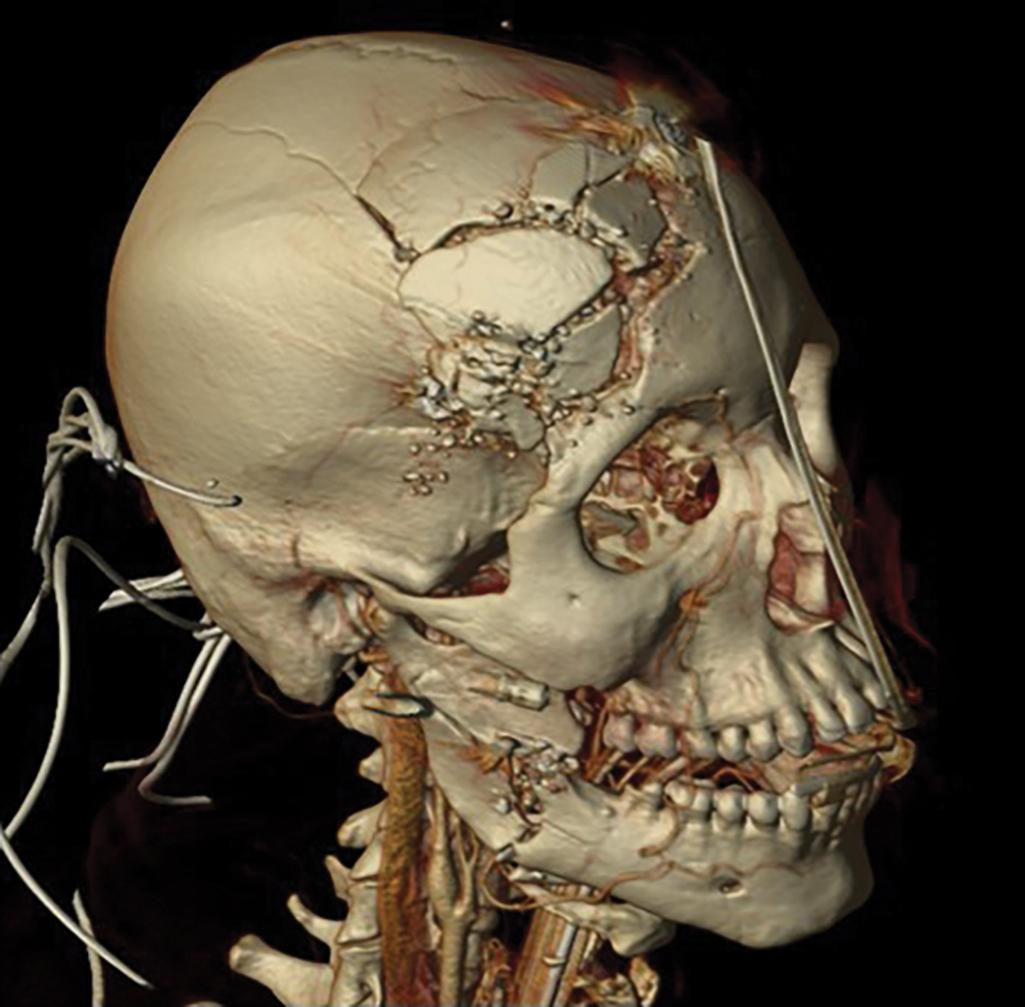
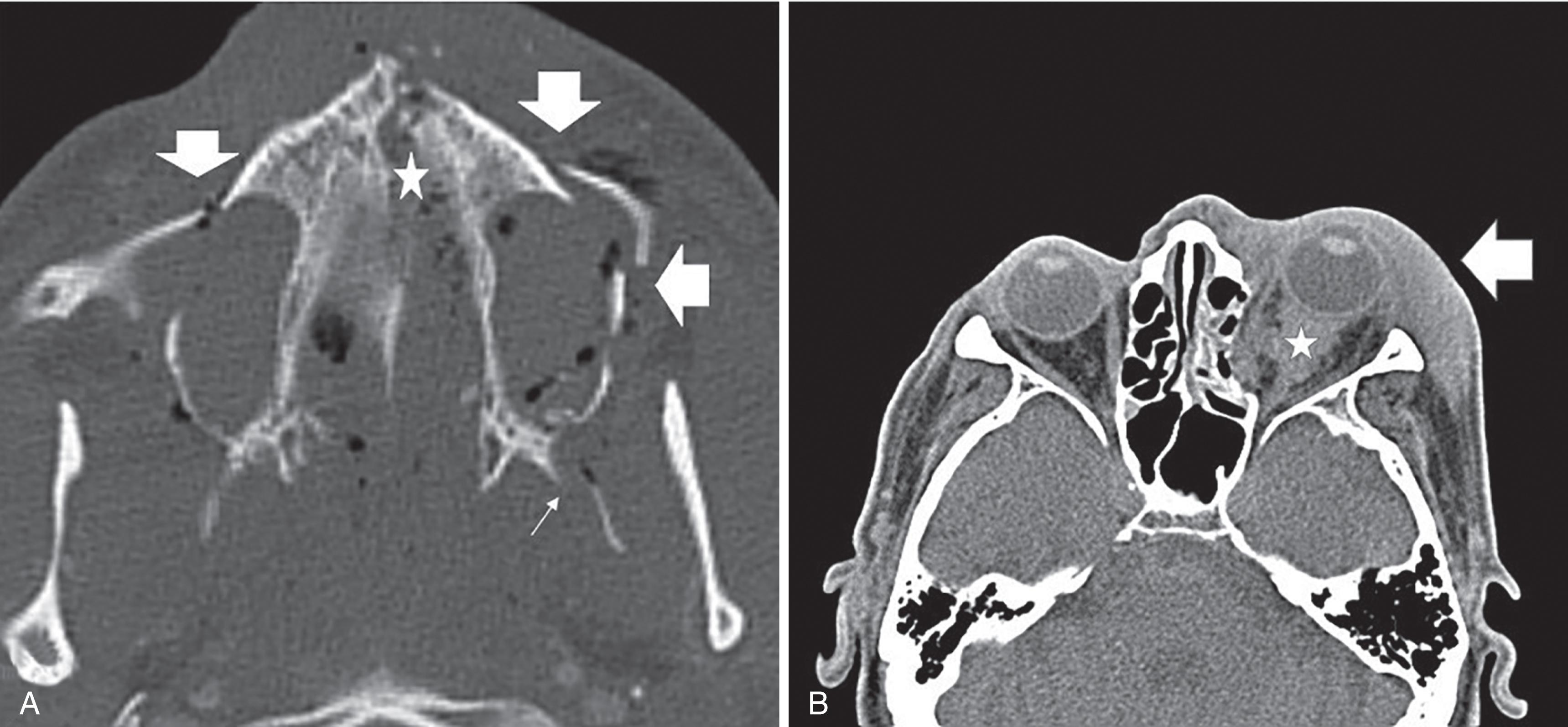
Any patient with signs of facial trauma, abrasions, swelling, gross deformities, and lacerations should undergo facial CT. Coronal and sagittal reconstructed images of the face aid in the diagnosis of subtle and nondisplaced fractures. Three-dimensional images have also been proven to be useful for surgical planning. Facial CT can identify injuries to the globe, retrobulbar hematomas, extraconal hematomas, muscular entrapment, and presence of foreign bodies ( Fig. 15 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here