Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Access video content for this chapter online at Elsevier eBooks+
Access video content for this chapter online at Elsevier eBooks+
“Robot” is a term derived from the Czech word robota , meaning “forced labor”. It was Karel Čapek (a Czech writer, playwright, and critic) who introduced the term to the English language and to science fiction in his play Rossom's Universal Robots in 1921. Before entering the field of surgery, robots were heavily used in industry and by the military. It was the National Aeronautics and Space Administration (NASA) and the US Army who promoted the concept of robotics in surgery after they introduced the concept of telepresence surgery (operating remotely) for space applications and for use in wartime.
Robotic surgery started with PUMA 560 (Programmable Universal Machine for Assembly or Programmable Universal Manipulation Arm), which consisted of a standard industrial robotic arm. It was developed in 1978 by an engineer named Victor Scheinman at Unimation (the world's first robotics company). It was initially used by General Motors for die-cast handling and spot welding of car bodies. In 1985, Dr. Yik San Kwoh of Memorial Medical Center (Long Beach, CA) developed a computer program that allowed the PUMA arm to perform CT-guided stereotactic brain surgery with improved accuracy. This accomplishment launched the “Age of Medical Robotics” around the world. Three years later, PUMA 560 was used by Davies et al . for a transurethral resection of the prostate. This platform led to the development of the PROBOT by Integrated Surgical Supplies Ltd., a robotic system specifically designed for transurethral resection of the prostate. It directed a rotating blade to complete the process of prostatic resection. Although the PROBOT did not gain a wider clinical appeal, a similar concept was concurrently being explored by orthopedic surgeons. They took advantage of robots’ greater precision, and successfully designed a system that augments the precision of hip replacement surgery, called the RoboDoc (Integrated Surgical Systems, Inc., Sacramento, CA). It was used to prepare the femoral canal for prosthesis placement. That was the first surgical robot approved by the FDA. It was adopted almost immediately in Europe (and then in the US), with the first procedures performed in 1992.
Based on the initial success of robots in augmenting surgical precision, several other systems were researched, then commercially developed, and approved by the FDA to be used in various fields. These included the Automated Endoscopic System for Optimal Positioning (AESOP) platform (Computer Motion, Inc., Santa Barbara, CA), which enabled surgeons to voice-control the positioning of a laparoscopic camera. Further modifications led to the birth of comprehensive surgical robotic systems, ZEUS (Computer Motion, Inc., Santa Barbara, CA) followed by Da Vinci (Intuitive Surgical, Inc., Mountain View, CA). These two rival platforms, ZEUS and Da Vinci, formed an oligopoly in the field of robotic surgery for about a decade – trading world-firsts and pushing the frontiers of minimally invasive surgery further. The ZEUS platform consisted of three arms. One of its three arms held the camera and the two others were used to hold surgical instruments. It continued to use the voice-activated AESOP camera system. The Da Vinci system, on the other hand, consisted of three to four arms, with a central arm holding a binocular lens (enabling 3D vision). More importantly, the arms articulated at the wrist, providing seven degrees of freedom. This unique feature was the innovation that was crucial to its subsequent dominance of the robotic market. The Da Vinci platform has three components: a vision tower that holds a dual light source and dual three-chip camera, a master console where the operating surgeon sits, and a moveable patient-side cart where the instrument arms and the camera arm are mounted ( Fig. 34.1 ).
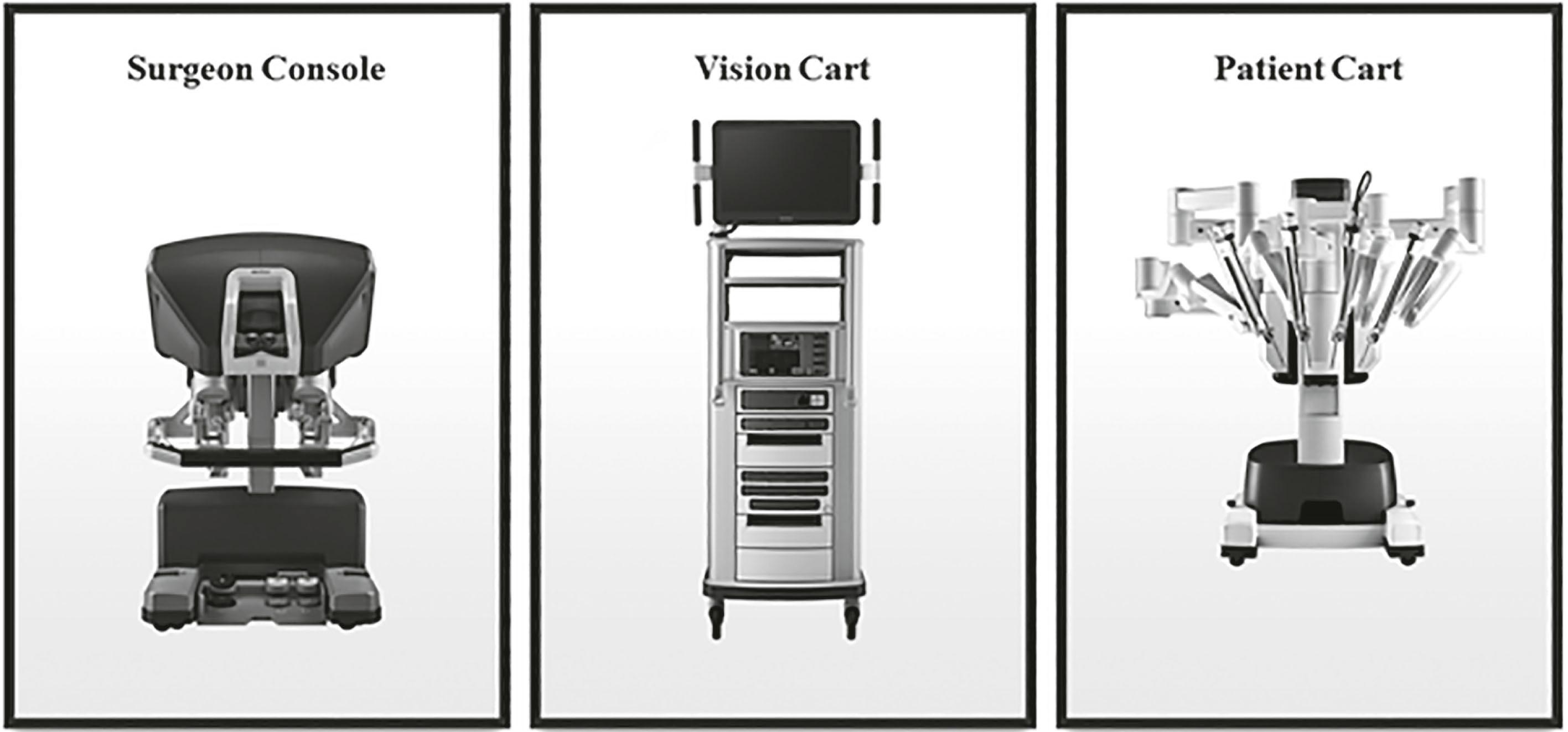
The ZEUS and Da Vinci systems were unified when Computer Motion and Intuitive Surgical merged in 2003. As a result, further improvements were centered on the Da Vinci platform, which subsequently dominated robotic surgery for the past decade.
The Da Vinci robot was used in a multitude of minimally invasive surgeries, including cholecystectomies, mitral valve repairs, radical prostatectomies, reversal of tubal ligations, in addition to many gastrointestinal surgeries, nephrectomies, and kidney transplants. Advantages were many: The robotic platform allowed the surgeon to overcome the technical constraints of laparoscopy and made surgeries that were previously technically unfeasible possible. These benefits included increased dexterity, improved visualization, restoration of proper hand–eye coordination, elimination of physiologic tremor, motion scaling, seven degrees of freedom, and an ergonomic surgeon position. In addition, a set of augmented reality systems are possible to integrate, including TilePro (Intuitive Surgical, Inc., Sunnyvale, CA) which allows image-within-image viewing and Firefly (Intuitive Surgical, Inc., Sunnyvale CA), with real-time infrared laser angiography to visualize microvascular capillaries, lymphatic vessels, bile ducts, or lymph nodes.
Inspired by the success of robotics in these surgical specialties, the senior author (JCS) began exploring the use of robots in plastic surgery in 2003. Over the subsequent two decades, multiple applications of robotics in plastic surgery were developed: (1) transoral robotic reconstructive surgery (TOR R S) for head and neck reconstruction, allowing complex oropharyngeal reconstruction without dividing the lip or mandible; (2) robotic microvascular, microneural, and microlymphatic anastomoses, extending the capabilities of the human hand to “supra-human” precision; and (3) minimal access harvest of the latissimus dorsi muscle, rectus abdominis muscle, and the deep inferior epigastric perforator flaps, significantly decreasing donor site morbidity and improving cosmesis. In this chapter, we highlighted the clinical experience of robotic surgery in those areas. We also present an overview of some of the novel applications of robotic surgery that are still emerging (namely cleft palate surgery). We finish by discussing robotic skills development and provide a framework for robotic microsurgical training to widen the adoption of this technology in plastic surgery.
The treatment of cancerous lesions of the oropharynx and base of tongue remains challenging. The need for morbid procedures (lip and mandible splitting) to resect those tumors led to the adoption of primary chemoradiation therapy as an alternative therapy. However, the toxicity of chemoradiation turned out to be substantial, and functional outcomes were not improved. Hence a clinical need for a less morbid yet effective treatment modality arose. Robotic surgery (or more specifically transoral robotic surgery, TORS), with its minimally invasive approach, allowed resection of those tumors, achieving locoregional control while obviating the need for mandibulotomies and chemoradiotherapy. This resulted in the return of surgery as a primary treatment modality, optimizing both oncologic and functional outcomes.
The TORS approach, however, created a reconstructive challenge. This is because the cylinder of the oropharynx remained almost entirely closed during the resection. Access is thus severely limited when attempting to inset and contour a flap. A mandibulotomy or a wide lateral pharyngotomy is usually needed to access the hypopharynx, or the region between the uvula and the epiglottis. Transoral robotic reconstructive surgery (TOR R S), whether using free flaps, local flaps, or primary closure, allowed access to this difficult anatomical area enabling plastic surgeons to achieve the goals of head and neck reconstruction: coverage of vasculature, preservation of a competent velopharyngeal sphincter, maintaining a watertight seal between the pharynx and neck (in cases of through-and-through defects), restoration of tongue volume and sensations at its base, optimization of the physiological function of the oropharynx and larynx. Set-up involves positioning the patient-side cart at about 60° from the head of the bed. A mouth retractor is then used to establish the interdental opening and the endoscope along with two instrument arms are passed into the mouth, converging to the target anatomy ( Fig. 34.2 ).
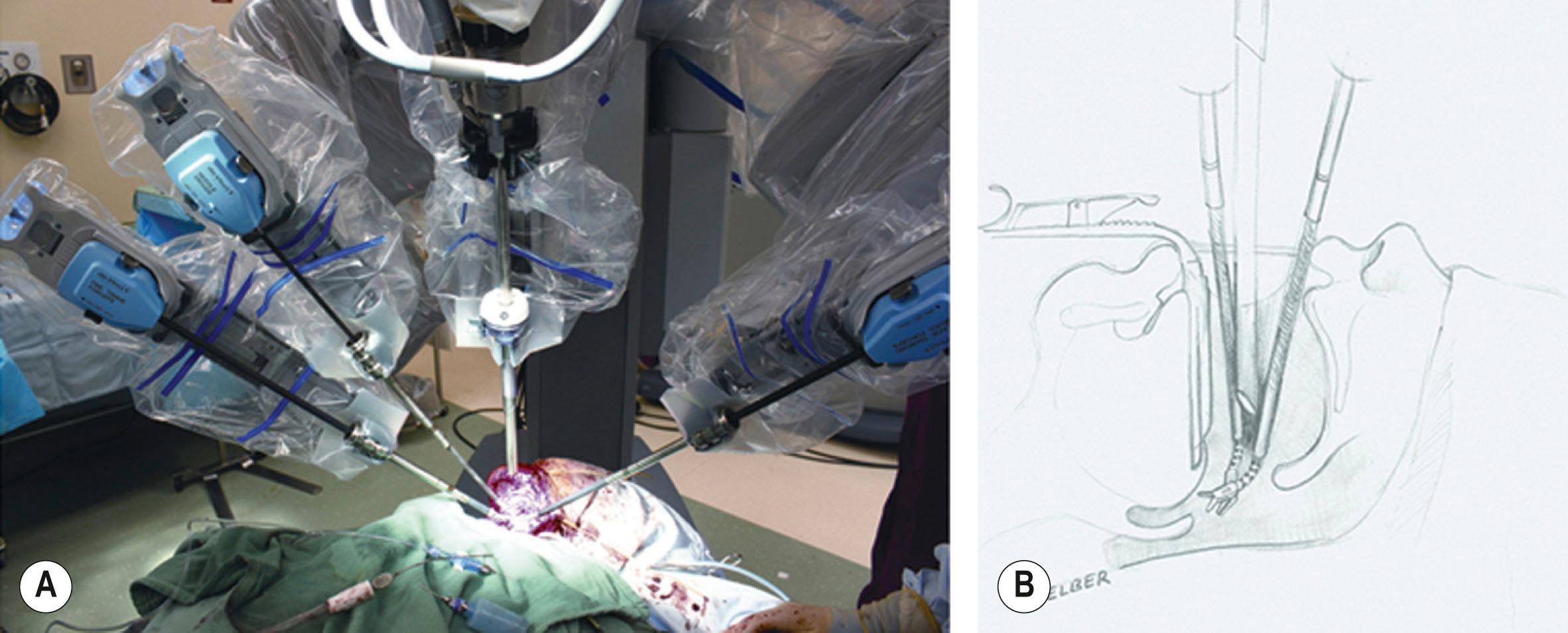
The “remote center” of the instruments which is the virtual, three-dimensional point around which the instruments pivot, is placed at the level of the mouth opening to minimize movement around the teeth. Complex manipulation of tissue, including delicate suturing, can then be performed. This approach is superior in select cases and carries a potential to increase the indications for minimally invasive resective and reconstructive procedures. Combining transoral robotic flap inset with manual inset through the pharyngotomy defect is also possible. In such cases, however, it is important to note that the pharyngotomy is considerably smaller than the traditional wide pharyngotomy required for accessing such tumors (since access to the upper pharynx is achieved robotically rather than through the neck). The primary author has documented the value of TOR R S for challenging defects of the head and neck, and has shown both feasibility and effectiveness of this reconstructive method. The main advantages included improved visualization in a confined space, reduction of tremor when manipulating delicate structures, and high-resolution high-magnification images for performing “high-risk” microsurgical anastomoses. In addition, by using this approach, plastic surgeons are able to provide a reliable reconstructive support for the robotic head and neck surgeon to resect larger, deeper, and more complex tumors (i.e., those involving the tonsils, base of tongue and soft palate) that would be very challenging to reconstruct through traditional methods.
Some groups defined indications for TOR R S application. Longfield et al . proposed an algorithm for the use of TOR R S based on tumor site, tumor extent, and patient-specific factors. Similarly, de Almeida et al . introduced in 2014 a defect classification system based on tumor site (tonsil, tongue base, pharynx or soft palate) and adverse features (internal carotid artery exposure, neck communication or >50% of soft palate resection). As a general rule reconstruction is planned whenever coverage of major vessels is needed or whenever a significant through-and-through neck defect is created. Local flaps include the facial artery musculomucosal flap, the infrahyoid myocutaneous flap, the nasoseptal flap after tonsillectomy. Distant flaps range from temporalis transfers to pectoralis major flaps to anterior lateral thigh flaps.
One of the most promising applications of robots in plastic surgery is microsurgery. With complete tremor elimination and up to 5 : 1 motion scaling, the surgical robot is capable of supra-human levels of precision. In no area in surgery is this level of precision more crucial for successful outcomes. Furthermore, with its high-definition three-dimensional optics and up to 10–15× magnification, the robotic platform provides an almost ideal setup for delicate microvascular manipulations. Robotic microsurgery was first investigated in a rat model. Although it showed a significantly slower time to anastomosis versus conventional microsurgery, there were no differences in technical failures or patency rates. Knight et al . compared 30 standard and 31 robot-assisted anastomoses and also showed excellent patency rates for both methods. As for the learning curve of robotic microsurgery, Katz et al . showed a rapid decrease of robotic anastomotic time from 67 to 20 minutes after 6 trials. Morita et al . reported a quantitative assessment of robotic versus freehand tasks in a microsurgical setting. A simulated deep and superficial surgical field was created and robot assistance was more successful for the deep microvascular tasks.
In the senior author’s initial series of transoral robotic reconstruction of oropharyngeal defects, the robot was used to perform some of the microvascular anastomoses. The facial artery (the most common recipient artery) passes beneath the hypoglossal nerve and digastric sling, often high under the body of the mandible. If a tracheostomy and a ventilator tube is also present, the space available to perform the anastomosis may be limited. The robot's precision and visualization in confined spaces make it well suited for such challenging anastomoses. Song et al . also used the robot for head and neck microvascular anastomosis. They employed a radial forearm flap (recipient vessel: facial artery) to reconstruct a defect following resection of a tonsillar tumor (T3 N0 M0, stage III). The neck dissection was performed through a retro-auricular incision. With this approach, it is even more difficult to perform a microanastomosis (through that narrow space) using the conventional microscope.
Set-up for robotic microsurgery is relatively straightforward. The robotic arms are placed at about 45° above the target anatomy and in direct proximity to the external incision ( Fig. 34.3 ). Black Diamond Micro Needle drivers (Intuitive Surgical, Inc., Sunnyvale, CA) replace the larger jawed needle drivers used during the inset, and a 9-0 nylon suture is used for the anastomosis.
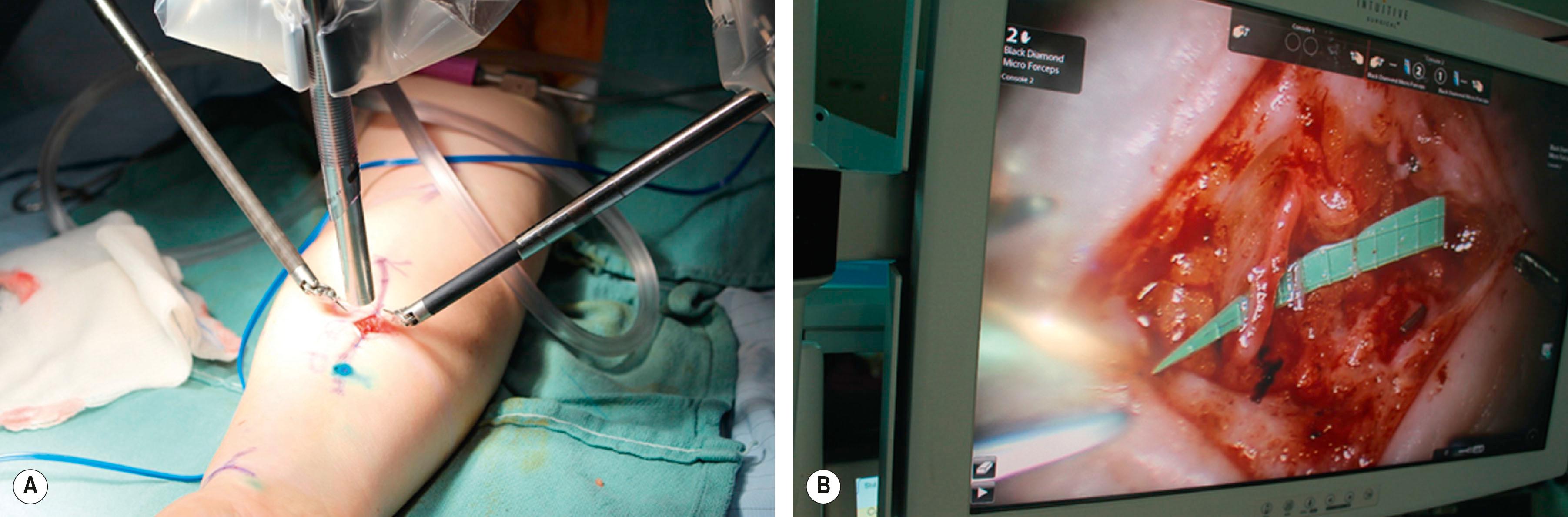
An additional third arm with a “fine tissue forceps” can be used as a stationary assistant, and the surgeon is able to toggle back and forth between arms 1 and 3, depending on which arm is being used to position the vessel, and which is being used to suture. This eliminates the need for a skilled microsurgical assistant which is yet another advantage of using the robotic platform for microsurgery.
The main highlights of robot-assisted surgery (ultra-precision and 100% tremor filtration) were recently expanded to the field of super-microsurgery, specifically to lymphedema surgery. Lymphatico-venular bypasses are generally performed end-to-end using 11-0 or 12-0 nylon sutures on a 50 µm needle. These are extremely technically challenging cases, and in certain cases exceed the limits of human precision. The supra-human precision provided by the robot can be of great benefit in this setting. The robot can help overcome these human limitations, allowing a potential breakthrough in super-microsurgery. Such a breakthrough can be in the form of an increase in access to care, or a democratization, for patients suffering from lymphedema since the robot will now allow surgeons with suboptimal microsurgical dexterity (i.e., surgeons who do not perform microsurgery on a routine basis) to perform these challenging procedures with less tremor and more reliability. The senior author performed several lymphovenous bypass surgeries using the Da Vinci robot and found it to be promising for this application. In addition, the robotic platform allowed rapid transitioning of the visual system between near-infrared laser visions (when using indocyanine green to identify lymphatics) to normal bright field vision. This is an advantage for lymphatic surgery when identifying lymphatic vessels using physiologic flow.
More recently, two dedicated robotic microsurgical platforms have emerged. A group of microsurgeons from Maastricht University Medical Center (MUMC+, Maastricht, The Netherlands) along with technical engineers from Eindhoven University of Technology (TUe, Eindhoven, The Netherlands) developed a dedicated robotic platform for super-microsurgery, MicroSure’s MUSA (MicroSure, Eindhoven, The Netherlands). The MUSA makes use of existing microsurgical instruments in holders with remote manipulation. It showed safety and feasibility in a preclinical animal study. Van Mulken et al . conducted a prospective randomized pilot study to compare robot-assisted vs. conventional super-microsurgical lymphaticovenous anastomosis in treating breast cancer-related lymphedema. Improvement of quality of life scores and a decrease in arm volume were detected at 3 months follow-up. Despite an initial longer time to perform the anastomosis in the robot-assisted group, a significant decline in that time was observed in the robot-assisted group after 8 trials (from ~31 min to ~16 min). Another unique, dedicated microsurgical platform, SYMANI (MMI, Pisa, Italy), has been developed. It employs its own unique instrumentation that can handle significantly smaller, more delicate structures and has articulation at the wrist, unlike the MUSA. Preclinical and clinical data are available. Both these systems have achieved the European CE Mark and are in production. Penetrance into the US is forthcoming.
The advantages of robotics over endoscopy (disappearance of physiological tremor, three-dimensional vision, high definition, magnification, and superior ergonomics) led to its use for micro-neural and brachial plexus surgery. Nectoux et al . demonstrated the feasibility of robotic micro-neural repair in experiments on fresh nerves (rat thigh sciatic nerve, pig upper limb median nerve, and human wrist median nerve, human wrist ulnar nerve, and human finger collateral nerve) using either an anatomical (epi-perineural repair) or a neurotrophic technique (nerve regrowth guide). Repairs were all performed without any damaging movements. They concluded that the robot allowed very safe and precise peripheral nerve repairs by counteracting physiological tremor and improving the overview of the surgical field. These promising results allowed them to use the robot for intraneural dissection of peripheral nerve tumors. The robotic approach permitted, in addition to a minimally invasive approach, a higher degree of precision, allowing identification of the fascicles with greater accuracy and safety. More recently, Chang et al . described the technical feasibility of robot-assisted sympathetic nerve reconstruction using a sural nerve graft in a case series of patients with postoperative compensatory sweating. They noted that the robotic platform led to an easier identification of the healthy proximal stump of the sympathetic trunk and facilitated meticulous suturing with 8-0 nylon in a limited anatomical space.
As for the more complex brachial plexus surgery, the advent of robotic surgery revealed new perspectives in treatment algorithms. To date, the traditional approach was to treat closed injuries with watchful waiting as access to the brachial plexus traditionally requires a long incision, with significant dissection, which often resulted in substantial scarring and adhesions. This algorithm, however, could lead to a delay in exploration for up to 3–6 months post-injury. An early intervention might allow a better assessment of the lesion and potential repair of graftable nerve roots (obviating the need for secondary nerve transfers), much like laparoscopy can be used to assess the need for a laparotomy. Although endoscopic approaches could allow early exploration by reducing incisional morbidity, this technique did not turn out to be suitable for finely tuned microneural repairs. A minimally invasive approach with three-dimensional, high definition, high magnification vision that allows early exploration of the brachial plexus to diagnose the injuries, and also to reliably perform higher-quality micro-neural repairs without the open incision is needed. Liverneaux and Mantovani reported their technique and favorable results of upper brachial plexus injury intervention using the Da Vinci platform with a supraclavicular approach. Tetik and Uzun also reported their favorable experience with robot-assisted axillary exposure of the brachial plexus region (especially for lower injuries) in a cadaveric model. Facca et al . presented their experimental and clinical experience in robotic-assisted surgery of the shoulder girdle and brachial plexus. In their cadaveric studies, they were able to dissect the supraclavicular brachial plexus and adjacent anatomical structures (jugular vein, omohyoid muscle, phrenic nerve, scalene muscles, and nerve roots from C4 to C7). A complete dissection and full exposure of the supraclavicular portion of the brachial plexus was successfully achieved. They were also able to graft a nerve segment into an artificially created gap by performing separate epi- and perineural repairs with 10-0 nylon.
Finally, it is worth noting that new directions for robotic microsurgical applications are being utilized in the field of urology. These include microsurgical vasectomy reversal, intra-abdominal vasovasostomy (for patient with prior inguinal hernia-related inguinal vasal obstruction), microsurgical subinguinal varicocelectomy, microsurgical testicular sperm extraction (MicroTESE), and targeted microsurgical denervation of the spermatic cord for chronic orchialgia. The reader is referred to an elegant review by Gudeloglu et al . for more detailed information.
Robotic microvascular anastomosis is a burgeoning application whose future in microsurgery is promising. Preliminary results suggest that surgical robots could play a key role in microsurgery in the future. The current limitations of the robotic platform for microsurgery include inferior optics of the endoscope compared to the operating microscope, instruments that are still not completely suited for fine microsurgical instrumentation, and lack of haptic (sensory) feedback. Only one study has objectively assessed haptics and concluded it was not crucial to completing an accurately tied knot with a microsurgical suture. Subjects tied knots and tightened them with eyes open and closed with robotic assistance. No difference in suture breakage and poor tightening was observed. The primary author’s experience is that microsurgery is 90% visual, and most of what we imagine we are feeling, we are actually seeing, and our brain is supplying the illusion of sensation. The 10% of haptic feedback that is real, however, is a very important component and may constitute a barrier to robotics playing a more active role in microsurgery. Fortunately, there have been some recent technological advances in designing newer robotic platforms that feature haptic feedback. Titan Medical, Inc. (Toronto, ON, Canada) is developing a multi-port surgical system called Amadeus, which provides tactile feedback. The Sofie (Surgeons Operating Force-feedback Interface Eindhoven) is another robot that is being developed by Eindhoven University of Technology in The Netherlands which also features tactile feedback.
The robotic endoscope is limited, however, in both its clarity, resolution and magnification compared to the optics of more traditional operating microscopes. These differences can be overcome with better quality, fixed-distance lenses fitted to the stereoscopic optical system of the robot. Finally, more delicate and adapted instruments are required which hold greater resemblance to traditional microsurgical instruments and that would accommodate some form of haptic feedback (see discussion below on “Limitations”).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here