Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Rotational and orbital atherectomy facilitate stent implantation in undilatable, rigid, or heavily calcified lesions.
Cutting or scoring balloons slip less often than conventional balloons and may be useful for treating restenotic or ostial lesions.
Laser angioplasty uses a thermomechanical mechanism to prepare rigid or undilatable lesions for stent implantation.
Atheroablative devices do not reduce restenosis.
Atheroablative devices increase the risk of coronary perforation.
The safe use of lesion-modifying approaches requires advanced technical skills.
Several devices that ablate or section atheromatous plaque have been developed during the past 30 years to optimize the acute results of percutaneous coronary intervention (PCI) and reduce restenosis. After dozens of clinical trials found that the routine use of plaque modification did not produce better clinical outcomes than percutaneous transluminal coronary angioplasty (PTCA) alone, clinical practice guidelines recommended against the routine use of ablative approaches during PCI. However, in selected circumstances, atheroablative approaches may be the only means of achieving procedural and clinical success. This chapter analyzes the results of clinical trials and illustrates the complementary but crucial role that atheroablative devices fill in current practice in the preparation of rigid, highly calcified or undilatable lesions for stent implantation.
Before the modern era of coronary stenting, the search for treatments to overcome the shortcomings of PTCA was based on experimental findings that suggested that the healing response of treated coronary arteries was directly proportional to the degree of imposed injury. This was supported by angiographic analyses that suggested that late restenosis was directly proportional to the acute gain achieved during treatment with different treatments. Based on research that suggested that plaque excision could improve clinical outcomes and lower the rate of restenosis after PCI, directional coronary atherectomy (DCA) entered clinical trials in 1987. Excimer laser coronary angioplasty (ELCA) and percutaneous transluminal rotational atherectomy (PTRA) appeared in 1988. Holmium laser angioplasty (HLA) premiered in 1990, cutting balloon angioplasty (CBA) debuted in 1991, and the newest ablative device, orbital atherectomy (OA), entered investigation in 2008.
Although each device used a different mechanism for modifying atheromatous plaque, the common goal was to obtain larger lumens, higher success rates, and lower restenosis rates than could be achieved with balloon PTCA alone. When evidence from randomized trials ( Table 35.1 ) seemed to refute the ablation hypothesis, coronary stenting (see Chapter 15 ) rapidly replaced atheroablative therapies. Clinical practice guidelines currently specify that no atheroablative device should be used routinely during PCI, but the guidelines add the provision that rotational atherectomy is reasonable for fibrotic or heavily calcified lesions that might not be crossed by a balloon catheter or adequately dilated with high-pressure balloons before stent implantation (class IIa). Cutting and scoring balloons may be considered to reduce slippage and trauma during PCI for in-stent restenosis (ISR) or ostial lesions in side branches (class IIb). Laser angioplasty might be considered for fibrotic or moderately calcified lesions that cannot be crossed or dilated with high-pressure balloons before stent implantation (class IIb). Because treatments that ablate or section atheromatous plaque continue to be used in many interventional programs, each approach is reviewed in the following sections.
| Acronym | Definition | Primary End Point a | Patients ( n ) | Year b | Indications | Comparison |
|---|---|---|---|---|---|---|
| AMIGO | Atherectomy Before Multi-Link Improves Luminal Gain and Clinical Outcomes |
Binary restenosis | 753 | 2002 | Native vessel | DCA vs. PTCA |
| AMRO | Amsterdam Rotterdam Randomized Trial | 6-month MACE | 308 | 1993 | Native vessel | ELCA vs. PTCA |
| ARTIST | Angioplasty/Rotational Atherectomy for Treatment of Diffuse In-Stent Restenosis Trial | 6-month MACE | 298 | 2002 | ISR in native vessel | PTRA vs. PTCA |
| BETACUT | Beta Radiation Assisted by Cutting Balloon Angioplasty for In-Stent Restenosis | Binary restenosis | 100 | 2002 | ISR in native vessel | CBA vs. PTCA before BT |
| BOAT | Balloon/Optimal Atherectomy Trial | Binary restenosis | 989 | 1995 | Native vessel | DCA vs. PTCA |
| CAPAS | Cutting Balloon Atherotomy Versus Plain Old Balloon Angioplasty Study | Binary restenosis | 232 | 1997 | Native vessel | CBA vs. PTCA |
| CARAT | Coronary Angioplasty and Rotablator Atherectomy Trial | Postprocedure diameter stenosis | 222 | 2000 | Native vessel | PTRA vs. PTRA |
| CAVEAT-I | Coronary Angioplasty Versus Excisional Atherectomy Trial I | Binary restenosis | 1012 | 1992 | Native vessel | DCA vs. PTCA |
| CAVEAT-II | Coronary Angioplasty Versus Excisional Atherectomy Trial II | Binary restenosis | 305 | 1993 | SVG | DCA vs. PTCA |
| CBASS | Cutting Balloon for Small-Size Vessels c | Binary restenosis | 99 | 1999 | Native vessels <2.6 mm | CBA vs. PTCA |
| CCAT | Canadian Coronary Atherectomy Trial | Binary restenosis | 274 | 1992 | LAD | DCA vs. PTCA |
| COBRA | Comparison of Balloon Angioplasty/Rotational Atherectomy | Binary restenosis | 502 | 1996 | Native vessel | PTRA vs. PTCA |
| CUBA | Cutting Balloon Versus Conventional Balloon Angioplasty Trial c | Binary restenosis | 306 | 1997 | Native vessel | CBA vs. PTCA |
| DART | Dilation/Ablation Revascularization Trial | Binary restenosis | 446 | 1998 | Small vessel | PTRA vs. PTCA |
| ERBAC | Excimer Rotablator Balloon Angioplasty Comparison | Procedural success | 454 | 1996 | Native vessel | ELCA vs. PTCA |
| ERBAC | Excimer Rotablator Balloon Angioplasty Comparison | Procedural success | 453 | 1996 | Native vessel | PTRA vs. PTCA |
| GRT | Global Randomized Trial | Binary restenosis | 1238 | 1997 | Native vessel | CBA vs. PTCA |
| LAVA | Laser Angioplasty/Coronary Angioplasty | 6-month MACE | 215 | 1997 | Native vessel | HLA vs. PTCA |
| REDUCE 1 | Restenosis Reduction by Cutting Balloon Evaluation 1 c | Binary restenosis | 802 | 2001 | Native vessel | CBA vs. PTCA |
| REDUCE 2 | Restenosis Reduction by Cutting Balloon Evaluation 2 c | Binary restenosis | 492 | 2002 | ISR | CBA vs. PTCA |
| REDUCE 3 | Restenosis Reduction by Cutting Balloon Evaluation 3 | Binary restenosis | 521 | 2003 | Stenting | CBA vs. PTCA |
| RESCUT | Restenosis Cutting Balloon Evaluation |
Binary restenosis | 428 | 2002 | ISR | CBA vs. PTCA |
| ROSTER | Rotational Atherectomy Versus Balloon Angioplasty for Diffuse In-Stent Restenosis | Target-lesion revascularization | 200 | 2001 | ISR | PTRA vs. PTCA |
| ROTAXUS | Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease | In-stent late lumen loss | 120 | 2013 | Native vessel calcified lesions | PTRA vs. PTCA |
| SPORT | Stenting Post Rotational Atherectomy Trial c |
30-day MACE | 735 | 1999 | Native vessel calcified lesions | PTRA vs. PTCA |
| STRATAS | Study to Determine Rotablator System and Transluminal Angioplasty Strategy | Acute success | 497 | 2000 | Native vessel | PTRA vs. PTRA |
a If the primary end point was not stated or if multiple primary end points were listed, the end point used in power calculations for sample size estimation was used.
b Year that patient recruitment was completed. Otherwise, the year the study was reported or published.
c Unpublished, with data approved by investigators where cited.
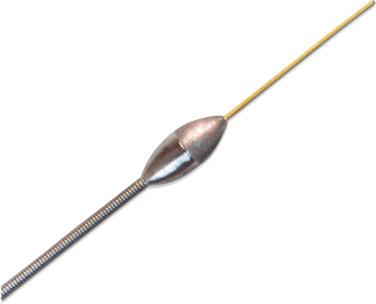
PTRA removes tissue and reduces lesion rigidity by attacking calcified atherosclerotic plaque like a dental drill, which bores into enamel but leaves pulp unharmed. Based on the theory of differential cutting, rotary ablation pulverizes rigid atherosclerotic plaque, which is not able to deflect, and yet preserves the integrity of the flexible artery wall. The hard plaque is abraded into small particles that average 5 μm in diameter and are taken up by the reticuloendothelial system.
The Rotablator system (SciMed/Boston Scientific, Natick, MA) contains: (1) a preconnected burr with an advancing device that houses an air turbine and drive shaft; (2) a console that regulates an air supply and monitors the rotation of the burr; and (3) a DynaGlide foot pedal to activate the device. The burr has an abrasive tip that is welded to a long flexible drive shaft covered by a plastic sheath, and it tracks over a central coaxial RotaWire (0.009-inch diameter, 3.3-m length) that has a flexible radiopaque platinum distal part (20 mm-length) that does not rotate during abrasion. The wire and the burr can be advanced independently.
The elliptical nickel-coated brass burr has 2000 to 3000 microscopic diamond crystals on its leading face ( Fig. 35.1 ). The diamond crystals are 20 μm in size, with only 5 μm protruding from the nickel coating, and the trailing edge of the burr is smooth. Burrs are available in various diameters that range from 1.25 to 2.50 mm in 0.25-mm increments.
During use, a rotaflush solution containing a lubricant, verapamil 5 mg, 5000 units of heparin, and 1000 μg of nitroglycerin per 500 mL saline irrigates the catheter sheath to lubricate and cool the rotating parts. In recent years, many centers omit all but the lubricant to avoid hypotension. The number of revolutions per minute (rpm) is measured by a fiberoptic light probe and is displayed on a control panel. The advancer has preset delimiters for retraction and advancement. The wireClip Torquer and guidewires are critical components of the system. RotaGlide lubricant may be useful for crossing resistant lesions.
All patients are pretreated with aspirin and an anticoagulant. The size of the guide catheter depends on the size of the burr. A 6-Fr Runway Guide (Boston Scientific) with a 0.070-inch inner lumen accommodates a 1.25-mm burr, allowing conversion to PTRA if an undilatable or uncrossable lesion is encountered during PTCA, even during transradial PCI with low-profile 5/6-Fr sheaths.
Rotary ablation is preceded by placing the RotaWire across the lesion and parking the unfolded wire tip in a straight segment of the distal vessel, not in a side branch. Many practitioners initially place their workhorse wire into the distal vessel and use a microcatheter to replace it with the RotaWire. During rotablation, protection of side branches is unnecessary because a “snow plow” effect is rare. When treating large coronary arteries, particularly the right coronary artery, many cardiologists insert a prophylactic temporary pacemaker because of the possibility of bradyarrhythmias. Before advancing the burr into the guide catheter, the rotational speed of the burr is checked outside the body at the Y-adaptor with flush running. An outside-body speed of 155,000 rpm translates to an unimpeded speed of 140,000 rpm within the coronary artery. A recent study has suggested no difference in clinical or angiographic outcomes at speeds of 140,000 or 190,000.
The burr and drive shaft are manually advanced over the guidewire to a proximal segment of the target vessel. Before rotablation, a three-point checklist is completed to prevent abrupt burr advancement and vessel dissection: (1) the advancer knob is loosened and moved back and forth approximately 5 mm to unload stored tension on the drive cable; (2) the Y-adaptor is loosened to unload stored tension in the Rotablator sheath; and (3) the DynaGlide mode is activated at lower rotational speeds to unload any residual tension within the system and then switched off to allow the burr to advance into the vessel at ablative speeds. The burr is moved into the target lesion with a pecking motion that maintains a high speed of rotation. Decelerations of greater than 5000 rpm are avoided to reduce the risk of vessel trauma, heat formation, and large particle generation. Ablation runs are limited to 15 to 20 seconds each. If the lesion cannot be crossed after five attempts, downsizing of the burr may be required. After successful crossing, several polishing runs complete lesion preparation for stent delivery. Occasionally, an initial smaller burr is exchanged for a larger diameter burr for more complete ablation prior to stent implantation, although the benefit of this approach has not been demonstrated (see later).
Several trials have defined the optimal use of PTRA. The Study to Determine Rotablator and Transluminal Angioplasty Strategy (STRATAS) trial compared an aggressive debulking strategy (burr/artery ratio of 0.7 to 0.9 followed by balloon inflation of less than 1 atm or no inflation) with a moderate debulking strategy (burr/artery ratio of less than 0.7 followed by conventional balloon angioplasty). The clinical success was similar, but the aggressive strategy caused more myocardial infarctions (11% vs. 7%) and a higher rate of restenosis (58% vs. 52%). The Coronary Angioplasty and Rotablator Atherectomy Trial (CARAT) compared a large-burr strategy (burr/artery ratio >0.7) with a small-burr strategy (burr/artery ratio <0.7). The large-burr strategy achieved similar immediate lumen enlargement and rate of target-vessel revascularization (TVR) as the small-burr strategy but caused more angiographic complications (12.7% vs. 5.2%, P < .05). Taken together, these two trials are the basis for recommending a single burr for each procedure, selecting a burr/artery ratio of 0.5 to 0.6, and avoiding burr over-sizing.
In a series of multicenter randomized trials, rates of major adverse cardiac events (MACEs) at 30 days and rates of angiographic restenosis were higher after PTRA than after balloon PTCA alone ( Fig. 35.2 ).
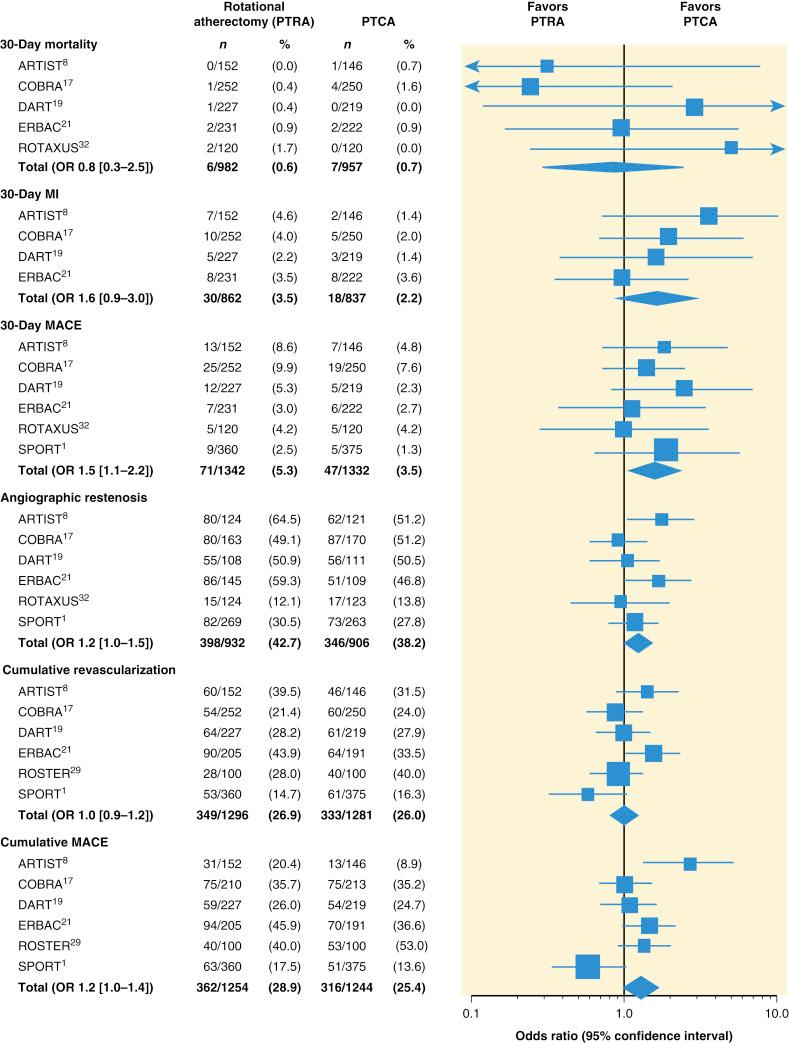
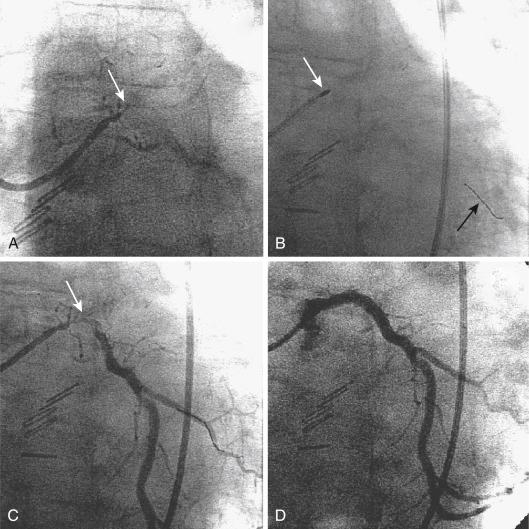
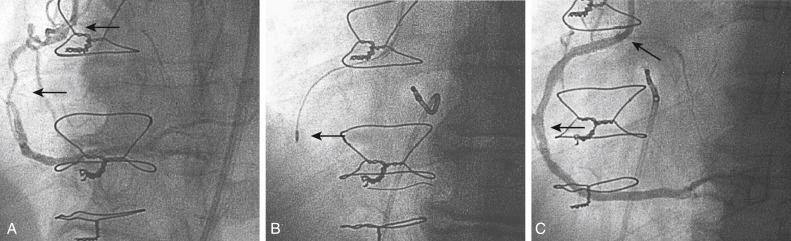
Because PTRA confers a slightly increased risk, it should be used selectively. Most practitioners find that they must maintain expertise with PTRA because undilatable lesions are encountered regularly. Approximately 1% to 3% of lesions that can be crossed with a guidewire are uncrossable with balloon catheters or are undilatable at pressures higher than 20 atm. Rotational atherectomy successfully improves the compliance of most rigid lesions, allowing balloon dilation and stent implantation to be completed successfully ( Fig. 35.3 ). For long calcified lesions, small burrs are recommended to alter the compliance of the vessel to allow balloon angioplasty and spot stenting of the segments with dissection ( Fig. 35.4 ).
Certain precautions need emphasis. Angulated lesions in bends of more than 60 degrees are a relative contraindication to the use of PTRA, and lesions in a bend greater than 90 degrees are an absolute contraindication because dissection or perforation may occur. When PTRA is performed in nonangulated lesions, the rotating burr should never be advanced to the point of contact with the spring tip of the RotaWire. The rotating burr should not be allowed to remain in one location within the artery; a gentle retraction and readvancement motion is needed to avoid dissection or welding. The rotating burr should not be advanced within the guide catheter. Rotational atherectomy should be avoided in dissected segments after balloon angioplasty, in lesions with visible thrombus, and in degenerated saphenous vein grafts (SVGs). Hemodynamic support with either balloon pumping or Impella (see Chapter 22 ) may be needed for patients with ischemic cardiomyopathy and borderline hemodynamics that might deteriorate in the presence of microembolization.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here