Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Rickettsia rickettsii, the etiologic agent of Rocky Mountain spotted fever (RMSF), is a small, obligately intracellular, gram-negative, rod-shaped organism that is among the most pathogenic of all known bacteria. Although R. rickettsii can infect several different cell types, its primary targets in mammalian hosts are the endothelial cells lining capillaries, arterioles, and venules of all major tissues and organ systems ( Fig. 178.1 ). Damage to the endothelium of the dermis, skeletal muscle, and vital organs such as the brain, heart, lungs, kidneys, and gastrointestinal tract results in the systemic manifestations characteristically observed in people with RMSF.
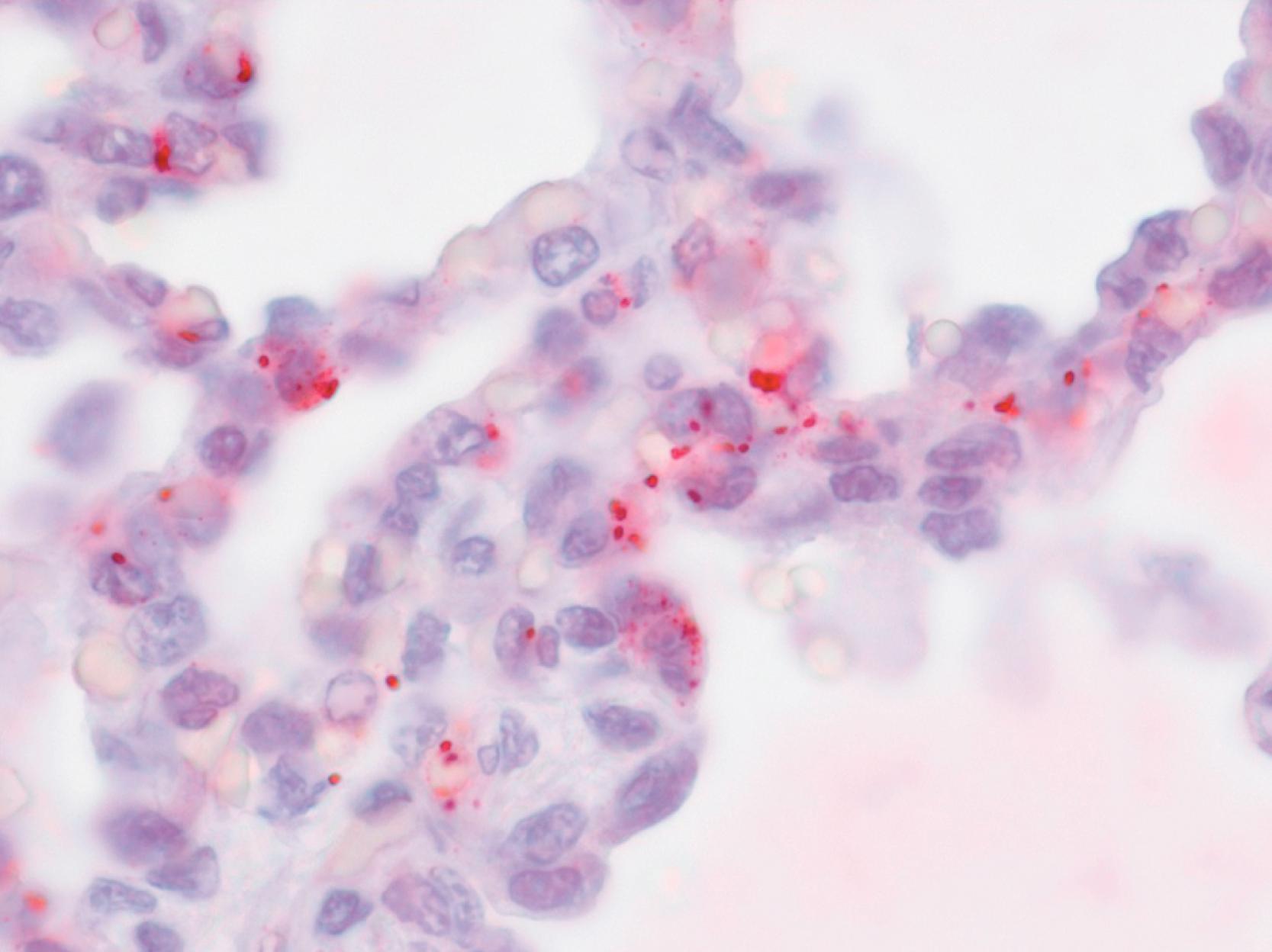
Ku70, a high molecular weight protein that is expressed at the plasma membrane of mammalian endothelial cells, monocytes, and macrophages, is believed to serve as a receptor for cell invasion by R. rickettsii . Several rickettsial outer membrane proteins, including rOmpB, rOmpA, and sca1 are involved in the attachment to and subsequent endocytosis by the mammalian host cell. Once inside the cell, R. rickettsii rapidly escapes a transient invasion vacuole to reside freely in the cytoplasm, where it replicates by binary fission. R. rickettsii is capable of intracellular movement that involves rickettsia-mediated polymerization of host-cell actin. , Rickettsiae that collide with the plasma membrane of the host cell create protrusions that extend into and become internalized by adjacent cells or may be released into the extracellular space and attach to neighboring cells. , R. rickettsii severely damages the plasma membrane of endothelial cells and disrupts the structural organization of intracellular organelles and cytoskeleton. Oxidative injury to host cells leads to diffuse microvascular injury and fluid leakage into extravascular spaces. Different strains of R. rickettsii produce varying levels of injury to cultured human endothelial cells; however, these differences have not been characterized in vivo, and severe or fatal disease has resulted from infections with at least 4 genetically distinct strains of R. rickettsii . , The genetic basis for the virulence of R. rickettsii is not well understood. ,
RMSF occurs throughout most countries of North, Central, and South America and is maintained in nature by several species of ticks and mammals. Humans are incidental hosts and become infected with R. rickettsii from the bite of an infected tick. Throughout much of the US, Dermacentor andersoni (the Rocky Mountain wood tick) and Dermacentor variabilis (the American dog tick) are considered primary tick vectors. Rhipicephalus sanguineus sensu lato (the brown dog tick) is another important vector in northern and central Mexico and the southwestern US ( Fig. 178.2 ). In South America, other hard tick species, particularly Amblyomma sculptum and Amblyomma aureolatum , are important vectors. ,

The reported incidence of RMSF and other tickborne spotted fever group rickettsioses, including Rickettsia parkeri rickettsiosis and Rickettsia 364D rickettsiosis (also known as Pacific Coast tick fever), have increased steadily during the last two decades: from 2000 to 2017, the annual incidence of these nationally notifiable infections rose from 1.7 to 19.2 cases per million persons. , The highest annual incidence rates of all spotted fever group rickettsioses in the US are reported consistently from midwestern and mid-Atlantic regions, particularly Arkansas, Missouri, North Carolina, Oklahoma, and Tennessee. Most cases are reported during April–September, corresponding with periods of increased host-seeking activity of adult-stage Dermacentor ticks.
Evaluations of well-documented cases identified from recent outbreaks of RMSF reveal that age-specific incidence rates are highest among persons aged ≤18 years. , During 1983–2007, approximately 15% of all reported fatal cases of RMSF were in children aged <10 years. , More recently, an analysis of 205 patients diagnosed with RMSF in Arizona from 2002–2011 determined that this cohort comprised approximately 47% of all deaths. With notable historical and contemporary exceptions, most cases of RMSF occur sporadically. , , , Small case clusters can occur among families and within communities when R. rickettsii infections in focal tick populations are localized around a home, neighborhood, or park. As a result, multiple infections can occur simultaneously or sequentially in the same household after identification of an index case.
RMSF can be difficult to diagnose clinically in its early stages. While most patients seek care within the first 3 days after the onset of fever, prodromal signs and symptoms are nonspecific, and even in areas where disease awareness is high, most patients with RMSF are not diagnosed correctly on their first visit for medical care. The early signs, symptoms, and laboratory features of RMSF often are insufficient to differentiate this disease from other viral or bacterial illnesses which present as an acute sepsis syndrome. Epidemiologic clues such as the history of outdoor activity, contact with animals (particularly dogs), or exposure to ticks may be helpful. However, a history of tick bite or exposure is absent in 40%–50% of patients included in several US pediatric case series ( Table 178.1 ). , ,
| Haynes et al., 1970 | Bradford and Hawkins, 1977 | Buckingham et al., 2007 | De Lara Huerta and Barragán, 2008 | Alvarez- Hernandez et al., 2015 | |
|---|---|---|---|---|---|
| Location | Ohio, North Carolina | North Carolina, Virginia | Arkansas, Kentucky, Missouri, North Carolina, Tennessee | Coahuila (Mexico) | Sonora (Mexico) |
| Years of evaluation | 1947−1968 | 1935−1974 | 1990−2002 | 1975−2007 | 2004−2013 |
| No. of patients | 78 | 138 | 92 | 115 | 104 |
| Male/female ratio | 39/39 | 77/61 | 43/61 | 54/61 | 57/47 |
| Known tick bite or exposure | 58% | 61% | 49% | NR | 89% |
| Clinical Features | |||||
| Fever | 100% | 100% | 98% | 100% | 100% |
| Any rash | 100% | 96% | 97% | 100% | 89% |
| Headache | 41% | NR | 61% | 70% | 80% |
| Nausea or vomiting | 28% | NR | 73% | NR | NR |
| Myalgia | 27% | NR | 45% | 70% | 80% |
| Abdominal pain | 23% | NR | 36% | 30% | NR |
| Periorbital edema | 24% | NR | 12% | 35% | 71% |
| Peripheral edema | 22% | NR | 25% | NR | 61% |
| Seizure | 4% | NR | 17% | NR | 6% |
| Coma | 4% | NR | 10% | NR | NR |
| Death | 4% | 13% | 3% | 55% | 20% |
Most patients develop symptoms within 2–10 days after the bite of an infected tick. , The disease begins with the abrupt onset of fever, often accompanied by severe frontal headache, nausea or vomiting, and generalized myalgia. The temperature generally is high and often >40°C. Headache, which may be less commonly noted in young children, is often intractable to therapy. Other findings recorded with varying frequency in pediatric case series include calf tenderness, abdominal pain, irritability, splenomegaly, conjunctivitis, periorbital edema, and peripheral edema, particularly involving the dorsum of the hands ( Table 178.1 and Fig. 178.3 ). ,
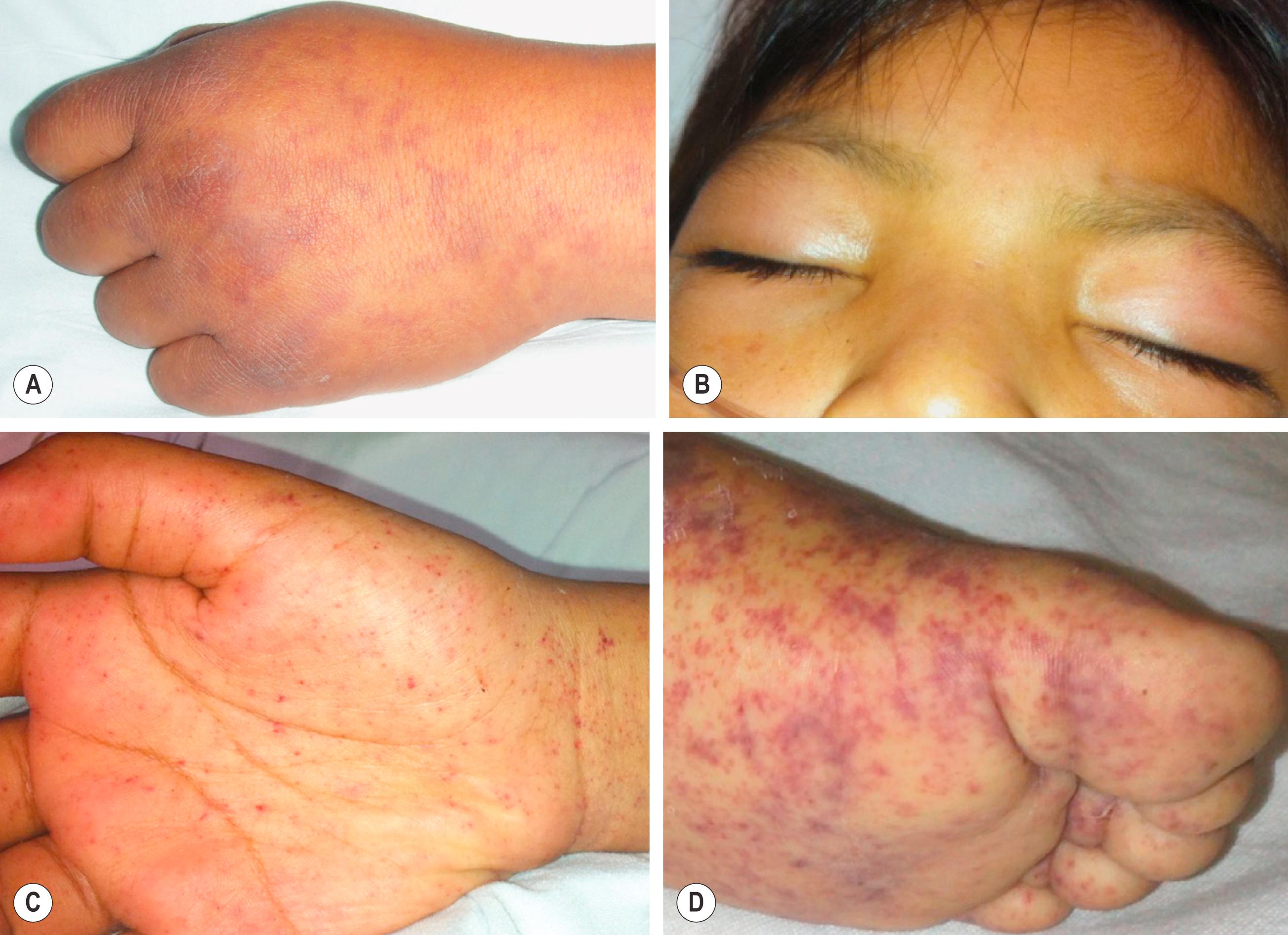
A generalized maculopapular rash, consisting of discrete, 1–5 mm blanching macules, is considered a hallmark feature of RMSF. However, the rash may be absent until 2–5 days after fever onset, especially in older patients, and may be difficult to identify in patients with dark skin. Therefore, clinicians must not await the appearance of the distinctive rash to establish a diagnosis and initiate therapy. In most cases, the rash begins on the wrists, ankles, and forearms then spreads centrally to involve the legs, buttocks, trunk, and face. In more advanced cases of the disease, the rash frequently involves the palms and soles ( Fig. 178.3 ). In some patients, the mucous membranes of the palate and pharynx also can be affected. , With the progression of the disease, the rash becomes more petechial, and individual lesions often enlarge and coalesce to form ecchymoses. In approximately 10% of patients, the rash is evanescent, atypical in distribution, or entirely absent. , Other exanthematous diseases in children that can resemble RMSF include rat-bite fever, staphylococcal sepsis, meningococcemia, secondary syphilis, leptospirosis, Kawasaki disease, measles, and papular purpuric gloves and socks syndrome.
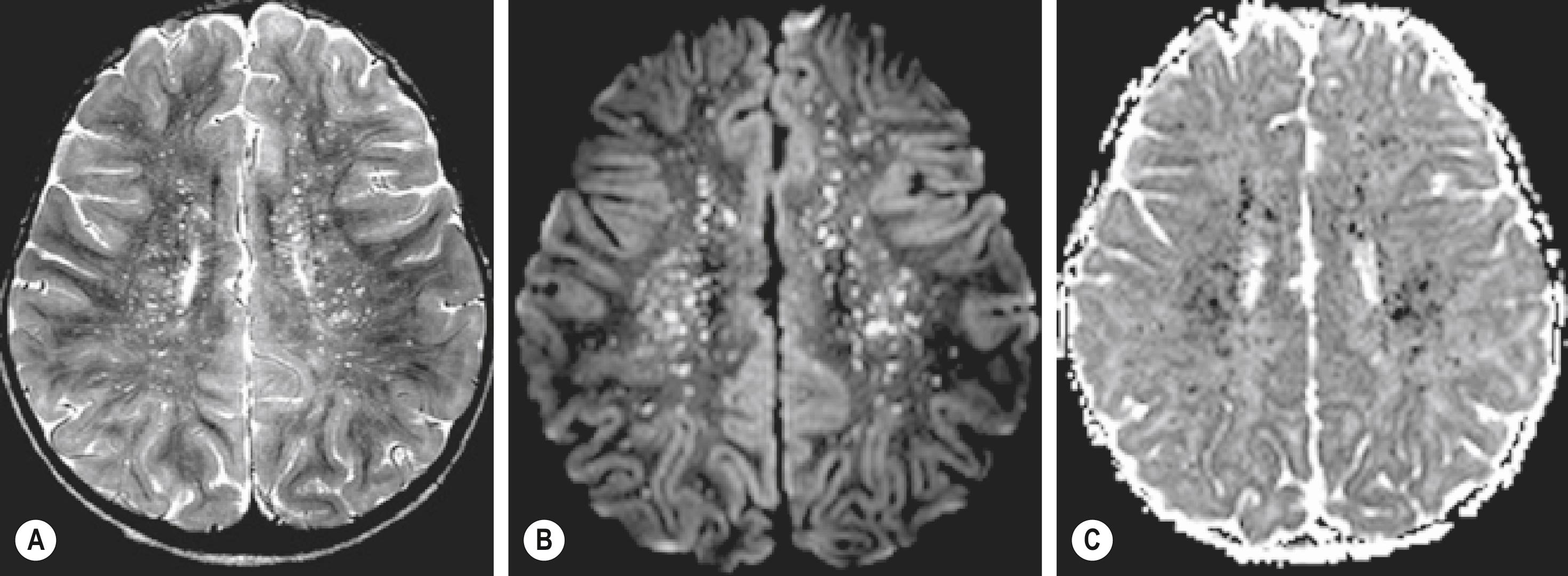
RMSF can progress rapidly to catastrophic disease involving multiple organ systems. Severe infections often are characterized by concomitant life-threatening manifestations that may include acute respiratory distress syndrome, first-degree atrioventricular block, cardiac failure, disseminated intravascular coagulopathy, gangrene, acute renal failure, seizure, coma, or tonsillar herniation. , , , , , Central nervous system disease generally occurs later in the illness and manifests as parenchymal edema, perivascular infarcts, and leptomeningeal vasculitis. Computed tomography is generally unremarkable, although images may demonstrate sulcal effacement consistent with generalized edema, or low-density white matter foci consistent with infarcts. Magnetic resonance imaging is generally more distinctive and may identify punctate perivascular infarcts in the deep white matter referred to as a “starry sky” pattern that is identified more often in children than adults ( Fig. 178.4 ). ,
During the years immediately preceding the introduction of effective anti-rickettsial therapies, an estimated 19% of all US patients infected with R. rickettsii died. With advances in treatment and medical care, US deaths attributable to RMSF declined considerably. Nonetheless, during one well-characterized US outbreak involving approximately 200 patients from 2002 to 2011, the case-fatality rate was 7%. In many countries in Latin America, case-fatality rates are much higher, including recent estimates from pediatric case series in Mexico that document fatal outcomes in 20%–55% of infected children. , , Most RMSF deaths are attributable to delayed diagnosis and failure to initiate specific antimicrobial therapy within the first 5–6 days of illness. , , Timely administration of appropriate therapy is critical because at least one-half of all deaths occur within 8 days of disease onset. , Patients with glucose-6-phosphate dehydrogenase deficiency typically experience severe and accelerated illness that often culminates in death in ≤5 days.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here