Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Understanding the physiology of neonatal respiratory control has served as an effective guide to common therapeutic approaches.
Both the acute and longer-term consequences of intermittent hypoxemia and apnea of prematurity are subjects of intense interest.
Intermittent hypoxemia may contribute to an inflammatory stress response.
Although caffeine is the mainstay of apnea therapy, there remains considerable controversy regarding optimal treatment regimens.
Innovative newer approaches to stabilize respiratory control and improve lung function may hold promise as future interventions.
There are a multitude of reasons why immaturity in respiratory control is of interest to scientists and clinicians alike. From a biological perspective, it represents a unique link between the developing respiratory and central nervous systems. The resultant apnea precipitates repetitive hypoxemia episodes, and hence, preterm infants serve as a novel biological model for studying the consequences of such episodic hypoxemia. From a clinical perspective, the combination of immature respiratory control, an immature lung, and the resultant therapeutic ventilatory support predisposes these infants to chronic respiratory morbidity. Finally, there is a need to optimize and provide safe pharmacotherapy that enhances respiratory neural output in this high-risk population of neonates. These are some of the current high-profile issues and controversies that this review will address.
The ability to challenge respiratory neural output with hypoxic or hypercapnic exposures is quite limited in human infants. Therefore, one must rely on older studies to better understand the maturation of peripheral and central components of chemoreception. We are also very dependent on neonatal animal models, particularly data derived from rodents, although, unfortunately, such models rarely exhibit long spontaneous apnea or periodic breathing as seen in preterm infants.
The neural circuitry that generates respiratory rhythm and governs inspiratory and expiratory motor patterns is distributed throughout the pons and medulla. The medulla contains a specialized region known as the pre-Bötzinger complex, which contains neurons that exhibit intrinsic pacemaker activity capable of producing rhythmic respiratory motor output without sensory feedback. Although a fundamental feature of this network is that it enables breathing to occur automatically, this systematic central rhythmicity may fail in preterm infants. Meanwhile, central and peripheral sensory inputs from multiple sources allow adjustments to the patterns of inspiratory and expiratory activity in response to changing metabolic conditions. For example, inhibitory sensory inputs from the upper airway may be particularly prominent in early postnatal life to serve a protective function, although this may trigger potentially clinically significant apnea.
A poorly understood concept is the relationship between periodic breathing, i.e., repetitive cycles of respiratory output and pauses of approximately 5 to 10 seconds’ duration, and apneic episodes typically of 10 to 20 seconds’ duration that do not exhibit a cyclic pattern. Available data suggest that periodic breathing occurs predominantly in quiet sleep while apnea is more common in active sleep. This would suggest a different central or peripheral biological basis for those breathing patterns as discussed later.
Excitatory and inhibitory neurotransmitters and neuromodulators mediate the rhythmogenic synaptic communications between neurons of the medulla. Glutamate, acting on AMPA and NMDA receptors, is the major neurotransmitter mediating excitatory synaptic input to brainstem respiratory neurons. Gamma-aminobutyric acid (GABA) and glycine are the two primary inhibitory neurotransmitters in the network, mediating the waves of inhibitory postsynaptic potentials during the silent phase of respiratory neurons. Interestingly, during late embryonic and early postnatal development, GABA and glycine can mediate excitatory neurotransmission secondary to changes in the chloride gradient across the membrane. It is unclear how this phenomenon relates to the inhibition of respiratory output and resultant apnea seen in preterm infants. Neonatal rodent data suggest that caffeine, which is a nonselective adenosine receptor inhibitor, may block excitatory A 2A receptors at GABAergic neurons and so inhibit GABA output and contribute to the ability of caffeine to enhance respiratory drive. Serotonin may be of particular importance in the modulation of respiratory function. Serotonergic neurons and their projections may represent the neuroanatomic substrate for the integration of cardiorespiratory responses. Defects in the medullary serotonergic system likely contribute importantly to the pathogenesis of sudden infant death syndrome (SIDS). For future advances in the pharmacotherapy of neonatal apnea, greater understanding of the maturation of these neurotransmitters/neuromodulators is imperative.
Responsiveness to CO 2 is the major chemical driver of respiratory neural output. This is apparent in fetal life where breathing movements increase under hypercapnic conditions in animal models. As in later life, CO 2 /H + responsiveness is predominantly based in the brainstem, although peripheral chemoreceptors contribute to the ventilatory response and respond more rapidly. The reduced ventilatory response to carbon dioxide in small preterm infants, especially those with apnea, is primarily the result of decreased central chemosensitivity; however, mechanical factors such as poor respiratory function and an unstable, compliant chest wall may contribute. It is difficult to distinguish the neural from mechanical factors that contribute to respiratory failure in this population.
It has been known for many years that preterm infants respond to a fall in inspired oxygen concentration with a transient increase in ventilation over approximately 1 minute, followed by a return to baseline or even depression of ventilation. The characteristic response to low oxygen in infants appears to result from initial peripheral chemoreceptor stimulation, followed by overriding depression of the respiratory center as a result of hypoxemia. Such hypoxic respiratory depression may be useful in the hypoxic intrauterine environment where respiratory activity is only intermittent and not contributing to gas exchange. The nonsustained response to low inspired oxygen concentration may, however, be a disadvantage postnatally. Decreased peripheral chemoreceptor responsiveness to oxygen or central hypoxic depression of respiratory neural output may impair recovery from apnea. In contrast, excessive peripheral chemosensitivity has also been shown to compromise ventilatory stability and predispose to periodic breathing and even apnea in preterm infants. , Periodic breathing is thought to result from a combination of dominant peripheral chemosensitivity combined with a CO 2 level close to the apneic threshold, resulting in the characteristic cycles of breaths and pauses. These findings are consistent with the observed postnatal delay in onset of periodic breathing as peripheral chemoreceptors may be silenced in the initial postnatal period. ,
It is well recognized that apnea may be the first indication of neonatal sepsis. There is also considerable current biological interest in the role of inflammation on respiratory neural output at both central and peripheral levels. Although inflammatory cytokines probably do not readily cross the blood-brain barrier, systemic infection does upregulate inflammatory cytokines at the blood-brain barrier, resulting in activation of prostaglandin signaling and resultant inhibition of respiratory neural output. Chorioamnionitis is a major precipitant of preterm birth. It is possible that antenatal or postnatal exposure of the lung to a proinflammatory stimulus may activate brain circuits that destabilize respiratory neural output. In neonatal rodents, there is a response of proinflammatory cytokine gene expression in the brainstem after intrapulmonary lipopolysaccharide exposure, which is partially vagally mediated. This is accompanied by significant ventilatory depression to hypoxic exposure. An interesting related line of investigation is the role of intermittent hypoxia and resultant oxidant stress on inflammatory pathways that regulate respiratory neural output. Much work needs to be done to explore these potential interrelationships between impaired respiratory neural output and inflammation, intermittent hypoxemia (IH), and any resultant oxidant stress as discussed later.
During early postnatal life, apneic events are ubiquitous; they can vary widely in duration and are often accompanied by bradycardia and/or intermittent hypoxemia. Accordingly, American Academy of Pediatrics (AAP) guidelines have historically defined clinical apnea of prematurity as a respiratory pause of >20 seconds or shorter if accompanied by hypoxemia (O 2 sat <80–85%) and/or bradycardia (<80 bpm). It should be noted that even short respiratory pauses, approximating 10 seconds or less, may be associated with hypoxemia and/or bradycardia. Apnea is categorized as (1) central—with loss of central respiratory drive resulting in complete cessation of flow and absence of respiratory effort; (2) obstructive—with absence of flow in the presence of respiratory efforts; and (3) mixed—consisting of both central and obstructive components. Mixed apnea accounts for ∼40% to 50% of all events in preterm infants , and is often initiated by a loss of central drive, followed by a delay in resolution due to upper airway closure. However, airway narrowing and/or collapse, as measured by cardiac signal transmission on the flow waveform, can also occur during central apnea. Unfortunately, standard impedance monitoring, which reflects chest wall motion, may fail to differentiate obstructed versus unobstructed inspiratory efforts.
The true incidence of apnea during early postnatal life has been grossly underestimated due to the historical practice of using nursing documentation, shown to underreport the true frequency of clinical events by >50%. More accurate pneumogram recordings have revealed a high incidence of cardiorespiratory events even in convalescing infants. For example, in very low-birth-weight (VLBW) neonates, 91% of pneumograms performed within 72 hours of anticipated discharge revealed apnea accompanied by a fall in heart rate or oxygen saturation. This has been supported in an expanded cohort of 1211 infants <35 weeks’ gestation showing that preterm infants continue to experience short apnea with bradycardia and/or hypoxemia in the week prior to discharge. It is, therefore, not surprising that hospitalization is frequently prolonged due to concern about persistent cardiorespiratory events. NICU EMR databases are beginning to incorporate automated detection of apnea/bradycardia/hypoxemia in contrast to manual entries by the nursing staff. Since hospital discharge is often driven by the presence (or absence) of events, it is unclear how the anticipated increased frequency of documentation with automated bedside monitoring may affect the duration of hospitalization.
In extremely preterm infants, intermittent hypoxemia (IH) events are pervasive and transient during early postnatal life with a relatively low incidence during the first week of life, followed by a rapid increase during the second and third weeks and a plateau or decrease thereafter. Intermittent hypoxemia events are almost always preceded by body movement or a respiratory pause. The initiation, duration, and severity of IH can be influenced by many factors including baseline oxygen saturation, oxygen diffusion in the lung pulmonary oxygen stores, total blood oxygen carrying capacity, the slope of the hemoglobin oxygen dissociation curve, cardiac shunts, and oxygen consumption.
Intermittent hypoxemia events in neonates have been traditionally attributed to hypoventilation due to central or obstructive apnea, but these mechanisms mainly apply to spontaneously breathing infants. The occurrence of spontaneous IH in mechanically ventilated infants is often perplexing because they occur in spite of continued cycling of the ventilator and patency of the airway. These episodes of hypoxemia are characterized by a rapid decline in oxygen saturation (SpO 2 ) that become more frequent with advancing postnatal age. , ,
One of the most common mechanisms leading to spontaneous IH events in mechanically ventilated preterm infants consists of forced exhalations secondary to contractions of the abdominal musculature that impinge on the respiratory system and impair respiratory mechanics. The resultant decrease in lung volume and hypoventilation cause hypoxemia that becomes more severe and persistent with successive abdominal contractions. , ,
Electromyographic measurements show that these forced exhalations are caused by contractions of the abdominal muscles that produce a marked increase in abdominal and intrathoracic pressure, some of them in excess of 25 cm H 2 O. As shown in Fig. 8.1 , repeated contractions of the abdominal muscles can prolong the hypoxemia episodes and increase their severity. It is important to note that in mechanically ventilated infants, the endotracheal tube bypasses the glottis and eliminates the protective upper airway’s function to preserve lung volume during the rise in intrathoracic pressure.
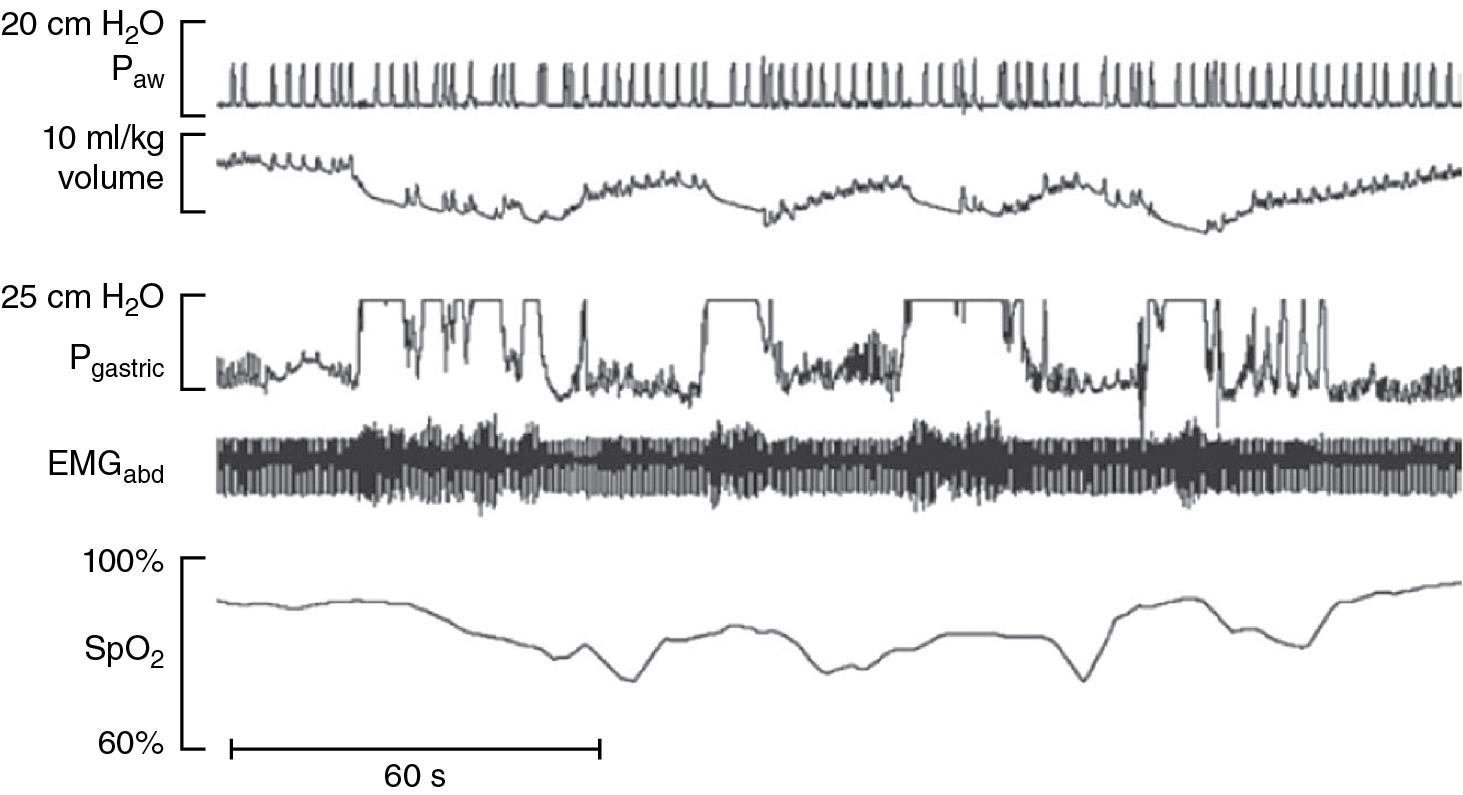
The factors that elicit these forced exhalations leading to loss in lung volume and hypoventilation with hypoxemia have not been clearly defined. However, behavioral disturbances appear to trigger IH. Increased body activity, agitation, squirming, and tachycardia are frequently present moments prior to the onset of the episodes. Increased frequency of IH has been observed during awake or indeterminate sleep states than in quiet or active sleep. This is important because indeterminate sleep is the most common sleep state in preterm infants. These observations correlate with the observed increased frequency of IH during the day compared to nighttime in mechanically ventilated extreme premature infants. The higher daytime IH frequency is likely related to increased patient activity accompanied by disrupted sleep as well as by more negative stimulation associated with routine care in the NICU. Prone position has also been associated with fewer and shorter IH episodes compared to supine. ,
The combination of increased activity leading to ventilation changes and poor lung function due to the underlying lung disease may aggravate the frequency and severity of IH. In addition, the low functional residual capacity and decreased lung compliance characteristic of mechanically ventilated infants combined with bypassing of the glottis by the endotracheal tube may increase the likelihood of reaching closing volume in some areas of the lung with even small decreases in lung volume.
The decline in SpO 2 during these episodes of hypoxemia is more abrupt than what is expected from a decline in ventilation alone and often persist after ventilation has been reestablished. This observation suggests that the initial loss in lung volume and hypoventilation may produce ventilation-perfusion inequalities and some degree of intrapulmonary shunting causing a rapid decline in SpO 2 . The onset of hypoxemia can also provoke an increase in pulmonary vascular resistance and induce right-to-left shunting through extrapulmonary channels. These circulatory changes can explain why episodes of IH are frequently observed in infants with chronic lung disease and increased pulmonary vascular reactivity. In many of these infants, normoxemia is restored only after the fraction of inspired oxygen (FiO 2 ) is increased, which may restore oxygenation not only by increasing the alveolar-capillary oxygen gradient but also by attenuating the hypoxia-induced pulmonary vasoconstriction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here