Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Magnetic resonance imaging (MRI) has an established role in evaluation of maternal conditions, placenta accreta spectrum, and fetal anomalies. Fetal malformations are addressed in other chapters; here we review the role of MRI, emphasizing appropriate utilization in order to maximize the impact on clinical management.
Expertly performed ultrasound (US) is the imaging modality of choice for fetal evaluation. It is readily available and comparatively inexpensive, provides superior spatial resolution compared to MRI, and allows real-time evaluation of the fetoplacental circulation and fetal well-being. MRI is an expensive, time-consuming, highly technical procedure that is not a routine screening examination and not a substitute for a poorly performed sonogram. It should only be considered if additional information will affect pregnancy management, including possible in utero intervention or mode of delivery, or provide prognostic information for parental counseling and postnatal care planning.
A unique advantage of MRI is superb contrast resolution, which complements the excellent spatial resolution of US. Gray-white matter differentiation improves detection of central nervous system (CNS) migrational abnormalities and gray matter heterotopia, which are difficult to detect even with dedicated neurosonography. Imaging in orthogonal planes can be challenging with two-dimensional US if fetal position, late gestational age, multiple pregnancy, or maternal habitus limit acoustic access. Three-dimensional (3D) volume acquisition allows reconstruction of sagittal or coronal images from an axial volume but is subject to the same limitations as 2D US.
MRI allows direct acquisition of any scan plane, is less compromised by maternal obesity and oligohydramnios, and provides a large field of view, which is particularly helpful for complex anomalies because the entire area of interest can be displayed (e.g., a sagittal view of the cerebellum and spine showing a Chiari II malformation with a lumbar myelomeningocele) ( Fig 19.1 ). Pediatric surgeons often request fetal MRI before a prenatal consultation; it is routine before fetal surgery and may provide adequate information for pediatric surgery planning, thus avoiding the need to sedate a potentially unstable neonate for postnatal imaging.
The American College of Radiology has published guidelines on the indications for, performance of, and safety of fetal MRI. Like US, there is no ionizing radiation, but the mother and the fetus are exposed to high static magnetic fields, gradient magnetic fields, and radiofrequency energy. At 1.5 Tesla (T), no deleterious effects to the developing fetus have been shown. MRI of the pregnant patient can also be performed at 3.0T with improved anatomical detail at the cost of increased artifacts and SAR (specific absorption rate of electromagnetic energy, which results in tissue heating). Risks and benefits of MRI should be discussed with the patient, although written consent is no longer considered necessary.
Gadolinium-based MRI contrast agents cross the placenta, enter the fetal circulation, and are ultimately excreted into the amniotic fluid. Although recent primate work shows that this may not be significant, the effect on the human fetus remains unknown. The U.S. Food and Drug Administration has classified gadolinium-based contrast agents as pregnancy category C drugs; their safety in pregnancy is not established, but they may be used in select circumstances when the potential benefits outweigh the risks, such as when gadolinium administration would be necessary to make the diagnosis of a serious maternal condition. There are no fetal indications for gadolinium administration.
Timing of the examination depends on the clinical circumstances. MRI before 18 weeks’ gestation provides limited diagnostic information and is not recommended. From 18 to 24 weeks’ gestation, MRI can provide additional details regarding the severity of a malformation if the patient is considering termination. If termination is not an option, scanning in the third trimester when organ development is more mature provides maximum information; this is particularly true for the brain.
Rapid MRI techniques are the mainstay of fetal imaging. Single-shot or other rapid acquisition techniques minimize the effects of fetal movement, while thin (3–5 mm) slice thickness and sequence selection to maximize soft tissue contrast provide anatomic detail. Specialized radiofrequency coils increase the signal-to-noise ratio, further improving image quality. A targeted scan can be completed in approximately 20 minutes.
A “feet first” position, keeping the patient’s head outside the bore of the magnet, and, if necessary, a mild sedative help with claustrophobia. A lateral decubitus position is often more comfortable than the supine position in later pregnancy. Table weight limits and bore diameter can be limiting factors in patients with extreme obesity.
Each study begins with scout images to determine the fetal position and establish the diagnostic imaging planes. Axial, sagittal, and coronal images through the area of interest may be supplemented with additional, nontraditional, oblique planes as determined by the supervising radiologist. The technologist may also need to adjust scan planes to compensate for changes in fetal position. These studies can be challenging, requiring multiple series to gain the information needed; a trained MRI technologist and an experienced radiologist need to spend time at the magnet tailoring each study to answer the clinical question.
Image acquisition is focused on the area of interest, but in most cases a fetal anatomic survey can be completed with the exception of the heart (because of its rapid motion) and the extremities, which are seldom completely visualized. In addition, placental location, cervical length, and a general assessment of amniotic fluid volume can be made.
T2-weighted imaging (T2WI) is the mainstay of fetal MRI. The contrast resolution on T2WI is far superior to that of US—the primary advantage of MRI. On T2WI, fluid is high in signal intensity (i.e., white), so the fetal stomach, urinary bladder, gallbladder, cerebrospinal fluid, and surrounding amniotic fluid are easily visible. Solid fetal organs vary in signal intensity; the liver, spleen, and adrenal glands are relatively low in signal intensity and appear as shades of gray. The lungs are intermediate in signal intensity in the second trimester, gradually increasing in signal intensity (lighter gray) with advancing gestation, a reflection of accumulating fluid within the enlarging alveoli. The kidneys are also intermediate in signal intensity, with corticomedullary differentiation often seen by the third trimester. In the brain, gray matter is lower in signal intensity (darker) than white matter. Flowing blood produces a signal void (black). Clotted blood and hemorrhagic lesions show varying signal intensity depending on the age of the clot and the sequence used. Typically blood products are high signal on T1-weighted imaging (T1WI) and low on T2WI.
T1WI does not show anatomy well. Fluid is low in signal intensity (dark gray), as are most of the soft tissues. However, meconium, fat, and blood products are high in signal intensity (white), so this sequence is extremely important when evaluating for conditions such as anal atresia or intracranial lipoma or hemorrhage. The liver and thyroid gland show mild increased signal intensity (light gray) on T1WI (see Fig. 19.1 ).
Diffusion-weighted imaging (DWI) and apparent diffusion coefficient mapping are more advanced MRI techniques particularly helpful for fetal brain imaging. Both reflect diffusion properties in tissue; restricted diffusion is seen in the setting of hypoxic-ischemic injury and can be altered in congenital brain malformations. Diffusion tensor imaging, spectroscopy, and functional MRI remain investigational but show promise in evaluating white matter tract destruction or maldevelopment. ,
The role of MRI is most established in evaluation of the brain. The anatomic detail is exquisite, and structures may be visualized with more confidence than with standard transabdominal US, particularly in the setting of limited acoustic access. MRI is unaffected by bone, so there is uniform signal intensity from the entire brain and spinal canal without blind spots from shadowing by the skull and vertebral bodies. Studies have shown that additional abnormalities are seen on MRI in 20% to 50% of cases. ,
Interpretation of fetal MRI requires an in-depth understanding of embryology and normal development throughout gestation. This is particularly important in the brain, where a smooth cerebral cortex is normal at 20 weeks’ gestation but at 36 weeks would indicate lissencephaly. Gyri and sulci are well seen by MRI and develop at predictable time intervals. The sylvian fissure, callosal sulcus, parietooccipital sulcus, and calcarine sulcus should all be visible by 24 weeks’ gestation, followed by the central sulcus at 26–27 weeks and the cingulate sulcus and convexity sulci at 28–29 weeks.
Many serious brain malformations are easily diagnosed by US even in the first trimester. MRI is most helpful when there are abnormal findings but the diagnosis is not clear even after performance of advanced neurosonography.
Three clinical situations in which MRI can help clarify the diagnosis are absent cavum septi pellucidi (CSP), mild ventriculomegaly, and a posterior fossa fluid collection, each described below.
The CSP is a rectangular, midline, fluid-filled space between the frontal horns of the lateral ventricles. The more posterior extension (CSP et vergae) should not be mistaken for an intracranial cyst. Demonstration of the CSP is required in routine US evaluation because it is a marker for normal midline development and should always be seen between 18 and 37 weeks’ gestation. The differential diagnosis for an absent CSP is extensive ( Box 19.1 ) and warrants a detailed workup.
Agenesis/dysgenesis of corpus callosum
Holoprosencephaly spectrum
Alobar
Semilobar
Lobar
Syntelencephaly
Septo-optic dysplasia
Isolated septal agenesis
Severe hydrocephalus
Chiari II malformation
Schizencephaly
Dysgenesis (abnormal development) and agenesis (complete absence) of the corpus callosum are important causes of an absent CSP. In dysgenesis, coronal views show a thin, abnormal corpus callosum connecting the cerebral hemispheres. In agenesis, coronal images show an obvious absence of the corpus callosum between the cerebral hemispheres, the frontal horns have a “Texas longhorn” or “trident” shape, and the third ventricle is elevated and contiguous dorsally with the interhemispheric fissure ( Fig 19.2 ). In the sagittal plane, the cingulate gyrus is absent and the remaining gyri are radially oriented in a “sun ray” appearance. A midline lipoma or cyst may be present. Cysts may be large and associated with the AVID complex ( a symmetric v entriculomegaly, i nterhemispheric cyst, and d ysgenesis of the corpus callosum) ( Fig. 19.3 ).

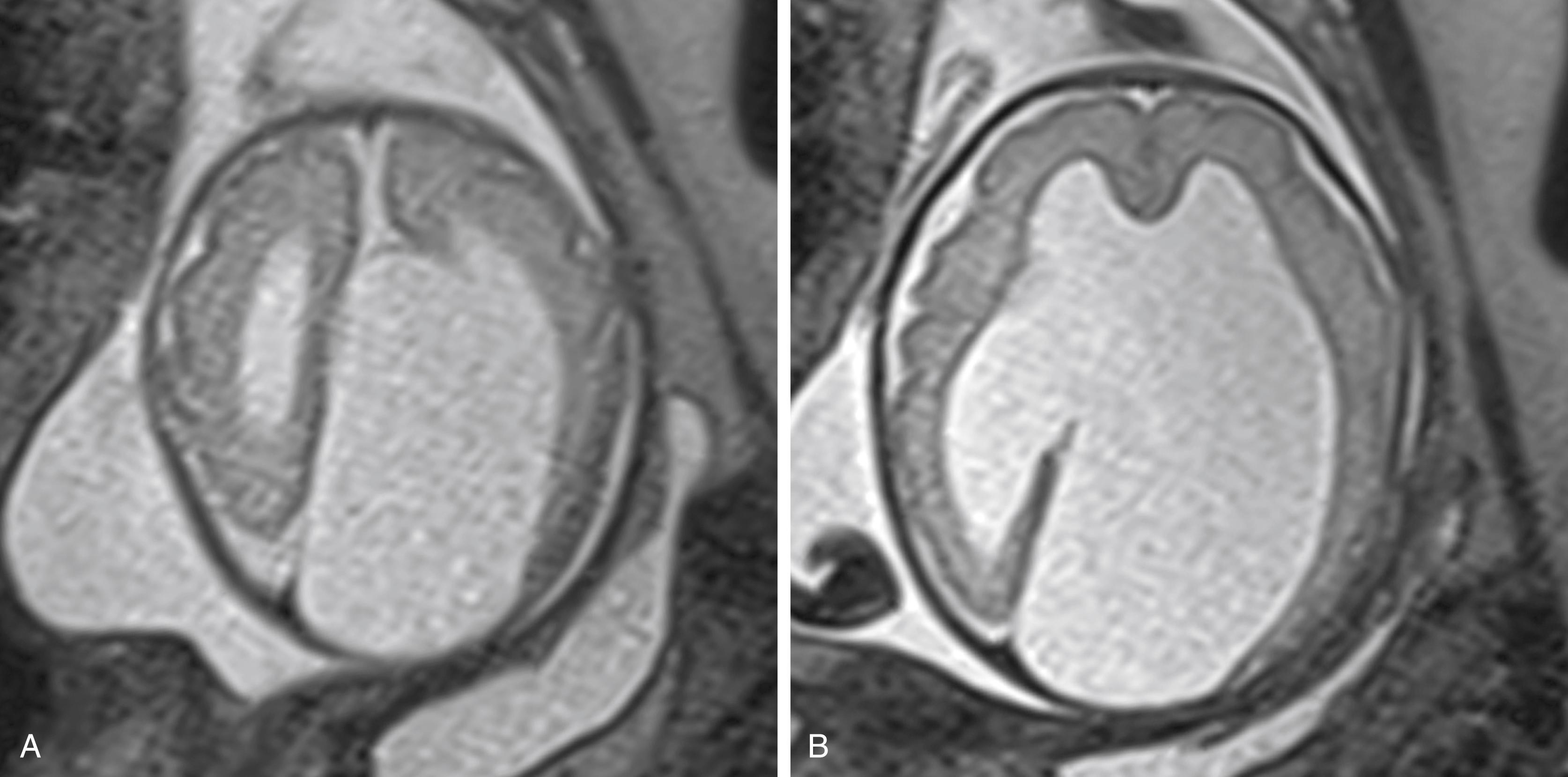
Holoprosencephaly, described as alobar, semilobar, and lobar in decreasing order of severity, is a disorder of midline development with absent CSP and varying degrees of communication of the ventricles across the midline. In syntelencephaly (the middle interhemispheric variant of holoprosencephaly), the CSP is absent and the posterior frontoparietal areas are fused across the midline, but the frontal and occipital horns are separate.
Septo-optic dysplasia (SOD) is a difficult prenatal diagnosis; apparently isolated absence of the CSP may be the only sonographic finding. On MRI, the corpus callosum is present but may be thinned. On the coronal view, the frontal horns have a flat-topped or squared shape and point inferiorly as they drape over the fornices. Fusion of the fornices may also occur (see Fig. 19.2D ). Optic nerve hypoplasia and pituitary insufficiency are common; thus it is important to evaluate the optic nerves, which should be approximately the same thickness as an extraocular muscle. Any fetus with the suspected diagnosis of SOD requires a thorough postnatal ophthalmologic examination and endocrine workup.
Ventriculomegaly is defined as mild, moderate, or severe based on the diameter of the lateral ventricle (≥10 mm but ≤12 mm, mild; 12–15 mm, moderate; >15 mm, severe). When severe, it may be difficult to differentiate from hydranencephaly, as shadowing and reverberation artifact from the cranium may make it difficult to determine whether there is residual cerebral mantle. MRI is unaffected by the bony skull vault and provides excellent images of both hemispheres. It readily differentiates the lethal hydranencephaly (absence of supratentorial cerebral tissue) from severe hydrocephalus with thinned cerebrum (e.g., aqueductal stenosis), which can be shunted after delivery ( Fig. 19.4 ). Schizencephaly is a disorder of neuronal migration that results in a wedge-shaped, gray matter–lined cleft that extends from the inner table of the skull to the lateral ventricle. The cleft may be very large (giant open-lip defect), which may be confused with ventriculomegaly, or quite subtle (smaller open-lip or closed-lip defects). MRI easily shows the low-signal-intensity gray matter lining the cleft, which confirms schizencephaly. In an area of porencephaly (tissue destruction) the defect is not lined by gray matter.

Mild to moderate ventriculomegaly has a broad differential diagnosis ( Box 19.2 ). The Society for Maternal-Fetal Medicine recommends consideration of MRI in these cases “when this modality and expert radiologic interpretation are available” while acknowledging that “it may be of less value if the patient has had a detailed ultrasound performed by an individual with specific experience and expertise in sonographic imaging of the fetal brain.” A 2019 meta-analysis confirmed a lower rate of CNS anomaly detection exclusively on MRI in fetuses that had undergone dedicated neurosonography but also concluded that early MRI had an excellent diagnostic performance in identifying additional CNS anomalies and that third-trimester MRI was better for cortical, white matter, and hemorrhagic abnormalities. A 2020 international multicenter study supported the practice of MRI assessment in every fetus with a prenatal diagnosis of ventriculomegaly noting that, if ventriculomegaly was isolated on neurosonography, parents could be reassured of the low risk of an associated anomaly. If dedicated neurosonography is not available, referral for fetal MRI at designated facilities may provide critical additional information for appropriate counseling.
Obstructive hydrocephalus (may be severe)
Aqueductal stenosis
Chiari II malformation
Dandy-Walker malformation
Tumor
Agenesis of corpus callosum
Septo-optic dysplasia
Aneuploidy (trisomies 21, 18, 13)
Congenital infection
Schizencephaly
Ischemia/encephalomalacia
Idiopathic
While the CSP is absent in some of these entities, ventriculomegaly is often the more obvious finding; agenesis of the corpus callosum is a classic example. An elevated third ventricle or cerebrospinal fluid between the cerebral hemispheres can be mistaken for the CSP, but the teardrop-shaped, dilated atria of the lateral ventricles (colpocephaly) on axial views and the steer horn configuration on coronal views are characteristic findings that point to the correct diagnosis. That said, even fetuses with apparently isolated complete or partial agenesis of the corpus callosum by dedicated neurosonography still benefit from MRI. A systematic review and meta-analysis from 2021 noted that “cortical and posterior fossa anomalies are among the most common anomalies detected exclusively at MRI, thus confirming the crucial role of fetal MRI in determining the prognosis of these fetuses.”
Abnormal cortical development can present with mild ventriculomegaly. Forms of cortical dysplasia include lissencephaly (lack of gyral formation with an abnormally smooth brain), pachygyria (abnormally broad, flattened gyri with a thick cortex), polymicrogyria (multiple small gyri), cobblestone lissencephaly (fine nodular cortical development), and gray matter heterotopia (arrested neural migration anywhere between the germinal matrix and cortex). Gray matter heterotopia can form bands (a layer of gray matter between the ventricles and cortex creating a double cortex appearance) or nodules, which may be subependymal or subcortical. Subependymal tubers (hamartomas) in tuberous sclerosis do not follow gray matter signal intensity, whereas subependymal heterotopic nodules do; therefore MRI can differentiate these conditions. Migrational anomalies are often associated with syndromes (e.g., Miller-Dieker syndrome, Walker-Warburg syndrome) and autosomal recessive inheritance patterns, so making the most definitive diagnosis possible is crucial for patient counseling.
Hypoxic-ischemic injury often presents with mild ventriculomegaly, which becomes progressive with advancing gestation. Associated areas of hemorrhage are easily detected as increased signal intensity on T1WI with corresponding low signal intensity on T2WI ( Fig 19.5 ). More sophisticated techniques such as DWI can demonstrate diffuse ischemic injury earlier than US; this information can be very important in countries where termination is legal at any gestational age. In countries where late pregnancy termination is not allowed, knowledge of severe brain injury or malformation is still useful for delivery planning and proof that the insult did not occur during labor or delivery.

Idiopathic mild ventriculomegaly (10–12 mm) is more common in male fetuses, especially at greater than 20 weeks’ gestation, and is thought to be secondary to a larger calvarial volume. If truly isolated, the outcome is good, but this is a diagnosis of exclusion; subtle abnormalities that may only be seen on MRI must be excluded.
Detailed, multiplanar US evaluation of the posterior fossa and brainstem is difficult due to shadowing from the skull base. With MRI, the cerebellar hemispheres, vermis, fourth ventricle, midbrain, medulla, and pons are easily evaluated.
A posterior fossa fluid collection presents a diagnostic challenge; the differential is broad ( Box 19.3 ), and arriving at a final diagnosis can be difficult even with postnatal MRI. The first step is to determine whether the posterior fossa is too big (e.g., expanded by a cyst) or the cerebellum is too small (e.g., cerebellar hypoplasia).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here