Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The term respiratory distress is used to indicate signs and symptoms of abnormal respiratory pattern. A child with nasal flaring, tachypnea, chest wall retractions, stridor, grunting, dyspnea, and wheezing has respiratory distress. Taken together, the magnitude of these findings is used to judge clinical severity. Nasal flaring is nonspecific, but the other signs are useful in localizing the site of pathology (see Chapter 400 ). Respiratory failure is defined as inability of the lungs to provide sufficient oxygen (hypoxic respiratory failure) or remove carbon dioxide (ventilator failure) to meet metabolic demands. Therefore, whereas respiratory distress is determined by a clinical impression, the diagnosis of respiratory failure is indicated by inadequacy of oxygenation or of ventilation, or both. Respiratory distress can occur in patients without respiratory disease, and respiratory failure can occur in patients without respiratory distress.
A careful physical examination must be performed when managing a child in respiratory distress. Nasal flaring , although nonspecific, is an extremely important sign of distress in infants. It may indicate discomfort, pain, fatigue, or breathing difficulty. The state of responsiveness is another crucial sign. Lethargy, disinterest in surroundings, and poor cry are suggestive of exhaustion, hypercarbia, and impending respiratory failure. Abnormalities of the rate and depth of breathing can occur with both pulmonary and nonpulmonary causes of respiratory distress. In diseases of decreased lung compliance, such as pneumonia and pulmonary edema, breathing is characteristically rapid and shallow (decreased tidal volume). In obstructive airway diseases, such as asthma and laryngotracheitis, breathing is deep with increased tidal volume, but less rapid. Rapid and deep breathing without other respiratory signs should alert the physician to possible nonpulmonary or nonthoracic causes of respiratory distress, such as response to metabolic acidosis (e.g., diabetic ketoacidosis, renal tubular acidosis) or stimulation of the respiratory center (e.g., encephalitis, ingestion of central nervous system stimulants). Chest wall, suprasternal, and subcostal retractions are manifestations of increased inspiratory effort, weak chest wall, or both. Inspiratory stridor indicates airway obstruction above the thoracic inlet, whereas expiratory wheezing results from airway obstruction below the thoracic inlet. Grunting is most commonly heard in diseases with decreased functional residual capacity (e.g., pneumonia, pulmonary edema) and peripheral airway obstruction (e.g., bronchiolitis).
Clinical examination is important in localizing the site of pathology (see Chapter 400 ). Extrathoracic airway obstruction occurs anywhere above the thoracic inlet. Inspiratory stridor, suprasternal, chest wall, and subcostal retractions; and prolongation of inspiration are hallmarks of extrathoracic airway obstruction. By comparison, features of intrathoracic airway obstruction are prolongation of expiration and expiratory wheezing. Typical manifestations of alveolar interstitial pathology are rapid, shallow respirations, chest wall retractions, and grunting. The site of pathology can be localized and the differential diagnosis established on the basis of the clinical signs and symptoms ( Tables 89.1 and 89.2 ).
| SITE OF PATHOLOGY | RESPIRATORY RATE | RETRACTIONS | AUDIBLE SOUNDS |
|---|---|---|---|
| Extrathoracic airway | ↑ | ↑↑↑↑ | Stridor |
| Intrathoracic extrapulmonary | ↑ | ↑↑ | Wheezing |
| Intrathoracic intrapulmonary | ↑↑ | ↑↑ | Wheezing |
| Alveolar interstitial | ↑↑↑ | ↑↑↑ | Grunting |
| LUNG | RESPIRATORY PUMP |
|---|---|
| CENTRAL AIRWAY OBSTRUCTION | THORACIC CAGE |
|
|
| PERIPHERAL AIRWAY OBSTRUCTION | BRAINSTEM |
|
|
| ALVEOLAR-INTERSTITIAL DISEASE | SPINAL CORD |
|
|
| NEUROMUSCULAR | |
|
Although respiratory distress most frequently results from diseases of lungs, airways, and chest wall, pathology in other organ systems can manifest as respiratory distress and lead to misdiagnosis and inappropriate management ( Table 89.3 ). Respiratory distress resulting from heart failure or diabetic ketoacidosis may be misdiagnosed as asthma and improperly treated with albuterol, resulting in worsened hemodynamic state or ketoacidosis. Careful history and physical examination provide essential clues in avoiding misdiagnosis.
| SYSTEM | EXAMPLE(S) | MECHANISM(S) |
|---|---|---|
| Cardiovascular |
|
|
| Central nervous |
|
Stimulation of brainstem respiratory centers |
| Metabolic |
|
Stimulation of central and peripheral chemoreceptors |
| Renal | Renal tubular acidosis | Stimulation of central and peripheral chemoreceptors |
| Hypertension | Left ventricular dysfunction → increased pulmonary blood/water content | |
| Sepsis |
|
|
A child with cardiovascular pathology may present with respiratory distress caused by either decreased lung compliance or cardiogenic shock ( Table 89.4 ). Diseases that result in increased pulmonary arterial blood flow (e.g., left-to-right shunts) or increased pulmonary venous pressure (e.g., left ventricular dysfunction from hypertension or myocarditis, obstructed total anomalous pulmonary venous return) cause an increase in pulmonary capillary pressure and transudation of fluid into the pulmonary interstitium and alveoli. The increased pulmonary blood and water content lead to decreased lung compliance and result in rapid shallow breathing.
Decreased lung compliance
Left-to-right shunts
Ventricular septal defect, atrial septal defect, patent ductus arteriosus, atrioventricular canal, truncus arteriosus
Cerebral or hepatic arteriovenous fistula
Ventricular failure
Left heart obstructive lesions
Aortic stenosis
Coarctation of the aorta
Mitral stenosis
Interrupted aortic arch
Hypoplastic left heart syndrome
Myocardial infarction
Anomalous left coronary artery arising from the pulmonary artery
Hypertension
Acute glomerulonephritis
Inflammatory/infectious
Myocarditis
Pericardial effusion
Idiopathic
Dilated cardiomyopathy
Hypertrophic obstructive cardiomyopathy
Pulmonary venous obstruction
Total anomalous pulmonary venous return with obstruction
Cor triatriatum
Shock resulting in metabolic acidosis
Left heart obstructive lesions
Acute ventricular failure
Myocarditis, myocardial infarction
It is important to recognize that interstitial lung edema cannot only manifest as alveolar fluid, but as small airway obstruction as well. Wheezing as a sign of congestive cardiac disease is common in infants and young children and should be recognized. Patients with cardiac lesions, resulting in low cardiac output, often present in shock. For example, obstructive lesions of left side of the heart and acquired or congenital cardiomyopathy result in decreased perfusion and metabolic acidosis, as well as respiratory distress because of chemoreceptor and baroreceptor stimulation. The likelihood of a particular cardiovascular illness manifesting as respiratory distress depends on age at presentation ( Table 89.5 ).
| AGE | MECHANISM | DISEASE |
|---|---|---|
| Newborn (1-10 days) | ↑ Arteriovenous pressure difference | Arteriovenous fistula (brain, liver) |
| Ductal closure | Single ventricle lesions or severe ventricular outflow obstruction | |
| Independent pulmonary and systemic blood flow | Transposition of the great arteries | |
| Pulmonary venous obstruction | Total anomalous pulmonary venous return (TAPVR) | |
| Young infant (1-6 mo) | ↓ Pulmonary vascular resistance | Left-to-right shunt |
| ↓ Pulmonary artery pressure | Anomalous left coronary artery to the pulmonary artery | |
| Any age | Rate disturbance | Tachy- or bradyarrhythmias |
| Infection | Myocarditis, pericarditis | |
| Abnormal cardiac myocytes | Cardiomyopathy | |
| Excess afterload | hypertension |
Central nervous system (CNS) dysfunction can lead to alterations in respiratory patterns and manifest as respiratory distress. Increased intracranial pressure (ICP) may manifest as respiratory distress. Early rise in intracranial pressure (ICP) results in stimulation of respiratory centers, leading to increases in the rate ( tachypnea ) and depth ( hyperpnea ) of respiration. The resultant decrease in arterial blood partial pressure of carbon dioxide (Pa co 2 ) and elevation of cerebrospinal fluid (CSF) pH lead to cerebral vasoconstriction and amelioration of intracranial hypertension. Stereotypical respiratory patterns are associated with dysfunction at multiple levels of the brain. Cerebral hemisphere and midbrain lesions result in hyperpnea as well as tachypnea. In such situations, arterial blood gas (ABG) measurements typically show respiratory alkalosis without hypoxemia. Pathology affecting the pons and medulla manifests as irregular breathing patterns such as apneustic breathing (prolonged inspiration with brief expiratory periods), Cheyne-Stokes breathing (alternate periods of rapid and slow breathing), and irregular, ineffective breathing or apnea ( Table 89.6 ). Along with respiratory changes, other manifestations of CNS dysfunction and increased ICP may be present, such as focal neurologic signs, pupillary changes, hypertension, and bradycardia (see Chapter 85 ). Occasionally, severe CNS dysfunction can result in neurogenic pulmonary edema and respiratory distress, which may follow excessive sympathetic discharge resulting in increased pulmonary venous hydrostatic pressure as well as increased pulmonary capillary permeability. Central neurogenic hyperventilation is characteristically observed in CNS involvement by illnesses such as urea cycle defects and encephalitis. Bradycardia and apnea may be caused by CNS-depressant medications, poisoning, prolonged hypoxia, trauma, or infection (see Table 89.2 ).
| INJURY | PATTERN * | COMMENTS |
|---|---|---|
| Normal |  |
Variable V t with normal respiratory pauses and sighs |
| Cortex |  |
Hyperpnea and tachypnea |
| Midbrain |  |
Cheyne-Stokes breathing : Gradually increasing and decreasing V t |
| Pons |  |
Apneustic breathing : Prolonged inspiration followed by prolonged expiration |
| Medulla and pons |  |
Biot's breathing : Rapid and irregular respirations with pauses |
Direct stimulation of respiratory centers resulting in respiratory alkalosis is encountered in intoxication involving agents such as salicylates and theophylline. Similarly, intoxication with general CNS stimulants, such as cocaine and amphetamines, may result in increased respiration. Presence of endogenous and exogenous toxins, such as organic acidemias, ingestion of methanol and ethylene glycol, and late stages of salicylism, cause metabolic acidosis and compensatory hyperventilation, which can manifest as respiratory distress. ABG measurements show decreased pH and compensatory hypocarbia with normal oxygenation. Metabolic disorders causing hyperammonemia, on the other hand, cause respiratory alkalosis (decreased Pa co 2 with increased pH) because ammonia stimulates the respiratory centers. Carbon monoxide and cyanide poisoning or methemoglobinemia may produce respiratory distress.
Sepsis and septic shock may cause an acute respiratory distress syndrome (ARDS) with hypovolemic stimulation of baroreceptors, cytokine stimulation of respiratory centers, and lactic acidosis. Other indirect causes of lung injury include systemic inflammatory conditions, trauma, transfusion-related acute lung injury, and pancreatitis. Similarly, renal disease may manifest as respiratory distress by causing metabolic acidosis (e.g., renal tubular acidosis or renal failure) or hypertensive left ventricular failure and fluid overload.
Respiratory failure occurs when oxygenation and ventilation are insufficient to meet the metabolic demands of the body. Respiratory failure may result from an abnormality in (1) lung and airways, (2) chest wall and muscles of breathing, or (3) central and peripheral chemoreceptors. Clinical manifestations depend largely on the site of pathology. Although respiratory failure is traditionally defined as respiratory dysfunction resulting in arterial partial pressure of oxygen (Pa o 2 ) <60 mm Hg when breathing room air and Pa co 2 >50 mm Hg resulting in acidosis, the patient's general state, respiratory effort, and potential for impending exhaustion are more important indicators than ABG values.
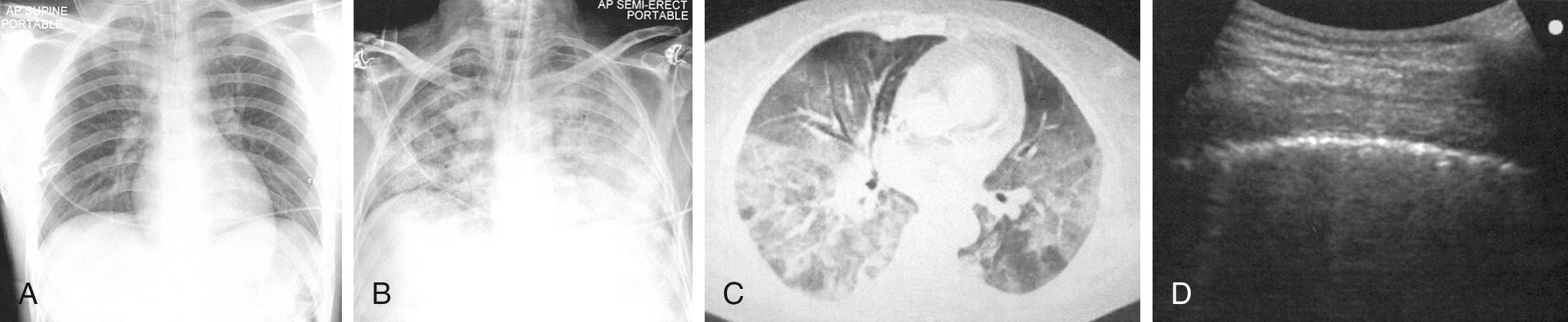
The Berlin definition of ARDS was once used to describe pediatric patients with ARDS, even though the pathophysiology is different between children and adults. The current pediatric definition differs in chest imaging findings, definition of oxygenation, consideration of both noninvasive and invasive mechanical ventilation, and consideration of special populations ( Table 89.7 and Fig. 89.1 ).
| BERLIN DEFINITION | PARDS | |
|---|---|---|
| Age | Adults and children | Excludes patients with perinatal-related lung disease |
| Timing | Within 1 wk of known clinical insult or new or worsening respiratory symptoms | Within 1 wk of a known clinical insult |
| Origin of edema | Respiratory failure not fully explained by cardiac failure or fluid overload. Need objective assessment (e.g., echocardiography) to exclude hydrostatic edema, even if no risk factor present. | Respiratory failure not fully explained by cardiac failure or fluid overload |
| Chest imaging a | Bilateral opacities not fully explained by effusions, lobar/lung collapse, or nodules. (Illustrative clinical cases and chest radiographs have been provided.) | Chest imaging findings of new infiltrate(s) consistent with acute pulmonary parenchymal disease |
| Oxygenation b | ||
| Mild | 200 mm Hg < Pa o 2 /F io 2 ≤300 mm Hg with PEEP, or CPAP ≥5 cm H 2 O c | Noninvasive mechanical ventilation:
Invasive mechanical ventilation f : |
| Moderate | 100 mm Hg < Pa o 2 /F io 2 ≤200 mm Hg with PEEP ≥5 cm H 2 O | |
| Severe | Pa o 2 /F io 2 <100 mm Hg with PEEP ≥5 cm H 2 O | |
a Chest radiograph or CT scan in Berlin criteria only.
b If altitude is >1,000 m, the correction factor should be calculated as follows: Pa o 2 /F io 2 × Barometric pressure/760.
c This may be delivered noninvasively in the mild acute respiratory distress syndrome group.
d Use P ao 2 -based metric when available. If Pa o 2 not available, wean F io 2 to maintain Sp o 2 <97% to calculate OSI or SF ratio.
e For nonintubated patients treated with supplemental oxygen or nasal modes of noninvasive ventilation, refer to reference below for “At Risk Criteria.”
f ARDS severity groups stratified by OI or OSI should not be applied to children with chronic lung disease who normally receive invasive mechanical ventilation or children with cyanotic heart disease.
Respiratory failure can be classified into hypoxic respiratory failure (failure of oxygenation) and hypercarbic respiratory failure (failure of ventilation). Systemic venous (pulmonary arterial) blood is arterialized after equilibration with alveolar gas in the pulmonary capillaries and is carried back to the heart by pulmonary veins. The ABG is influenced by the composition of inspired gas, effectiveness of alveolar ventilation, pulmonary capillary perfusion, and diffusion capacity of the alveolar capillary membrane. Abnormality in any of these steps can result in respiratory failure. Hypoxic respiratory failure results from intrapulmonary shunting and venous admixture or insufficient diffusion of oxygen from alveoli into pulmonary capillaries. This physiology can be caused by small airways obstruction, increased barriers to diffusion (e.g., interstitial edema, fibrosis), and conditions in which alveoli are collapsed or filled with fluid (e.g., ARDS, pneumonia, atelectasis, pulmonary edema). In most cases, hypoxic respiratory failure is associated with decreased functional residual capacity and can be managed by lung volume recruitment with positive pressure ventilation. Hypercarbic respiratory failure is caused by decreased minute ventilation (i.e., tidal volume multiplied by respiratory rate). This physiology can result from centrally mediated disorders of respiratory drive, increased dead space ventilation, or obstructive airways disease. Hypoxic and hypercarbic respiratory failure may coexist as a combined failure of oxygenation and ventilation.
For exchange of O 2 and CO 2 to occur, alveolar gas must be exposed to blood in pulmonary capillaries. Both ventilation and perfusion are lower in nondependent areas of the lung and higher in dependent areas. The difference in perfusion (Q̇) is greater than the difference in ventilation (V̇). Perfusion in excess of ventilation results in incomplete arterialization of systemic venous (pulmonary arterial) blood and is referred to as venous admixture . Perfusion of unventilated areas is referred to as intrapulmonary shunting of systemic venous blood to systemic arterial circulation. Conversely, ventilation that is in excess of perfusion is wasted; that is, it does not contribute to gas exchange and is referred to as dead space ventilation . Dead space ventilation results in return of greater amounts of atmospheric gas (which has not participated in gas exchange and has negligible CO 2 ) back to the atmosphere during exhalation. The respiratory dead space is divided into the anatomic dead space and the alveolar dead space. The anatomic dead space includes the conducting airways from the nasopharynx to the terminal bronchioles, ends at the alveoli, and has no contact with the pulmonary capillary bed. The alveolar dead space refers to areas of the lung where alveoli are ventilated but not perfused. Under normal conditions, this physiology usually occurs in West zone I, where alveolar pressure is greater than pulmonary capillary pressure. Under clinical conditions, this physiology may result from dynamic hyperinflation, high levels of positive end-expiratory pressure (PEEP), or large tidal volume in ventilated patients. Additionally, decreased pulmonary artery perfusion from pulmonary embolism or decreased cardiac output and hypovolemia can result in alveolar dead space. The end result is a decrease in mixed expired CO 2 (P eco 2 ) and an increase in the Pa co 2 – P eco 2 gradient. Dead space as a fraction of tidal volume (V d /V t ) is calculated as:
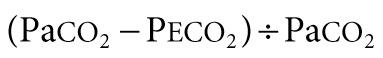
Normal V d /V t is approximately 0.33. Venous admixture and intrapulmonary shunting predominantly affect oxygenation, resulting in a alveolar oxygen (P ao 2 ) to Pa o 2 (A-a o 2 ) gradient without elevation in Pa co 2 . This physiology is caused by greater ventilation of perfused areas, which is sufficient to normalize Pa co 2 but not Pa o 2 because of their respective dissociation curves. The relative straight-line relationship of the hemoglobin-CO 2 dissociation allows for averaging of capillary P co 2 (Pc co 2 ) from hyperventilated and hypoventilated areas. Because the association between oxygen tension and hemoglobin saturation plateaus with increasing Pa o 2 , the decreased hemoglobin-O 2 saturation in poorly ventilated areas cannot be compensated for by well-ventilated areas where hemoglobin-O 2 saturation has already reached near-maximum. This physiology results in decreased arterial oxyhemoglobin saturation (Sa o 2 ) and Pa o 2 . Elevation of Pa co 2 in such situations is indicative of coincident alveolar hypoventilation. Examples of diseases leading to venous admixture include asthma and aspiration pneumonia, and those of intrapulmonary shunt include lobar pneumonia and ARDS.
Even if ventilation and perfusion are matched, gas exchange requires diffusion across the interstitial space between alveoli and pulmonary capillaries. Under normal conditions, there is sufficient time for the pulmonary capillary blood to equilibrate with alveolar gas across the interstitial space. When the interstitial space is filled with inflammatory cells or fluid, diffusion is impaired. Because the diffusion capacity of CO 2 is 20 times greater than that of O 2 , diffusion defects manifest as hypoxemia rather than hypercarbia. Even with the administration of 100% oxygen, P ao 2 increases to approximately 660 mm Hg from 100 mm Hg at sea level, and the concentration gradient for diffusion of O 2 is increased by only 6.6 times. Therefore, with diffusion defects, lethal hypoxemia will set in before clinically significant CO 2 retention results. In fact, in such situations, Pa co 2 is often decreased because of the hyperventilation that accompanies hypoxemia. Presence of hypercarbia in diseases that impair diffusion is indicative of alveolar hypoventilation from coexisting airway obstruction, exhaustion, or CNS depression. Examples of disease that impair diffusion are interstitial pneumonia, ARDS, scleroderma, and pulmonary lymphangiectasia.
It cannot be overemphasized that clinical observation is the most important component of monitoring. The presence and magnitude of abnormal clinical findings, their progression with time, and their temporal relation to therapeutic interventions serve as guides to diagnosis and management (see Chapter 400 ). As much as possible, the child with respiratory distress or failure should be observed in the position of greatest comfort and in the least threatening environment.
Pulse oximetry is the most commonly used technique to monitor oxygenation. Noninvasive and safe, it is the standard of care in bedside monitoring of children during transport, procedural sedation, surgery, and critical illness. It indirectly measures arterial hemoglobin-O 2 saturation by differentiating oxyhemoglobin from deoxygenated hemoglobin using their respective light absorption at wavelengths of 660 nm (red) and 940 nm (infrared). A pulsatile circulation is required to enable detection of oxygenated blood entering the capillary bed. Percentage of arterial oxyhemoglobin is reported as Sa o 2 ; however, the correct description is oxyhemoglobin saturation as measured by pulse oximetry (Sp o 2 ). Such precision is needed because Sp o 2 may not always reflect Sa o 2 . It is important to be familiar with the hemoglobin-O 2 dissociation curve (see Chapter 400 ) to estimate Pa o 2 at a given oxyhemoglobin saturation. Because of the shape of the hemoglobin-O 2 dissociation curve, changes in Pa o 2 above 70 mm Hg are not readily identified by pulse oximetry. Also, at the same Pa o 2 , there may be significant change in Sp o 2 at a different blood pH value. In most situations, Sp o 2 >95% is a reasonable goal, especially in emergency care. In some adult studies of ARDS, the recommended saturation is 94–96% to avoid oxygen toxicity. There are exceptions, such as in patients with single-ventricle cardiac lesions, in whom the pulmonary and systemic circulations are receiving blood flow from the same ventricle (e.g., after Norwood procedure for hypoplastic left heart syndrome), or with large left-to-right shunts (e.g., ventricular septal defect, patent ductus arteriosus). In these types of pathophysiologic situations, a lower Sp o 2 is desired to avoid excessive blood flow to the lungs and pulmonary edema from the pulmonary vasodilatory effects of oxygen, in the patient with a single ventricle, diverting blood flow away from the systemic circulation. Because most commercially available pulse oximeters recognize all types of hemoglobin as either oxyhemoglobin or deoxygenated hemoglobin, they provide inaccurate information in the presence of carboxyhemoglobin and methemoglobin. In carbon monoxide poisoning, carboxyhemoglobin absorbs light in the same (red) wavelength as oxyhemoglobin, leading to overestimation of oxygen saturation. Methemoglobin absorbs light in both the oxygenated and deoxygenated wavelengths, which can cause either an overestimation or underestimation of oxygen saturation. Data suggest that increasing methemoglobin concentrations tend to drive Sp o 2 toward 85%, no matter the actual percent of oxyhemoglobin. At lower methemoglobin levels, the pulse oximetry reading is falsely low, whereas high levels lead to a falsely high pulse oximetry reading. Newer pulse oximeters may have the ability to distinguish dyshemoglobinemias and to prevent false readings, but these are not currently in widespread use. It should be recognized that dangerous levels of hypercarbia may exist in patients with ventilatory failure, who have satisfactory Sp o 2 if they are receiving supplemental oxygen. Pulse oximetry should not be the only monitoring method in patients with primary ventilatory failure, such as neuromuscular weakness and CNS depression. It is also unreliable in patients with poor perfusion and poor pulsatile flow to the extremities. Despite these limitations, pulse oximetry is a noninvasive, easily applicable, and effective means of evaluating the percentage of oxyhemoglobin in most patients.
Volumetric capnography (end-tidal CO 2 [Pet co 2 ] measurement) is helpful in non-invasively determining the effectiveness of ventilation and pulmonary circulation. The Pet co 2 can be used to determine the alveolar dead space fraction and is calculated as follows: [(Pa co 2 − Pet co 2 )/Pa co 2 ]. Changes in the alveolar dead space fraction usually correlate well with changes in the gradient of Pa co 2 and Pet co 2 (Pa co 2 – Pet co 2 ). Thus a change in Pa co 2 – Pet co 2 can be used as an index of changes in alveolar dead space. In healthy children the gradient is smaller than in adults and is usually <3 mm Hg. Diseases resulting in increased alveolar dead space (e.g., dynamic hyperinflation) or decreased pulmonary blood flow (e.g., pulmonary embolism, low cardiac output) lead to decreases in Pet co 2 and an increase in Pa co 2 – Pet co 2 . Pet co 2 alone may overestimate adequacy of ventilation.
Arterial blood gas analysis offers valuable assistance in diagnosis, monitoring, and management of a child in respiratory distress and failure. Because of technical difficulties in obtaining an arterial sample in children, a capillary blood gas (CBG) sample is most often obtained in emergency situations. A properly arterialized CBG sample obtained by warming the digit and obtaining free-flowing blood is acceptable. The blood sample needs to be processed without delay. CBG provides a good estimate of Pa co 2 and arterial pH, but less so for Pa o 2 . In patients who mainly require monitoring of ventilation (especially those whose oxygenation is being monitored with pulse oximetry) a venous blood gas sample provides reliable estimate of arterial pH and Pa co 2 values, provided tissue perfusion is reasonably adequate. Venous P co 2 (Pv co 2 ) is approximately 6 mm Hg higher and pH approximately 0.03 lower than the arterial values. Pv o 2 has a poor correlation with Pa o 2 . Mixed venous O 2 saturation obtained from a central venous catheter in the right atrium is an excellent marker of the balance between oxygen delivery and oxygen consumption. In patients with a constant arterial O 2 content and O 2 consumption, mixed venous O 2 saturation offers valuable information about cardiac output.
Blood gas analysis is important not only for determining the adequacy of oxygenation and ventilation but also for determining site of respiratory pathology and planning treatment (see Chapter 400 ). Briefly, in the presence of pure alveolar hypoventilation (e.g., airway obstruction above carina, decreased CO 2 responsiveness, neuromuscular weakness), the blood gas will show respiratory acidosis with an elevated Pa co 2 but a relative sparing of oxygenation. V̇/Q̇ mismatch (peripheral airway obstruction, bronchopneumonia) will be reflected in increasing hypoxemia and variable levels of Pa co 2 (low, normal, high) depending on severity of disease. Intrapulmonary right-to-left shunting and diffusion defects (alveolar-interstitial diseases such as pulmonary edema, ARDS) will be associated with a large A-a o 2 gradient and hypoxemia with relative sparing of CO 2 elimination, unless there is coincident fatigue or CNS depression.
It is crucial to analyze the magnitude and appropriateness of changes in pH, Pa co 2 , and bicarbonate concentration ([HCO 3 − ]) because they provide useful clues to the underlying pathophysiology and presence of more than 1 disorder. To do so, it is useful to assume baseline values of pH 7.40, Pa co 2 40 mm Hg, and [HCO 3 − ] 24 mEq/L. Newborns have lower renal threshold for bicarbonate and therefore have slightly different baseline values of pH 7.38, Pa co 2 35 mm Hg, and [HCO 3 − ] 20 mEq/L.
Patients with metabolic acidosis have decreased pH resulting from decreased serum [HCO 3 − ]. Chemoreceptor stimulation results in hyperventilation and respiratory compensation that may clinically manifest as respiratory distress. Normal compensation does not completely correct the pH but rather minimizes a change in pH that would otherwise occur without compensation. The adequacy of respiratory compensation is judged by the extent of the decline in Pa co 2 in response to the decline in [HCO 3 − ] or pH. A normal compensation for metabolic acidosis results in a fall in Pa co 2 by 1.2 mm Hg for every 1 mEq/L fall in [HCO 3 − ]. The most commonly used method to analyze the adequacy of respiratory compensation is Winter's formula:
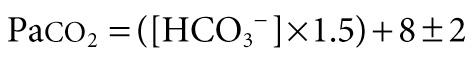
A quick method is to look at the last 2 digits of pH (provided it is not <7.10), which should be within 2 mm Hg of Pa co 2 . For example, pH 7.27, Pa co 2 26 mm Hg, and [HCO 3 − ] 12 mEq/L represents metabolic acidosis with a normal respiratory compensation response. On the other hand, pH 7.15, Pa co 2 30 mm Hg, and [HCO 3 − ] 10 mEq/L constitutes metabolic acidosis with inadequate respiratory compensation. The reasons for inadequate compensation include decreased CO 2 responsiveness (e.g., narcotic poisoning, cerebral edema), abnormalities of lungs and airways, or neuromuscular weakness. A decrease in Pa co 2 that is greater than what could be expected as a normal compensatory response to metabolic acidosis is indicative of a mixed disorder. A pH 7.20, Pa co 2 15 mm Hg, and [HCO 3 − ] 7.5 mEq/L represents metabolic acidosis with a concomitant respiratory alkalosis because the decline in Pa co 2 is greater than what can be expected as normal compensation. Combination of metabolic acidosis and respiratory alkalosis is often encountered in serious conditions such as cardiogenic shock (e.g., anxiety, stimulation of baroreceptors), sepsis, or toxic-metabolic states (e.g., salicylates, organic acidemia).
Patients with respiratory acidosis have decreased pH as a result of elevated Pa co 2 . An acute increase in Pa co 2 of 10 mm Hg results in a decrease in pH by 0.08. Thus a child with severe status asthmaticus and a Pa co 2 of 60 mm Hg will have blood pH of approximately 7.24. Chronically elevated (>3-5 days) Pa co 2 is accompanied by renal compensation and increase in serum [HCO 3 − ], limiting the fall in pH to 0.03 for every 10 mm Hg rise in Pa co 2 . Thus an infant with bronchopulmonary dysplasia who has a basal Pa co 2 of 60 mm Hg will have blood pH of approximately 7.34. These findings are helpful in distinguishing acute from chronic changes in Pa co 2 . Also, for a given level of CO 2 accumulation, a decrease in pH that is greater than expected is indicative of concomitant metabolic acidosis, and a decline in pH that is less than expected is caused by accompanying metabolic alkalosis.
For standardizing management, following clinical progress, and determining prognosis for patients with defects in oxygenation or ventilation, the following indicators have been proposed, each with its strengths and limitations:
A-a o 2 gradient is calculated by the subtraction, P ao 2 − Pa o 2 . For the comparison to be valid, both values must be taken at the same time and with the same fraction of oxygen in the inspired gas (F io 2 ).
Pa o 2 /F io 2 ratio (P/F) is calculated by dividing Pa o 2 by F io 2 . In hypoxic respiratory failure, a Pa o 2 /F io 2 value <300 mm Hg is consistent with acute lung injury, and a value <200 mm Hg is consistent with ARDS. Although the intent is to measure V̇/Q̇ mismatch, intrapulmonary shunt, and diffusion defect, the status of alveolar hypoventilation could have a significant impact on Pa o 2 /F io 2 .
Sp o 2 /F io 2 ratio is a surrogate measure of oxygenation when Pa o 2 is not available. It is calculated by dividing the pulse oximeter saturation by the F io 2 . P/F ratios of 200 mm Hg and 300 mm Hg correlate approximately with S/F ratios of 235 and 315 respectively. This relationship is most valid for Sp o 2 values between 80% and 97%.
Pa o 2 /P ao 2 ratio is determined by dividing Pa o 2 by P ao 2 . The level of alveolar ventilation is accounted for in the calculation of Pa o 2 . Therefore, Pa o 2 /P ao 2 is more indicative of V̇/Q̇ mismatch and alveolar capillary integrity.
Oxygenation index (OI) is aimed at standardizing oxygenation to the level of therapeutic interventions, such as mean airway pressure (MAP) and F io 2 used during mechanical ventilation, which are directed toward improving oxygenation. None of the indicators of oxygenation mentioned above takes into account the degree of positive pressure respiratory support.
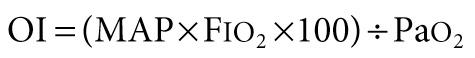
The limitation of OI is that level of ventilation is not accounted for in the assessment.
Ventilation index (VI) is aimed at standardizing alveolar ventilation to the level of therapeutic interventions, such as peak inspiratory pressure (PIP), positive end-expiratory pressure (PEEP), and ventilator rate ([R) directed toward lowering Pa co 2 .

The goal of management for respiratory distress and respiratory failure is to ensure a patent airway and provide necessary support for adequate oxygenation of the blood and removal of CO 2 . Compared with hypercapnia, hypoxemia is a life-threatening condition; therefore initial therapy for respiratory failure should be aimed at ensuring adequate oxygenation.
Supplemental oxygen administration is the least invasive and most easily tolerated therapy for hypoxemic respiratory failure. Nasal cannula oxygen provides low levels of oxygen supplementation and is easy to administer. Oxygen is humidified in a bubble humidifier and delivered via nasal prongs inserted in to the nares. In children, a flow rate <5 L/min is most often used because of increasing nasal irritation with higher flow rates. A common formula for an estimation of the F io 2 during use of a nasal cannula in older children and adults follows:
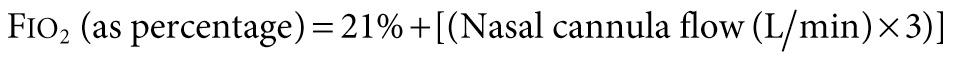
The typical F io 2 value (expressed as percentage rather than fraction of 1) using this method is between 23% and 40%, although the F io 2 varies according to the size of the child, the respiratory rate, and the volume of air moved with each breath. In a young child, because typical nasal cannula flow rates are a greater percentage of total minute ventilation, significantly higher F io 2 may be provided. Alternately, a simple mask may be used, which consists of a mask with open side ports and a valveless oxygen source. Variable amounts of room air are entrained through the ports and around the side of the mask, depending on the fit, size, and minute volume of the child. Oxygen flow rates vary from 5-10 L/min, yielding typical F io 2 values (expressed as percentage rather than fraction of 1) between 30% and 65%. If more precise delivery of oxygen is desired, other mask devices should be used.
A Venturi mask provides preset F io 2 through a mask and reservoir system by entraining precise flow rates of room air into the reservoir along with high-flow oxygen. The adapter at the end of each mask reservoir determines the flow rate of entrained room air and the subsequent F io 2 . (Adapters provide F io 2 of 0.30-0.50.) Oxygen flow rates of 5-10 L/min are recommended to achieve the desired F io 2 and to prevent rebreathing. Partial rebreather and non-rebreather masks use a reservoir bag attached to a mask to provide higher F io 2 . Partial rebreather masks have 2 open exhalation ports and contain a valveless oxygen reservoir bag. Some exhaled gas can mix with reservoir gas, although most exhaled gas exits the mask via the exhalation ports. Through these same ports, room air is entrained, and the partial rebreather mask can provide F io 2 up to 0.60, for as long as oxygen flow is adequate to keep the bag from collapsing (typically 10-15 L/ min). As with nasal cannulas, smaller children with smaller tidal volumes entrain less room air, and their Fio 2 values will be higher. Non-rebreather masks include 2 one-way valves, 1 between the oxygen reservoir bag and the mask and the other on 1 of the 2 exhalation ports. This arrangement minimizes mixing of exhaled and fresh gas and entrainment of room air during inspiration. The 2nd exhalation port has no valve, a safeguard to allow some room air to enter the mask in the event of disconnection from the oxygen source. A non-rebreather mask can provide F io 2 up to 0.95. The use of a non-rebreather mask in conjunction with an oxygen blender allows delivery of F io 2 between 0.50 and 0.95 ( Table 89.8 ). When supplemental oxygen alone is inadequate to improve oxygenation, or when ventilation problems coexist, additional therapies may be necessary.
| DEVICE | FLOW (L/min) | F io 2 DELIVERED |
|---|---|---|
| Nasal cannula | 0.1-6 | 0.21-0.4 |
| Simple face mask | 5-10 | 0.4-0.6 |
| Partial rebreather | 6-15 | 0.55-0.7 |
| Non-rebreather | 6-15 | 0.7-0.95 |
| Venturi mask | 5-10 | 0.25-0.5 |
| Hood/tent | 7-12 | 0.21-1.0 |
| High-flow systems | 1-40 | 0.21-1.0 |
* Individual delivery varies and depends on the patient's size, respiratory rate, and volume moved with every breath.
Maintenance of a patent airway is a critical step in maintaining adequate oxygenation and ventilation. Artificial pharyngeal airways may be useful in patients with oropharyngeal or nasopharyngeal airway obstruction and in those with neuromuscular weakness in whom inherent extrathoracic airway resistance contributes to respiratory compromise. An oropharyngeal airway is a stiff plastic spacer with grooves along each side that can be placed in the mouth to run from the teeth along the tongue to its base just above the vallecula. The spacer prevents the tongue from opposing the posterior pharynx and occluding the airway. Because the tip sits at the base of the tongue, it is usually not tolerated by patients who are awake or whose gag reflex is strong. The nasopharyngeal airway , or nasal trumpet , is a flexible tube that can be inserted into the nose to run from the nasal opening along the top of the hard and soft palate with the tip ending in the hypopharynx. It is useful in bypassing obstruction from enlarged adenoids or from contact of the soft palate with the posterior nasopharynx. Because it is inserted past the adenoids, a nasopharyngeal airway should be used with caution in patients with bleeding tendencies.
Helium-oxygen mixture (heliox) is useful in overcoming airway obstruction and improving ventilation. Helium is much less dense and slightly more viscous than nitrogen. When substituted for nitrogen, helium helps maintain laminar flow across an obstructed airway, decreases airway resistance, and improves ventilation. It is especially helpful in diseases of large airways obstruction in which turbulent airflow is more common, such as acute laryngotracheobronchitis, subglottic stenosis, and vascular ring. It is also used in patients with severe status asthmaticus. To be effective, helium should be administered in concentrations of at least 60%, so associated hypoxemia may limit its use in patients requiring >40% oxygen.
Inhaled nitric oxide (iNO) is a powerful inhaled pulmonary vasodilator. Its use may improve pulmonary blood flow and V̇/Q̇ mismatch in patients with diseases that elevate pulmonary vascular resistance, such as occurs in persistent pulmonary hypertension of the newborn, primary pulmonary hypertension, and secondary pulmonary hypertension as a result of chronic excess pulmonary blood flow (e.g., ventriculoseptal defect) or collagen vascular diseases. iNO is administered in doses ranging from 5 to 20 parts per million of inspired gas. Although administration of iNO to unintubated patients is possible, it is usually administered to patients undergoing mechanical ventilation via an endotracheal tube, because of the need for precision in iNO dosing.
Noninvasive positive pressure respiratory support is useful in treating both hypoxemic and hypoventilatory respiratory failure. Positive airway pressure helps with aeration of partially atelectatic or filled alveoli, prevention of alveolar collapse at end-exhalation, and increase in functional residual capacity (FRC). These actions improve pulmonary compliance and hypoxemia, as well as decrease intrapulmonary shunt. In addition, positive pressure ventilation is useful in preventing collapse of extrathoracic airways by maintaining positive airway pressure during inspiration. Improving compliance and overcoming airway resistance also improves tidal volume and therefore ventilation. A high-flow nasal cannula delivers gas flow at 4-16 L/min and up to 60 L/min, with newer systems for older children and adolescents, capable of providing significant continuous positive airway pressure (CPAP) . In this setting, the amount of CPAP provided is not quantifiable and varies with each patient, depending on the percentage of total inspiratory flow that is delivered from the cannula, airway anatomy, and degree of mouth breathing. In small children the relative amount of CPAP for a given flow is usually greater than in older children and may provide significant positive pressure. The F io 2 can be adjusted by provision of gas flow through an oxygen blender. Another benefit of a high-flow nasal cannula system is the washout of CO 2 from the nasopharynx, which decreases rebreathing of CO 2 and dead space ventilation. When delivering high-flow air or oxygen, adequate humidification is essential, by using a separate heated humidification chamber. CPAP can also be provided through snugly fitting nasal prongs or a tight-fitting face mask attached to a mechanical ventilator or other positive pressure device. Noninvasive CPAP is most useful in diseases of mildly decreased lung compliance and low FRC, such as atelectasis and pneumonia. Patients with diseases of extrathoracic airway obstruction, in which extrathoracic negative airway pressures during inspiration lead to airway narrowing (e.g., laryngotracheitis, obstructive sleep apnea, postextubation airway edema), may also benefit from CPAP. Potential risks include nasal irritation, hyperinflation from excessive CPAP in smaller patients, and abdominal distention from swallowed air.
Noninvasive positive airway pressure ventilation (NIPPV) provides positive airway pressure during exhalation, and bilevel modes can apply additional positive pressure during inspiration (see Chapter 89.1 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here