Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Our bodies are exposed continually to bacteria, viruses, fungi, and parasites, all of which occur normally and to varying degrees in the skin, mouth, respiratory passageways, intestinal tract, lining membranes of the eyes, and even the urinary tract. Many of these infectious agents are capable of causing serious abnormal physiological function or even death if they invade deeper tissues. We are also exposed intermittently to other highly infectious bacteria and viruses besides those that are normally present, and these agents can cause acute lethal diseases such as pneumonia, streptococcal infection, and typhoid fever.
Our bodies have a special system for combating the different infectious and toxic agents. This system is composed of blood leukocytes (white blood cells [WBCs]) and tissue cells derived from leukocytes. These cells work together in two ways to prevent disease: (1) by actually destroying invading bacteria or viruses by phagocytosis; and (2) by forming antibodies and sensitized lymphocytes that may destroy or inactivate the invader. This chapter discusses the first of these methods, and Chapter 35 discusses the second.
The leukocytes, also called white blood cells , are the mobile units of the body’s protective system. They are formed partially in the bone marrow ( granulocytes and monocytes and a few lymphocytes ) and partially in the lymph tissue ( lymphocytes and plasma cells ). After formation, they are transported in the blood to different parts of the body where they are needed.
The real value of WBCs is that most of them are specifically transported to areas of serious infection and inflammation, thereby providing a rapid and potent defense against infectious agents. As we see later, the granulocytes and monocytes have a special ability to “seek out and destroy” a foreign invader.
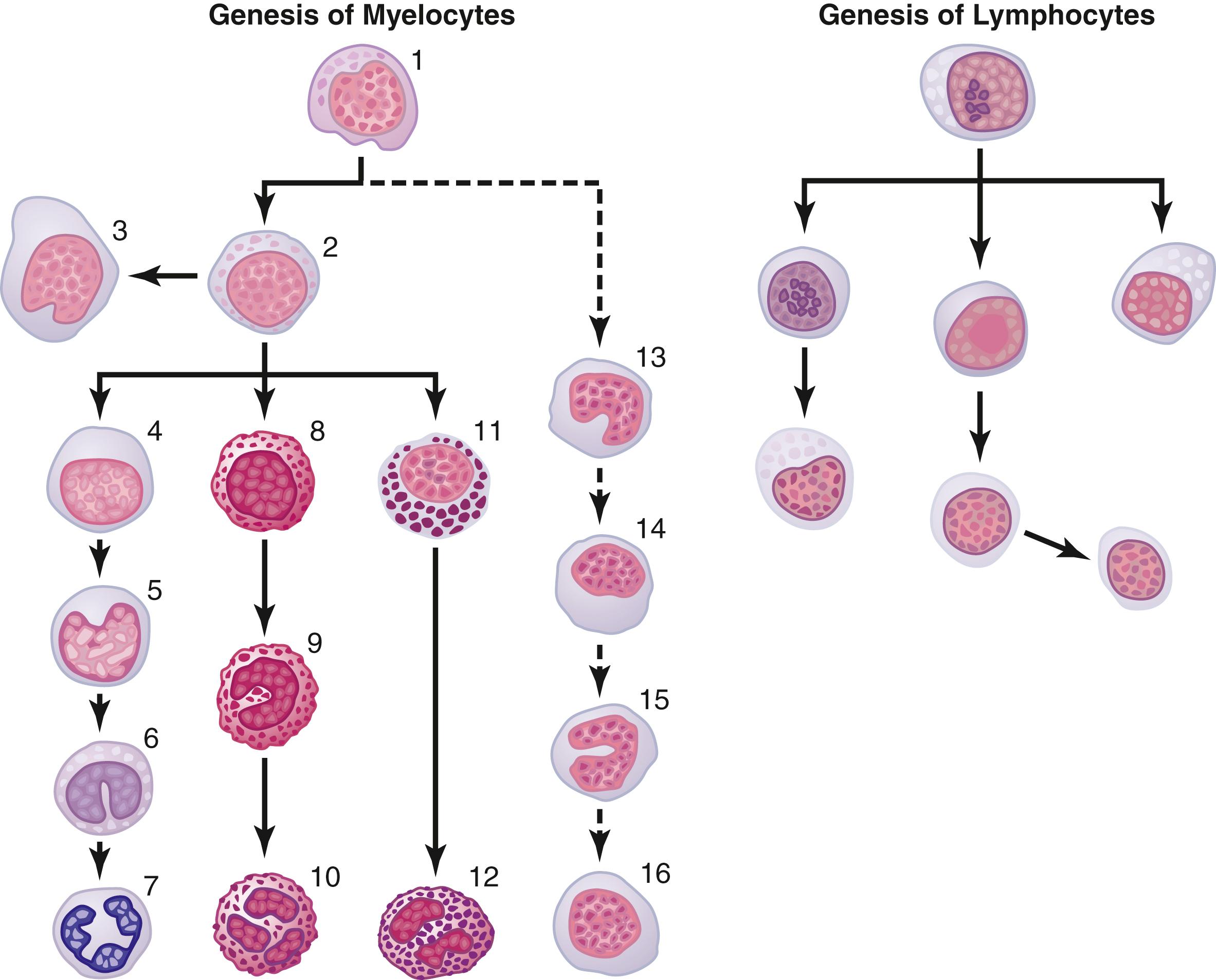
Six types of WBCs are normally present in the blood: neutrophils (polymorphonuclear), eosinophils ( polymorphonuclear), basophils ( polymorphonuclear), monocytes, lymphocytes and, occasionally, plasma cells . In addition, there are large numbers of platelets, which are fragments of another type of cell similar to the WBCs found in the bone marrow, the megakaryocyte . The first three types of cells, the polymorphonuclear cells, all have a granular appearance, as shown in cell numbers 7, 10, and 12 in Figure 34-1 , and for this reason they are called granulocytes .
The granulocytes and monocytes protect the body against invading organisms by ingesting them (by phagocytosis ) or by releasing antimicrobial or inflammatory substances that have multiple effects that aid in destroying the offending organism. The lymphocytes and plasma cells function mainly in connection with the immune system, as discussed in Chapter 35 . Finally, the function of platelets is specifically to activate the blood-clotting mechanism, discussed in Chapter 37 .
An adult human has about 7000 WBCs per microliter of blood (in comparison with 5 million red blood cells [RBCs] per microliter). Of the total WBCs, the normal percentages of the different types are approximately the following:
Neutrophils:62.0%
Eosinophils:2.3%
Basophils:0.4%
Monocytes:5.3%
Lymphocytes:30.0%
The number of platelets, which are only cell fragments, in each microliter of blood is normally between 150,000 and 450,000, averaging about 300,000.
Early differentiation of the multipotential hematopoietic stem cell into the different types of committed stem cells was shown in Figure 33-2 in the previous chapter. Aside from the cells committed to form RBCs, two major lineages of WBCs are formed, the myelocytic and lymphocytic lineages. The left side of Figure 34-1 shows the myelocytic lineage , beginning with the myeloblast; the right side shows the lymphocytic lineage , beginning with the lymphoblast .
The granulocytes and monocytes are formed only in the bone marrow. Lymphocytes and plasma cells are produced mainly in the various lymphogenous tissues—especially the lymph glands, spleen, thymus, tonsils, and various pockets of lymphoid tissue elsewhere in the body, such as in the bone marrow and in Peyer’s patches underneath the epithelium in the gut wall.
The WBCs formed in the bone marrow are stored in the marrow until they are needed in the circulatory system. Then, when the need arises, various factors cause them to be released (these factors are discussed later). Normally, about three times as many WBCs are stored in the marrow as circulate in the entire blood. This quantity represents about a 6-day supply of these cells.
The lymphocytes are mostly stored in the various lymphoid tissues, except for a small number that are temporarily being transported in the blood.
As shown in Figure 34-1 , megakaryocytes (cell 3) are also formed in the bone marrow. These megakaryocytes fragment in the bone marrow and the small fragments, known as platelets (or thrombocytes ), then pass into the blood. They are very important in the initiation of blood clotting.
The life of the granulocytes after being released from the bone marrow is normally 4 to 8 hours circulating in the blood and another 4 to 5 days in tissues where they are needed. In times of serious tissue infection, this total life span is often shortened to only a few hours because the granulocytes proceed even more rapidly to the infected area, perform their functions, and in the process, are themselves destroyed.
The monocytes also have a short transit time, 10 to 20 hours in the blood, before wandering through the capillary membranes into the tissues. Once in the tissues, they swell to much larger sizes to become tissue macrophages and, in this form, they can live for months unless destroyed while performing phagocytic functions. These tissue macrophages are the basis of the tissue macrophage system (discussed in greater detail later), which provides continuing defense against infection.
Lymphocytes enter the circulatory system continually, along with drainage of lymph from the lymph nodes and other lymphoid tissue. After a few hours, they pass out of the blood back into the tissues by diapedesis/extravasation . Then, they re-enter the lymph and return to the blood again and again; thus, there is continual circulation of lymphocytes through the body. Lymphocytes have life spans of weeks or months, depending on the body’s need for these cells.
The platelets in the blood are replaced about once every 10 days. In other words, about 30,000 platelets are formed each day for each microliter of blood.
It is mainly the neutrophils and tissue macrophages that attack and destroy invading bacteria, viruses, and other harmful agents. The neutrophils are mature cells that can attack and destroy bacteria, even in the circulating blood. Conversely, the tissue macrophages begin life as blood monocytes, which are immature cells while still in the blood and have little ability to fight infectious agents at that time. However, once they enter the tissues, they begin to swell—sometimes increasing their diameters as much as fivefold—to as great as 60 to 80 micrometers, a size that can barely be seen with the naked eye. These cells are now called macrophages , and they are extremely capable of combating disease agents in the tissues.
Neutrophils and monocytes can squeeze through gaps between endothelial cells of the blood capillaries and postcapillary venules by diapedesis . Although the intercellular gaps are much smaller than a cell, a small portion of the cell slides through the gap at a time; the portion sliding through is momentarily constricted to the size of the gap, as shown in Figure 34-2 (also see Figure 34-6 ).
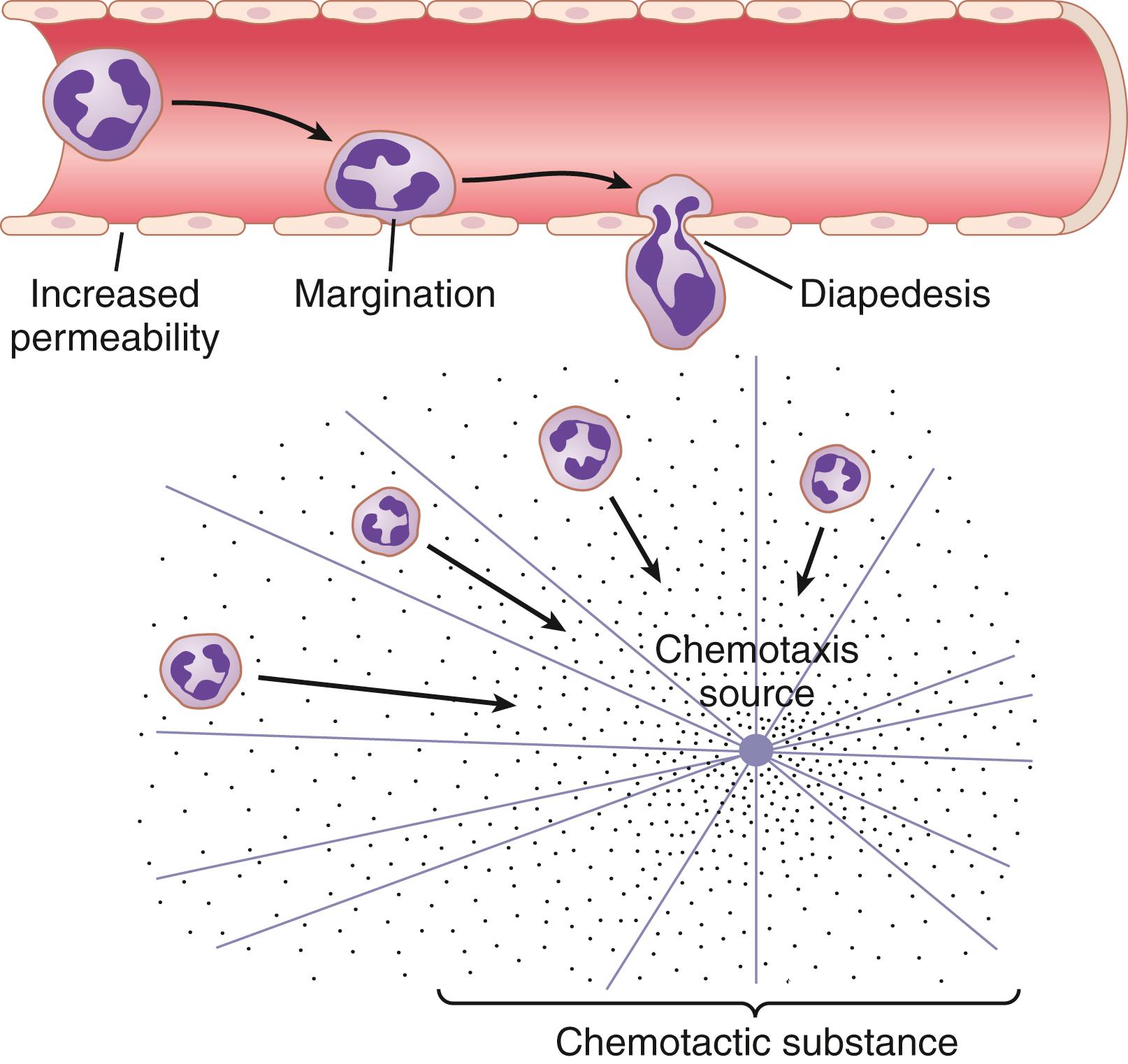
Both neutrophils and macrophages can move through the tissues by ameboid motion, described in Chapter 2 . Some cells move at velocities as great as 40 μm/min, a distance as great as their own length each minute.
Many different chemical substances in the tissues cause both neutrophils and macrophages to move toward the source of the chemical. This phenomenon, shown in Figure 34-2 , is known as chemotaxis . When a tissue becomes inflamed, at least a dozen different products that can cause chemotaxis toward the inflamed area are formed ( ). They include the following: (1) some of the bacterial or viral toxins; (2) degenerative products of the inflamed tissues; (3) several reaction products of the complement complex (discussed in Chapter 35 ) activated in inflamed tissues; and (4) several reaction products caused by plasma clotting in the inflamed area, as well as other substances.
As shown in Figure 34-2 , chemotaxis depends on the concentration gradient of the chemotactic substance. The concentration is greatest near the source, which directs the unidirectional movement of the WBCs. Chemotaxis is effective up to 100 micrometers away from an inflamed tissue. Therefore, because almost no tissue area is more than 50 micrometers away from a capillary, the chemotactic signal can easily move hordes of WBCs from the capillaries into the inflamed area.
A major function of the neutrophils and macrophages is phagocytosis, which means cellular ingestion of the offending agent. Phagocytes must be selective of the material that is phagocytized; otherwise, normal cells and structures of the body might be ingested. Whether phagocytosis will occur especially depends on three selective procedures ( Figure 34-3 ).
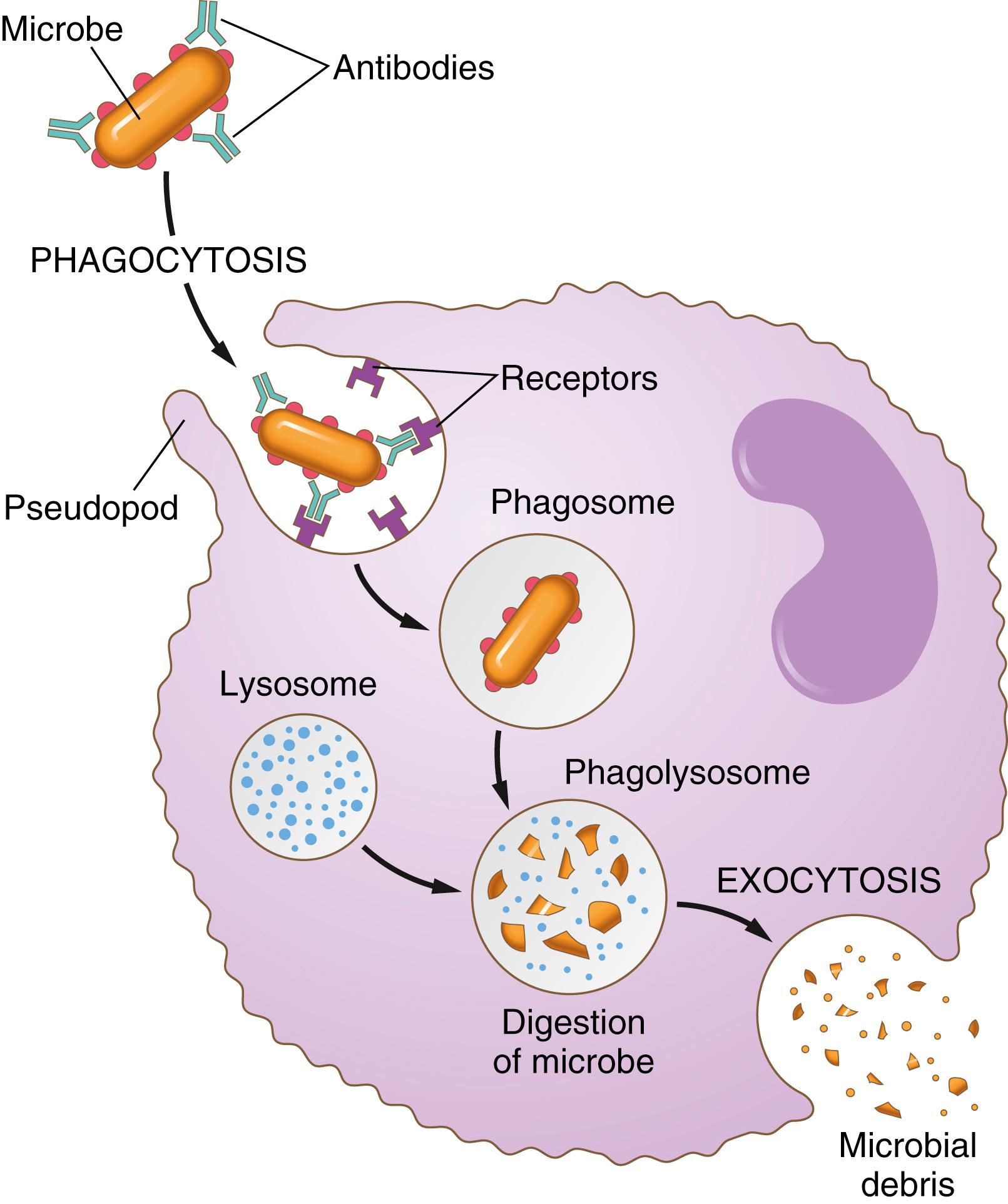
First, most natural structures in the tissues have smooth surfaces, which resist phagocytosis. However, if the surface is rough, the likelihood of phagocytosis is increased.
Second, most natural substances of the body have protective protein coats that repel the phagocytes. Conversely, most dead tissues and foreign particles have no protective coats, which makes them subject to phagocytosis.
Third, the immune system of the body (described in Chapter 35 ) develops antibodies against infectious agents such as bacteria. The antibodies then adhere to the bacterial membranes and thereby make the bacteria especially susceptible to phagocytosis. To do this, the antibody molecule also combines with the C3 product of the complement cascade , which is an additional part of the immune system discussed in the next chapter . The C3 molecules, in turn, attach to receptors on the phagocyte membrane, thus initiating phagocytosis. This process whereby a pathogen is selected for phagocytosis and destruction is called opsonization .
The neutrophils entering the tissues are already mature cells that can immediately begin phagocytosis. On approaching a particle to be phagocytized, the neutrophil first attaches itself to the particle and then projects pseudopodia in all directions around the particle. The pseudopodia meet one another on the opposite side and fuse. This action creates an enclosed chamber that contains the phagocytized particle. Then, the chamber invaginates to the inside of the cytoplasmic cavity and breaks away from the outer cell membrane to form a free-floating phagocytic vesicle (also called a phagosome ) inside the cytoplasm. A single neutrophil can usually phagocytize 3 to 20 bacteria before the neutrophil becomes inactivated and dies.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here