Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Shock can be defined as a state of circulatory failure to deliver sufficient oxygen to balance the demands of the tissues, which results in tissue hypoxia: a deficiency in the bioavailability of oxygen to the tissues of the body. Circulatory shock assessment is often a challenging and common clinical scenario (i.e., up to one-third of patients admitted to the intensive care unit [ICU] are in circulatory shock ). This should be considered a time-related syndrome needing prompt recognition to avoid multiorgan dysfunction and death. For this purpose, the assessment of clinical and nonclinical signs of acute circulatory dysfunction is of pivotal importance to define and target the therapeutic strategy at the bedside. First of all, the source of the hemodynamic instability should primarily be presumed by the physical examination of the patient and from details retrieved from the past and present medical history. For instance, in the large multicentric randomized European Sepsis Occurrence in Acutely Ill Patients II (SOAP II) trial, acute circulatory dysfunction was related to septic shock in the vast majority of the 1679 ICU patients enrolled (62%), whereas cardiogenic shock (16%), hypovolemic shock (16%), and other types of distributive (4%) or obstructive (2%) shock were less frequent. Considering this prevalence, a de novo acute circulatory failure presented in the emergency department should be primarily considered a septic event, and accordingly treated, in the absence of any evident clinical signs of a different pattern (i.e., an evident fluid or blood loss, signs and symptoms of severe right or left acute ventricle dysfunction). The challenge of shock management is to balance fluid administration (preload), heart contractility, and vascular tone (afterload) to avoid the detrimental effects of over-resuscitation or under-resuscitation. Basically, circulatory shock may be caused by a deficit related to the pump (heart function or heart flow) or by reduced circulatory flow. Hypovolemia can be “absolute” after severe hydration defects, plasma, or blood loss; it can be “relative” when fluid administration is insufficient to compensate for a loss in vascular tone in the context of sepsis or anaphylaxis (or use of large doses of sedative drugs). In that context, there is a discrepancy between the content (volume) and vascular capacity, and abnormal sympathetic tone is associated with altered capillary recruitment. The clinical examination is a cornerstone in shock, but is notoriously inaccurate in assessing the exact value of cardiac output (CO) and the intravascular volume status. , For these reasons, the goal of shock management can be achieved only adding bedside quantitative and qualitative evaluation of cardiovascular system function, based on an integrated approach of clinical examination, critical care echocardiography, and hemodynamic monitoring.
To better understand the systemic effects of the imbalance between oxygen delivery and consumption, some basic concepts of oxygen delivery to the cells and cellular energy metabolism should be appraised ( Fig. 80.1 ).
Global oxygen delivery (DO 2 ) is the total oxygen carried by blood to the cells and is calculated as the product of the oxygen content in arterial blood (CaO 2 ) and CO.
Dissolved oxygen contributes little to total oxygen content because of the limited solubility.
The DO 2 continuously balances the peripheral oxygen uptake or consumption (VO 2 ). Failure to maintain the DO 2 :VO 2 ratio is initially compensated by increased oxygen extraction and a fall in mixed venous oxygen content. The normal ratio of delivery to consumption (DO 2 :VO 2 ratio) is approximately 5:1. In fact, DO 2 and VO 2 are linked by a simple relationship called the oxygen extraction ratio (ER o 2 − %). Under physiologic conditions, DO 2 is larger than VO 2 and the ER o 2 is roughly 20% (<2.4 mL O 2 /kg/min for a 12 mL O 2 /kg/min DO 2 ). This DO 2 :VO 2 ratio is high enough that cellular respiration is not supply dependent, and VO 2 is predominantly a function of tissue oxygen demand: “consumption drives delivery.” VO 2 becomes supply dependent when the DO 2 :VO 2 ratio falls below 2:1, producing a biphasic DO 2 :VO 2 relationship. This critical level of the DO 2 :VO 2 relationship (the so-called “critical DO 2 ”) corresponds to the maximal oxygen extraction.
Cells require oxygen for the production of adenosine triphosphate (ATP), the principal energy source. ATP is hydrolyzed to adenosine diphosphate (ADP) and inorganic phosphate (Pi) by ATPs in the cytosol:
Energy released is used for the maintenance of membrane integrity, ionic pumps, and other specialized functions, such as contractility of muscle cells and impulse transmission in neurons.
Because the body’s stores of ATP will last no more than a few minutes, it must be continuously synthesized, and under physiologic conditions, the vast majority of ATP molecules are generated by the process of oxidative phosphorylation of glucose. Aerobic generation of ATP occurs in the mitochondria via oxidative phosphorylation along the electron transport chain in which reduced nicotine adenine dinucleotide (NADH) and reduced flavin adenine dinucleotide (FADH 2 ) are reduction-oxidized (redox) by molecular oxygen:
This yields 52.6 kcal/mol, which is used in the electron transport chain to create ATP from ADP + Pi + H + .
The first stage of oxidative phosphorylation is the conversion of glucose to pyruvic acid; this occurs in the cytoplasm. The second stage, the oxidation of pyruvic acid, can only occur in the mitochondria as part of the Krebs (citric acid) cycle ( Fig. 80.1 ). Oxidative phosphorylation is a more efficient process than substrate-level phosphorylation, yielding up to eight times more ATP than the anaerobic pathway per mole of glucose. In fact, this process produces a net 36 molecules of ATP (or 1270 kJ of available energy) for every glucose molecule oxidized.
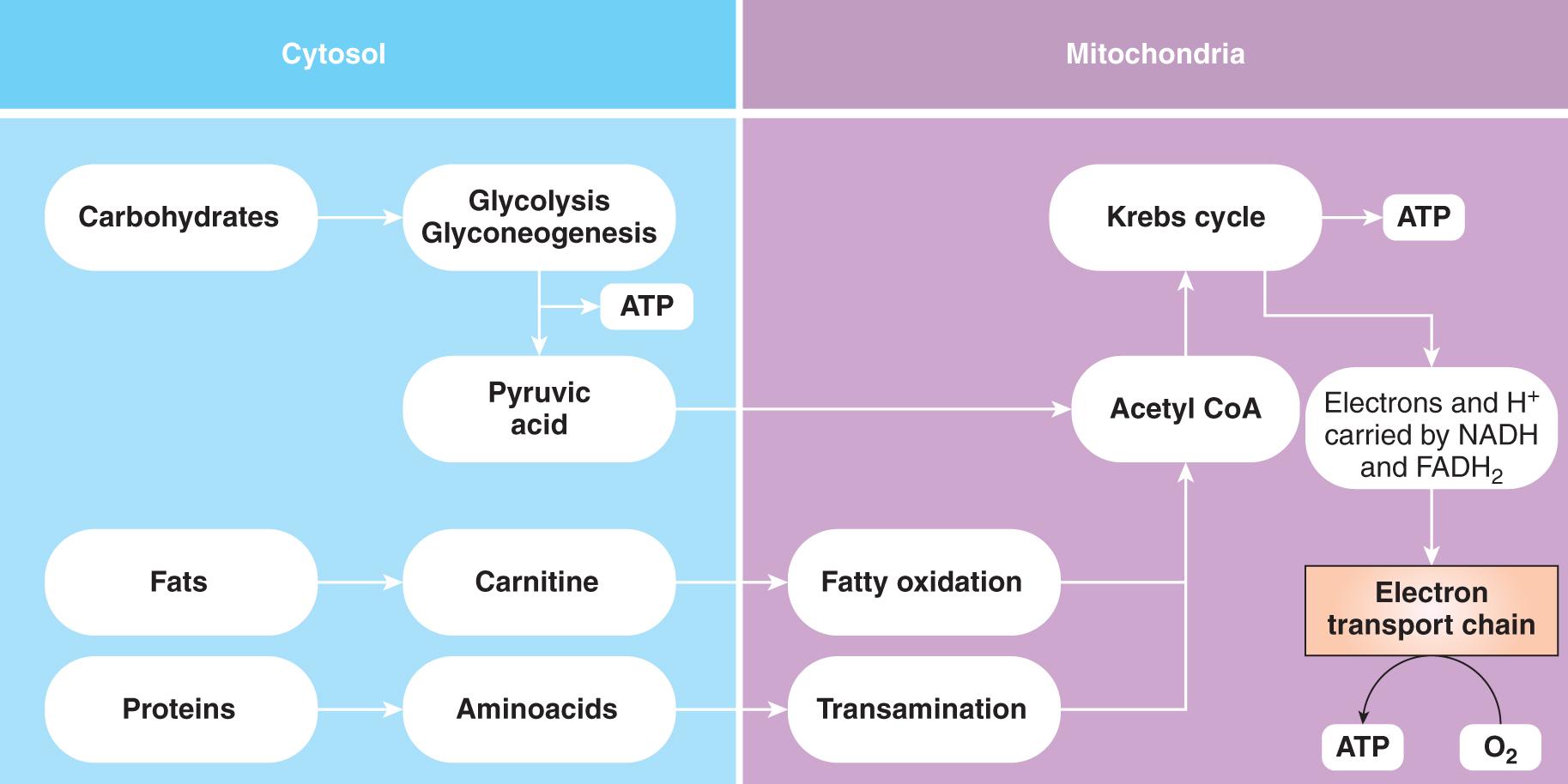
Anaerobic generation of ATP occurs in the cytosol and in the mitochondria via ATP-generating reactions that catalyze the following substrates with ADP into ATP:
* = Phosphoglycerate kinase (related enzyme)
** = Pyruvate kinase (related enzyme)
*** = Succinyl CoA synthase (related enzyme)
Lactate increase in blood level is the consequence of alterations in cellular energy metabolism caused by acquired derangements in cellular respiration. Lactate is produced according to the following cytoplasmic reaction:
This biochemical reaction promotes lactate formation, causing a 10-fold lactate/pyruvate ratio. Hence, the lactate increase in the blood is the result of a pyruvate production exceeding its use by the mitochondria. Because the pyruvate is essentially produced via glycolysis, any increase in glycolysis, regardless of its origin, can cause lactatemia. Pyruvate is principally metabolized into the mitochondria by means of the aerobic oxidation pathway, using the Krebs cycle. The synthesis of lactate in the cell is dependent on the ATP/ADP and NADH/NAD ratios, which are both related to the oxygen use in the mitochondria. In fact, hypoxia inhibits mitochondrial oxidative phosphorylation, leading to a decrease in the ATP/ADP ratio and an increase in the NADH/NAD ratio. This metabolic condition inhibits both the pyruvate carboxylase (converting pyruvate into oxaloacetate) and the pyruvate dehydrogenase (converting pyruvate into acetyl CoA). When the physiologic pathway of pyruvate use is affected by alterations in the redox potential of the cell, the excess of pyruvate concentration is shifted to lactate production and to the less efficient anaerobic glycolysis.
According to Connett and colleagues, it is possible to define three theoretical thresholds of cell hypoxia:
The first is crossed when cellular oxygen decreases but ATP production is maintained at a level sufficient to match ATP demand by metabolic adaptation (i.e., redox recruitment, alteration of phosphorylation states, increased glycolysis). The critical level of mitochondrial PO 2 for oxidative phosphorylation depends on the ability of a cell to adapt the phosphorylation process metabolically and the level of ATP demand.
The second occurs when steady-state ATP turnover can be maintained only by the production of ATP from anaerobic glycolysis by the Embden-Meyerhof pathway. This highly inefficient pathway generates only 2 molecules of ATP per 1 molecule of glucose metabolized. For highly metabolic tissues such as the brain, kidney, and liver, anaerobic glycolysis is too cumbersome to be effective, and these organs develop ATP depletion rapidly under hypoxic conditions. Dysoxia can be defined below this threshold.
The third threshold is crossed when glycolysis becomes insufficient to produce enough ATP to maintain cell function and structural integrity. This last step leads to cellular damage and death.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here