Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Living-donor hepatectomy is a major surgical operation performed on a healthy person only for the benefit of a recipient who requires liver transplantation (see Chapters 105 , 125 , and 128 ). In 1989 Strong (1999) performed donor left hepatectomy and removed segment IV of the liver on the back table before implantation of segments II and III into a pediatric recipient. In 1990 Tanaka et al. improvised using a right liver graft for a pediatric recipient because of unexpected precarious donor left hepatic arterial anatomy. Living-donor liver transplantation (LDLT) using left liver for adults was first performed by Makuuchi in 1993. The first case of right liver adult LDLT was performed by Fan in 1996. A priori, the right liver graft included the middle hepatic vein (MHV) to address the problems of small-for-size syndrome by providing good venous outflow of the right anterior section. The first seven recipients who underwent right LDLT all had acute liver failure before the transplantation; one died of candidiasis, and the other six survived. Subsequently, semiurgent and elective cases were accepted.
Donor workup is started after indication for LDLT for the potential recipient is established (see Chapter 105 ). The workup helps evaluate whether the donor will be psychologically and physically healthy in the long term after recovery from the organ donation. In a stepwise manner, expedience is achieved without omission. Only individuals of good health who have reached the age of consent are accepted.
A detailed medical history is taken to identify comorbidities, if any. A body mass index of 30 kg/m 2 or more (27 kg/m 2 for Asians) raises the concern for fatty liver and obesity-related comorbidities. Blood group compatibility is verified. Carriers of the human immunodeficiency virus, hepatitis B virus, or hepatitis C virus (see Chapter 11 ) are denied liver donation. Although a status of hepatitis B core antibody positivity by itself does not preclude liver donation, it mandates lifelong prophylaxis with lamivudine or entecavir in the recipient.
A psychological assessment is performed to verify the potential donor’s knowledge of the operation to be performed and coping abilities. The donor should have adequate knowledge of donor and recipient morbidity, mortality, and survival and should be apprised of the urgency of the transplant.
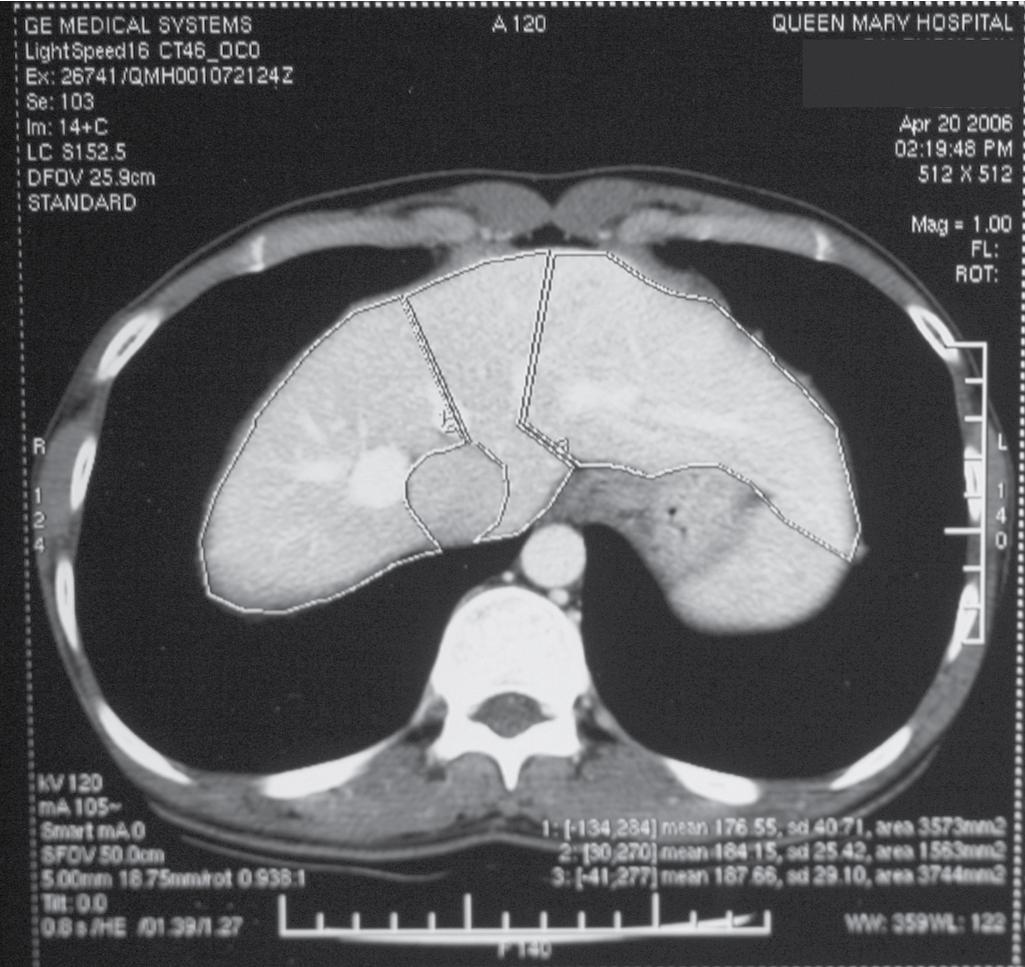
Chest radiographs are taken, and an electrocardiogram is performed. Computed tomography (CT) of the liver under sodium bicarbonate cover is also performed, and maximum-intensity projections (MIPs) of the hepatic veins (HVs) and portal veins (PVs) are produced (see Chapters 13 and 14 ). Volumetry of the donor liver by the Heymsfield method measures the volume of the right liver and left liver (for pediatric recipients, segments II and III), using the MHV as a demarcation line on the plane between the right and left liver ( Fig. 121.1 ) (see Chapters 4 , 101 , and 102 ). Attenuation of the liver parenchyma in comparison with the spleen on the plain film is appraised for detection of fatty change. HV anatomy (see Chapter 2 ) is determined by the axial cuts in the venous phase and by MIPs for easier appreciation. The presence of any inferior right HVs allows anticipation at operation of either preservation or division. Attention to the presence of the segment IVb HV or segment III HV draining into the MHV calls for a more caudal division of the MHV, to preserve adequate drainage of segment IV of the remnant left liver. The right, left, and segment IV hepatic arteries are also illustrated by three-dimensional reconstructions for images obtained during the arterial phase.
Liver biopsy is performed in selective donors and after genuineness and suitability of the potential donor is ascertained. Steatosis (see Chapter 69 ) of up to 15% for right liver donors and 20% for left liver donors are acceptable.
Informed consent is obtained from the donor and the donor’s relative. Donor and recipient morbidity and mortality risks are stated explicitly.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here