Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
As many as 10% of patients who had previous operations for primary hyperparathyroidism (PHPT) will experience persistent or recurrent disease after surgery ( Box 63.1 ). Due to the sequelae of PHPT, some of these patients will require reoperative parathyroid surgery. Unfortunately, in contrast to primary parathyroid surgery, which is extremely safe and has a high success rate, reoperative parathyroid surgery is associated with significant risks. Reoperative parathyroid surgery is a complex exercise that requires specialized skills and understanding to optimize outcomes. These reoperative procedures should be image driven, when possible, and the form of imaging available will vary by region and individual center. Surgeons performing reoperative parathyroid surgery need to be facile with a range of operative techniques to address the challenges that can present in these patients.
A set of guidelines published in 2018 was the first international multisociety and multidisciplinary effort to aggregate best practices and evidence to guide surgeons and patients on revision of surgery in cases of previously failed parathyroid surgery.
a) The first step in the evaluation of the reoperative parathyroid patient must include complete biochemical confirmation of primary hyperparathyroidism with hypercalcemia, relative or absolute hyperparathormonemia, and preferably hypercalciuria before reoperation.
b) The diagnosis of hyperparathyroidism must be secured through exclusion of nonparathyroid causes of hypercalcemia and/or hyperparathormonemia.
Detailed review of past surgical and pathologic data is essential to optimize surgical success.
Assessment of patient’s current health status and previous surgical complications, if any, must be made before planning for reoperative surgery.
Preoperative laryngeal assessment is required in all patients before reoperative parathyroid surgery.
a) Preoperative imaging of reoperative parathyroid surgical patients is mandatory.
b) Using the imaging modality that is readily available, most reliable, and cost effective in a given health system is recommended. This will vary depending on location.
Reoperative parathyroid surgery should be performed in the setting of an identified target gland(s) on preoperative imaging studies.
Parathyroid reoperations are challenging and should be undertaken by experienced surgeons.
Parathyroids should be approached through nondissected planes; when possible, most heavily scarred regions should be avoided.
Intraoperative adjuncts, such as neural monitoring and PTH assay, should be used during reoperative parathyroid surgery. Radioguidance, visible tracers, or ultrasound can be used, based on surgeon experience and availability.
During reoperative parathyroid surgery when postoperative hypoparathyroidism is a concern, primary auto transplantation should be considered after frozen section confirmation. Parathyroid cryopreservation can also be considered if available.
Nutritional and pharmacologic optimization should be performed in patients refusing or in patients who are not candidates for reoperative parathyroid surgery. Treatable metabolic abnormalities should also be addressed in operative patients.
One cause of unsuccessful primary parathyroid surgery is failure to diagnose the patient correctly. Although laboratory assessment in many patients with PHPT is clear and conclusive, in others the diagnosis can be challenging.
Several factors may influence biochemical results and lead to confusion in patient assessment. Vitamin D deficiency and calcium deficiency, particularly if severe, may mask PHPT because these patients may have a normal calcium level. In addition, 24-hour urine calcium excretion may be low in severely calcium-deficient individuals, and the PHPT biochemical picture can even mimic secondary hyperparathyroidism. Correction of any documented deficiencies and repeat testing after replacement therapy may reveal the true diagnosis of PHPT.
The use of thiazide diuretics and lithium, which may raise parathyroid hormone (PTH) and cause mild hypercalcemia, may mimic the diagnosis of PHPT. These medications should be discontinued when possible and the biochemical evaluation repeated 6 weeks after medication washout. Nonparathyroid-mediated hypercalcemia (i.e., lymphoma, granulomatous diseases, hyperthyroidism, metastatic bone disease, multiple myeloma, vitamin D toxicity, and milk-alkali syndrome) is distinguished from PHPT by an appropriately suppressed PTH paired with elevated calcium. PTH-related peptide (PTHrP) can be assayed when PTH is suppressed in the presence of hypercalcemia and there is a suspicion of malignancy.
Familial hypocalciuric hypercalcemia (FHH) must also be excluded with a 24-hour urine collection and estimation of the calcium-creatinine clearance ratio. This can be done based on a low urinary calcium-creatinine excretion ratio of < 0.01. If below 0.01, a genetic mutational analysis of the calcium-sensing receptor (CaSR), and possibly G alpha 11 and AP2S1 may be pursued. Conversely, a renal leak of calcium (hypercalciuria) can result in a compensatory raise in PTH, and it would cause persistent hyperparathormonemia, even after successful surgery for hyperparathyroidism.
In patients correctly diagnosed with PHPT, primary parathyroidectomy can fail for several reasons. Persistent hyperparathyroidism (HPT) is typically due to unsuccessful or inadequate surgery. Most commonly this occurs with a missed single adenoma, most frequently in a normal embryologic location rather than an ectopic location. Ectopic adenomas are found in common places (tracheoesophageal grove and thyrothymic ligament) that often are not dissected well by an occasional parathyroid surgeon. Intrathyroidal adenomas are infrequent causes for failed parathyroid surgery (< 1%). In the case of four-gland hyperplasia, a less than complete excision (< 3 glands) can result in a failure to cure. This often happens when all four parathyroid glands are never located during the initial surgery.
Recurrent HPT is usually due to a lack of appreciation for the underlying disease process, most commonly an asynchronous second adenoma or an unappreciated four-gland hyperplasia. Recurrent HPT in MEN-1 can be an expected outcome after adequate primary surgery that was initially successful. Recurrent HPT patients are more likely to have a family history of hyperparathyroidism and/or have had their initial parathyroid surgery performed at a young age.
Any patient with persistent elevated calcium or elevated PTH levels in the face of inappropriately normal calcium levels after a parathyroidectomy procedure is a potential candidate for reoperative parathyroidectomy. The biochemical diagnosis of persistent and recurrent PHPT is the same as for the initial diagnosis of PHPT. Persistent HPT is defined as hypercalcemia within 6 months of the initial parathyroidectomy; whereas, recurrent HPT is defined as hypercalcemia recurring 6 months or more after a successful parathyroidectomy.
National data sets reveal lower cure rates with reoperative parathyroid surgery—84% compared with 95% for first-time surgery. Not only are cure rates lower, but also, complication rates are higher with a six-fold increase in vocal cord paralysis and a doubling of the amount of bleeding compared with primary surgery. Permanent hypoparathyroidism rates of between 10% to 20% are also reported.
Institutional experience has been shown to influence operative success with higher volume institutions having more favorable outcomes. Regarding PHPT, surgeons having an annual volume in excess of 50 cases have better outcomes compared with lower volume surgeons, but there are no studies examining this question for reoperative surgery.
Due to the increased surgical risk of operating in a challenging, scarred surgical field, the threshold for reoperative surgery should be higher than for first-time surgery. Patients who have had parathyroid surgery typically meet one or more of the generally accepted guidelines for surgery. In these patients, the indications for surgery have not changed, except now that they have had unsuccessful parathyroid surgery, the increased risks associated with the reoperative procedure must be considered. In patients who underwent parathyroid surgery but didn’t meet any specific guidelines, the decision to consider another parathyroid operation should be even more carefully contemplated. Asymptomatic patients with PHPT have been followed for up to a decade without overt evidence of clinically significant disease progression.
As described earlier, an improper diagnosis of PHPT can lead to unsuccessful primary parathyroidectomy. Therefore critically, the first step for considering someone a candidate for reoperative surgery is to reaffirm conclusively the diagnosis of PHPT. Once confirmed, a determination of disease severity and possible clinical effects of the continued or recurrent HPT is required. An assessment of the degree of hypercalcemia [such as a serum calcium greater than 3.0 mmol/l (12.0 mg/dL)] may be complemented by an evaluation of kidney function (especially the glomerular filtration rate); renal imaging (abdominal ultrasound or computed tomography for nephrolithiasis or nephrocalcinosis); somatic symptoms (musculoskeletal aches and pains); assessment of bone health (with dual energy x-ray absorptiometry, [DEXA]); and an assessment of cardiovascular risk (computed tomography [CT] calcium cardiac scoring).
The decision to move forward with reoperative parathyroid surgery is nuanced, and it demands extensive counseling of the patient and close coordination between the patient’s physicians.
Typically, reoperative parathyroid surgery is elective, like the first parathyroid surgery. This is often true for both the need and the timing of the surgery. Extreme hypercalcemia, advanced osteoporosis (especially with a high risk of fractures or after a recent fracture), and unrelenting renal lithiasis with severe renal colic may make revision parathyroid surgery more acutely required.
The need for precision is imperative in reoperative parathyroid surgery, so having a specific imaged target(s) is essential. Often, thorough and more comprehensive parathyroid (cervical and upper thoracic) imaging than that which was used for the initial operation is the basis for successful parathyroid reoperation. Focused exploration and excision based on a preoperative image in conjunction with intraoperative PTH is the most effective way to manage the reoperative parathyroid patient. Unguided bilateral exploration of what can be a significantly scarred central neck is not recommended because it is challenging, often results in a second failure to treat hyperparathyroidism, and risks complications, such as hypoparathyroidism and bilateral vocal cord paralysis as well as tracheal and cervical esophageal injury.
If the first parathyroid operation was done by an inexperienced parathyroid surgeon who missed a well-localized adenoma, reoperation can be rather straightforward, because the first surgeon failed to go deep enough in to the neck for a superior adenoma or inferior enough within the thymus for an inferior parathyroid adenoma. A number of approaches are available to the reoperative parathyroid surgeon through less-dissected planes, which can make reoperation more likely to be successful and, at the same time, minimize the risks of complications.
If a patient has undergone a previous minimally invasive or targeted parathyroidectomy on one side and additional glands are localized on the contralateral side, it is perfectly reasonable to go through an original midline approach, especially if the strap muscles on the side in question have not been badly scarred to the underlying thyroid. If after dealing with an abnormal gland(s) on the previously unoperated side, the intraoperative PTH level does not fall appropriately, then it may be best to approach the original side by a lateral dissection (between straps and sternocleidomastoid [SCM] muscle) to avoid bleeding from peeling the scarred strap muscles off the thyroid.
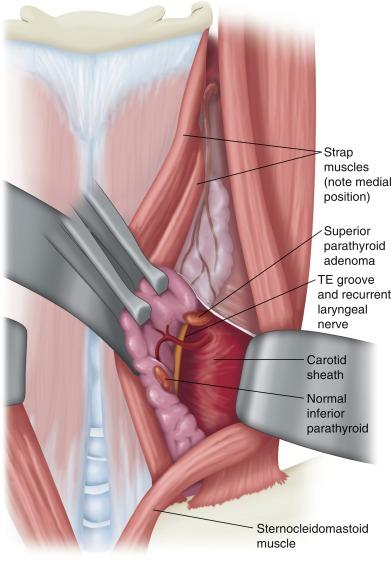
The lateral approach is ideal for missed superior parathyroid glands in patients who have previously undergone a standard midline approach ( Figure 63.1 ). One of the most commonly missed parathyroid adenomas is the superior adenoma posterior to the recurrent laryngeal nerve (RLN) deep within the tracheoesophageal groove or retroesophageal/prevertebral region. With this approach, a plane is developed between the sternocleidomastoid and the lateral strap muscle on the side in question. The contents of the carotid sheath are then retracted laterally, thus exposing the prevertebral fascia and the tracheoesophageal groove. Typically, the previously undisturbed RLN can be easily identified via this approach in a previously undissected plane and can be retracted medially. The lateral approach may be more difficult after prior low anterior cervical spine surgery because its plane of dissection is similar; however, this alone should not contraindicate the lateral approach.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here