Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
When one considers the complexity of the pulmonary and hemodynamic changes occurring after delivery, it is surprising that the majority of infants make the transition from intrauterine to extrauterine life so smoothly and uneventfully. Nonetheless, the staff working in the intensive care nursery spends a lion’s share of their time caring for neonates with respiratory problems that are responsible for much of the morbidity and mortality in this period.
Before birth, the lung is a fluid-filled organ receiving 10% to 15% of the total cardiac output. Within the first minutes of life, a large portion of the fluid is absorbed or expelled, the lung fills with air, and the blood flow through the lung increases eight- to tenfold. This considerable increase results from a decrease in pulmonary arterial tone and other physiologic changes that convert the circulation from a parallel arrangement to a series circuit.
The high vascular resistance in the fetal lung is caused by pulmonary arterial vasoconstriction. The pulmonary arterial vasodilation observed following delivery results in part from the large increase in oxygen tension, from the small decrease in CO 2 tension and the corresponding increase in pH, and from mechanical stretch associated with lung inflation at the onset of breathing.
At the same time, an adequate functional residual capacity (FRC = volume of air in the lungs at end expiration) is quickly attained. In healthy term infants, the first breaths are characterized by short deep inspirations and prolonged expirations through a partially closed glottis to ensure lung inflation. In preterm infants, continuous positive airway pressure (CPAP) enhances lung inflation. By 1 hour, the distribution of air with each breath in the newborn is already similar to that observed in later life. Specifically, lung compliance (change in lung volume expressed in mL of air/change in pleural pressure expressed in cm of H 2 O) and vital capacity increase briskly in the first hours of life, reaching values proportional to those in the adult.
Chemical control of respiration is, in general, similar in the newborn infant and the adult. As inspired (and arterial) p CO 2 is increased, both infants and adults increase their ventilation, although the neonatal ventilatory response is smaller. The ventilation of the newborn is also transiently increased when inspired gas mixtures contain less than 21% oxygen; this response suggests that the carotid body chemoreceptors are active at birth. The infant, however, differs from the adult in that if hypoxic exposure continues beyond about 1 minute, respiration is depressed during the first weeks of life. Hypoxia thus appears to depress the respiratory center, negating the hypoxic stimulation via peripheral chemoreceptors. This hypoxic respiratory depression in the newborn appears to depend on the presence of suprapontine structures in the brain. Even though hypoxic respiratory depression may be useful to the fetus (who maintains a normal Pa O 2 of 20–25 mm Hg), persistence of this phenomenon into postnatal life may enhance vulnerability of neonatal respiratory control.
The effects of pulmonary stretch receptor activity on the timing of respiration (Hering-Breuer reflex) are more readily elicited in the newborn than in the adult. In infants, a sustained increase in FRC causes a marked slowing of respiratory rate by prolonging expiratory time. In the first days of life, a brisk lung inflation causes a deep gasp (Head paradoxical gasp reflex) followed by apnea, which is, again, a manifestation of the Hering-Breuer inflation reflex. The deep gasp observed in the first day of life with low inflation pressures may explain the clinical observation that very low pressures (10–15 cm H 2 O) are often effective in resuscitating the apneic newborn at birth by stimulating a gasp reflex.
The partial pressure of carbon dioxide (p CO 2 ) reflects the ability of the lung to remove CO 2 . The HCO 3 concentration is controlled by the kidney. When the pH and CO 2 are determined, the HCO 3 can be calculated by using the Henderson-Hasselbalch equation:
If only the pH is measured, the cause of the acidosis or alkalosis cannot be determined. With metabolic acidosis, HCO 3 is decreased. To compensate for this, the infant hyperventilates, lowering arterial p CO 2 . With respiratory acidosis caused by pulmonary disease, apnea, or hypoventilation, the arterial p CO 2 increases. The kidney attempts compensation by retaining HCO 3 and excreting hydrogen ions. Only by measuring the p CO 2 and pH and calculating HCO 3 can the cause of an abnormality in acid–base balance be determined. The normal newborn quickly regulates his or her pH to near adult values, although HCO 3 may be lower than normal adult values.
Oxygen is carried in the blood in chemical combination with hemoglobin (Hb) and also in physical solution. The oxygen taken up by both processes depends on the partial pressure of oxygen (pO 2 ).
At ambient pressures, the amount of dissolved oxygen is only a small fraction of the total quantity carried in whole blood (0.3 mL O 2 /dL plasma/100 mm Hg at 37°C). Most of the oxygen in whole blood is bound to Hb (1 g of Hb can maximally bind to 1.34 mL of oxygen at 37°C). The quantity of oxygen bound to Hb depends on the partial pressure and is described by the oxygen dissociation curve ( Fig. 9.1 ). The blood is almost completely saturated (%sat. = mL O 2 bound to Hb/Hb (g) × 1.34X100) at an arterial oxygen tension (Pa O 2 ) exceeding 90 mm Hg.
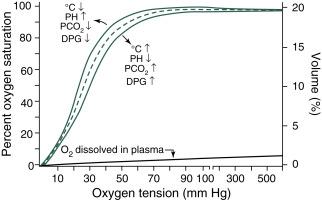
As an example, if the arterial pO 2 is 50 mm Hg, saturation is 90%, and Hb is 10 g/dL, then 9 g Hb is bound to oxygen. Thus the oxygen content of this 100-mL sample is 12.06 mL O 2 bound to Hb (1.34 × Hb 9) + 0.15 mL O 2 (0.3 × Hb 50/100) dissolved in plasma for a total of 12.21 mL O 2 . Naturally, if the Hb is doubled, then for the same saturation, the O 2 transported by Hb is also doubled (1.34 × Hb 18 = 24.12 mL O 2 ) without changing the amount dissolved. The dissociation curve of fetal blood is shifted to the left and, at any Pa O 2 less than 100 mm Hg, fetal Hb binds to more oxygen compared with adult Hb. The shift is the result of the lower affinity of fetal Hb for diphosphoglycerate. In contrast, the dissociation curve is shifted to the right by increasing acidosis and temperature. The clinical significance of the shift to the left for fetal Hb is that fetal blood will take up more oxygen at a given O 2 tension (pO 2 ). However, tissue pO 2 will need to decrease to a lower level to unload adequate oxygen. Thus oxygen delivery to the tissues is determined by a combination of cardiac output, total Hb concentration, and Hb oxygen affinity in addition to arterial pO 2 ( Fig. 9.1 ).
The shift in the dissociation curve induced by fetal Hb makes clinical recognition of hypoxia (insufficient amount of oxygen molecules in the tissues to cover the normal aerobic metabolism) more difficult because cyanosis is observed at a lower oxygen tension. Cyanosis is first observed at saturations from 75% to 85%, which correspond to oxygen tensions of 32 to 42 mm Hg on the fetal dissociation curve. Cyanosis in the adult is observed at higher tensions. The flattening of the upper portion of the S-shaped dissociation curve makes it almost impossible to monitor oxygen tensions above 60 to 80 mm Hg by following arterial oxygen saturation. Although the shape of the oxygen dissociation curve limits the usefulness of pulse oximetry to detect high Pa O 2 values, keeping saturation measured via pulse oximeter between 92% and 95% is one of the most effective and practical ways of reducing the risk of hyperoxemia.
The pO 2 in arterial blood not only depends on the ability of the lung to transfer oxygen, but also is modified by the shunting of venous blood into the systemic circulation through the heart or lungs. Breathing 100% oxygen for a prolonged time partially corrects desaturation resulting from alveolar hypoventilation, diffusion abnormalities, or ventilation/perfusion inequality. Measurements of Pa O 2 while breathing 100% oxygen are therefore useful diagnostically in determining whether arterial desaturation is caused by an anatomic right-to-left shunt, in which case oxygenation will fail to improve the condition (hyperoxia testing).
After birth, Pa O 2 increases to between 60 and 90 mm Hg. During the first days of life, 20% of the cardiac output is normally shunted from right to left in either the heart or lungs. When the normal adult breathes 100% oxygen, Pa O 2 increases to 600 mm Hg, compared with approximately 300 to 500 mm Hg in healthy neonates, which results from the substantial shunting in infants.
At the end of the first hour of life, perfusion of the lung is distributed in proportion to the distribution of ventilation. In the healthy newborn baby, oxygen saturations rise slowly over the initial few minutes of life; however, they approach 90% in the first 5 minutes. The postductal oxygen saturation levels are usually lower than the preductal measurements for as long as 15 minutes, indicating a persistence in elevated pulmonary vascular resistance for a significant period of time after birth ( Fig. 9.2 ). The speed with which pulmonary ventilation and perfusion is uniformly distributed is an indication of the remarkable adaptive capacities of the newborn infant for the maintenance of homeostasis.
Dawson et al defined the reference ranges for pulse oxygen saturation (SpO 2 ) values in the first 10 minutes after birth for 468 infants who received no medical intervention in the delivery room. For all 468 infants, the 3rd, 10th, 50th, 90th, and 97th percentile values at 1 minute were 29%, 39%, 66%, 87%, and 92%, respectively; those at 2 minutes were 34%, 46%, 73%, 91%, and 95%; and those at 5 minutes were 59%, 73%, 89%, 97%, and 98%. It took a median of 7.9 minutes (interquartile range: 5–10 minutes) to reach an SpO 2 value of greater than 90%. SpO 2 values for preterm infants increased more slowly than those for term infants (see also Chapter 3 ).
(Dawson JA, Kamlin CO, Vento M, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. 2010;125:e1340.)
![Fig. 9.2, Pre- and postductal Sp O 2 levels during the first 15 minutes after birth (median, interquartile range [IQR]); IQR postductal Sp O 2 levels were significantly lower than preductal Sp O 2 levels at 3, 4, 5, 10, and 15 minutes (∗ <.05). Fig. 9.2, Pre- and postductal Sp O 2 levels during the first 15 minutes after birth (median, interquartile range [IQR]); IQR postductal Sp O 2 levels were significantly lower than preductal Sp O 2 levels at 3, 4, 5, 10, and 15 minutes (∗ <.05).](https://storage.googleapis.com/dl.dentistrykey.com/clinical/RespiratoryProblems/1_3s20B9780323608541000092.jpg)
Oxygen supplementation is critical for the survival of many infants with respiratory problems. Previous restricted use resulted in an increase not only in mortality rate but also in neurologic handicaps. Additionally, recognition of the toxic effects of excessive or prolonged oxygen therapy is imperative when treating newborn infants. This has resulted in curtailed use of supplemental oxygen during neonatal resuscitation of both term and preterm infants (see Chapter 3 ). Therefore oxygen administration must be performed with great precision while carefully monitoring arterial oxygen tension or assessing oxygenation via noninvasive techniques.
For spontaneously breathing infants who require supplemental oxygen, a small hood to provide supplemental oxygen prevents fluctuations in inspired oxygen when opening the incubator. This has been largely replaced by low-flow nasal cannulae to deliver gas mixtures. Because improper oxygen administration can be detrimental, the following practical considerations should be highlighted:
Peripheral cyanosis may be present in a neonate with a normal or high arterial oxygen tension.
Inspired oxygen concentration should be monitored in all infants receiving supplementary oxygen or assisted ventilation.
Oxygen therapy without concurrent assessments of arterial oxygen tension is dangerous. A noninvasive monitoring device to measure oxygen saturation by pulse oximetry or transcutaneous pO 2 should be used continuously in infants receiving any supplemental oxygen. In the presence of an arterial line during the acute phase of illness, consider measuring Pa O 2 at least every 4 to 6 hours if the infant is receiving oxygen.
In preterm infants, arterial oxygen tension should be maintained between 50 and 80 mm Hg during the acute phase of respiratory failure. In the absence of available Pa O 2 monitoring, SaO 2 should be kept in the 90% to 95% range.
The development of retinopathy of prematurity (ROP) is related to high arterial oxygen tension levels, and these may rise above the normal range even with relatively low inspired oxygen concentrations. Whereas initial hyperoxia stunts retinal vascular development, later hypoxia appears to stimulate damaging vascular proliferation.
When infants receiving supplemental oxygen require bag-and-mask ventilation, both oxygen concentration and inflating pressures must be monitored closely.
Use of a nasal cannula for prolonged oxygen therapy allows greater mobility for the infant and enables oral feeding without manipulating oxygen concentration. Both inspired oxygen concentration and flow rate are precisely adjusted, and the infant’s oxygenation is closely monitored, typically via pulse oximetry. Administration of oxygen by nasal cannula requires close monitoring because in active infants, the cannula is easily displaced from the nose. Also, changes in respiratory pattern and oral breathing may entrain different amounts of room air around the prongs, changing the true inspired oxygen concentration. Finally, high gas flows via nasal prongs are favored by some centers in select patients.
When the infant with respiratory distress syndrome (RDS) is improving, environmental oxygen should be lowered in small decrements while continuously monitoring oxygenation.
Any inspired oxygen concentration above room air can be damaging to pulmonary tissue if maintained over several days. Oxygen therapy is continued only if necessary.
All premature infants 30 weeks’ gestation or less, or older if they had an unstable course or need for significant supplemental ventilation should be examined by an experienced ophthalmologist by the later of 31 weeks’ postmenstrual age (PMA) or 4 weeks after birth for treatable ROP.
Although oxygen targeting in extremely low–birth-weight infants has been under much investigation, the ideal saturation range for these most vulnerable infants remains unknown. Three recent international randomized controlled trials (RCTs) have addressed this clinical question, SUPPORT , BOOST-II , and COT , randomizing infants to low versus high saturation ranges and their effects on death and morbidities (ROP, necrotizing enterocolitis [NEC], bronchopulmonary dysplasia [BPD]). The results from these studies are conflicting, whereas SUPPORT and BOOST-II demonstrated higher mortality in the low saturation group with higher rates of ROP in the higher saturation group, the COT trial did not demonstrate any difference in their primary outcome of death or survival with one or more disabilities. A systematic review of the five available trials found an increase in relative risk of mortality in the lower saturation arm of 1.18 (95% confidence interval [CI]: 1.04–1.34). A more recent review states that even though the infants randomized to higher saturation goals demonstrated higher survival rates, the quality of the evidence was low, thus making the conclusion that lower saturation goals are dangerous is premature. Suffice it to say, at this time, a targeted saturation range of 90% to 95% may be safer than 85% to 89%, and has been widely adopted.
The debate on the optimal oxygen concentration for extremely preterm infants rages on with the June 2018 publication of the results of the Neonatal Oxygenation Prospective Meta-analysis (NeOProM) collaboration. Analyzing data of approximately 5000 babies from five different trials, there was no significant difference in the composite outcome of death or major disability between higher or lower oxygen saturation groups. There was, however, a higher rate of death and necrotizing enterocolitis in the lower-saturation group and a higher rate of retinopathy of prematurity in the higher-saturation group. The competing outcomes suggest that it is prudent to maintain the oxygen saturation in the 90% to 95% range as indicated above. Recognize that this is easier said than done and saturation levels will fluctuate so that the babies are out of range, both high and low, for a significant period of time. Newer automatic controlling devices responding to oxygen levels may reduce these fluctuations and hopefully lessen complications ( Askie LM, Darlow BA, Finer N, et al. Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA . 2018;319(21):2190-2201).
The initial objective is to establish an etiologic diagnosis for any observed respiratory symptoms. A major error in care can easily be made if other organ systems are not considered initially. Not every rapidly breathing infant has RDS or even respiratory disease. Hypovolemia, hyperviscosity (polycythemia), anemia, hypoglycemia, congenital heart disease, hypothermia, metabolic acidosis of any etiology, or even the effects of drugs or drug withdrawal may all mimic primary respiratory disorders. Appropriate care depends on the diagnosis. For example, rewarming should rapidly relieve respiratory symptoms in a mildly hypothermic infant; otherwise, sepsis must be strongly considered.
A working classification of some of these disorders is presented in Box 9.1 . Whenever faced with these respiratory symptoms, the next steps (following a history and physical examination) should be to obtain the following:
chest x-ray;
white blood cell count with differential and hematocrit (peripheral hematocrits can be higher than intravascular hematocrits);
blood sugar;
assessment of blood gas status via an arterial stick or capillary blood gas. (A capillary blood gas reliably estimates Pa CO 2 and pH, but not Pa O 2 .); and
pulse oximetry to assess oxygenation.
Pulmonary Disorders
Respiratory distress syndrome
Transient tachypnea
Meconium aspiration syndrome
Pneumonia
Air leak syndromes
Pulmonary hypoplasia
Systemic Disorders
Hypothermia
Metabolic acidosis
Anemia/polycythemia
Hypoglycemia
Pulmonary hypertension
Congenital heart disease
Anatomic Problems of the Respiratory System
Upper airway obstruction
Airway malformations
Space-occupying lesions
Rib cage anomalies
Phrenic nerve injury
Neuromuscular disease
The decision to catheterize the umbilical artery (see Appendix D ) depends on the infant’s condition. The umbilical artery and/or vein may need to be catheterized if significant metabolic acidosis or blood loss is suspected or if the infant remains severely distressed (as defined by continued hypoxemia and severe respiratory distress). On the other hand, if the infant has tachypnea and grunting with retractions but is active and pink, it is possible to withhold catheterization unless there is deterioration as manifested by marked respiratory distress and an oxygen requirement exceeding 40% to 50%.
Although the newborn has a relatively larger cardiac output and a lower peripheral resistance and blood pressure than the older child and adult, measurements of blood pressure must be routine. It has been shown that hypotension in sick preterm infants may not be associated with hypovolemia. Hypothermia or acidemia results in severe peripheral vasoconstriction and will confound blood volume estimates from measurement of blood pressure. In a hypovolemic infant, blood pressure often declines only after acidemia and hypoxemia are corrected. Blood pressure can be measured with a blood pressure cuff of correct size placed on one or all extremities (if coarctation of the aorta is suspected), employing either oscillometric or Doppler ultrasound techniques. Alternatively, direct arterial measurements may be made via indwelling catheters. Normal blood pressures and ranges may be found in Appendix C .
If the initial hematocrit is less than 30% without blood incompatibility, or if the blood pressure is reduced, it is reasonable to assume blood loss (e.g., an acute fetomaternal hemorrhage) and consider immediate correction of blood volume. With acute blood loss, hypotension prevails over anemia, whereas after chronic blood loss, anemia dominates the clinical picture and perfusion is less compromised. Saline is initially used, and blood is requested, starting with a push infusion of 10 mL/kg, observing blood pressure, heart rate, and the infant’s general condition. One must be extremely careful with rapid infusions of any solution in the critically ill premature infant because of the risk of increasing the incidence of intraventricular hemorrhage (IVH) by rapid volume expansion. Once a diagnosis has been made, it is necessary to determine whether the neonatal unit has all of the facilities that might be needed during the course of the illness. The following section discusses RDS in great depth, as this has been the primary model for understanding pathophysiology and management of neonatal respiratory disease.
RDS is still probably the most common initial problem in the intensive care nursery among premature infants weighing less than 1500 g. However, in preterm infants whose mothers have received antenatal steroids, and after postnatal intratracheal surfactant therapy, the characteristic clinical course for RDS may not be apparent. The following lists give the common symptoms, the physiologic abnormalities, and the pathologic findings.
Difficulty in initiating normal respiration. The disease should be anticipated if the infant is premature, the mother has diabetes, or if the infant has suffered perinatal asphyxia.
Expiratory grunting or whining (caused by closure of the glottis) is an important sign that sometimes may be the only early indication of disease; this maintains air in the immature lungs during expiration, and a decrease in grunting may be the first sign of improvement.
Sternal and intercostal retractions (secondary to decreased lung and increased rib cage compliance)
Nasal flaring
Cyanosis (if supplemental O 2 is inadequate)
Respirations—rapid (or slow when seriously ill)
Extremities edematous—after several hours (altered vascular permeability)
X-ray film showing reticulogranular ground glass appearance with air bronchograms
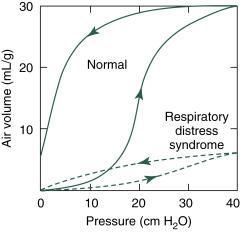
Lung compliance reduced to as much as one-fifth to one-tenth of normal ( Fig. 9.3 )
Large areas of lung not ventilated (right-to-left shunting of blood)
Large areas of lung not perfused
Decreased alveolar ventilation and increased work of breathing
Reduced lung volume
These changes result in hypoxemia, often hypercarbia, and, if hypoxemia is severe, metabolic acidosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here